Colour Preference of Post Hatchling Hawksbill (Eretmochelys imbricata) and Green (Chelonia mydas) Sea Turtles in Captivity
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Husbandry
2.2. Experimental Design
2.2.1. Set-Up
2.2.2. Recording Behaviour
2.3. Data Analysis and Statistics
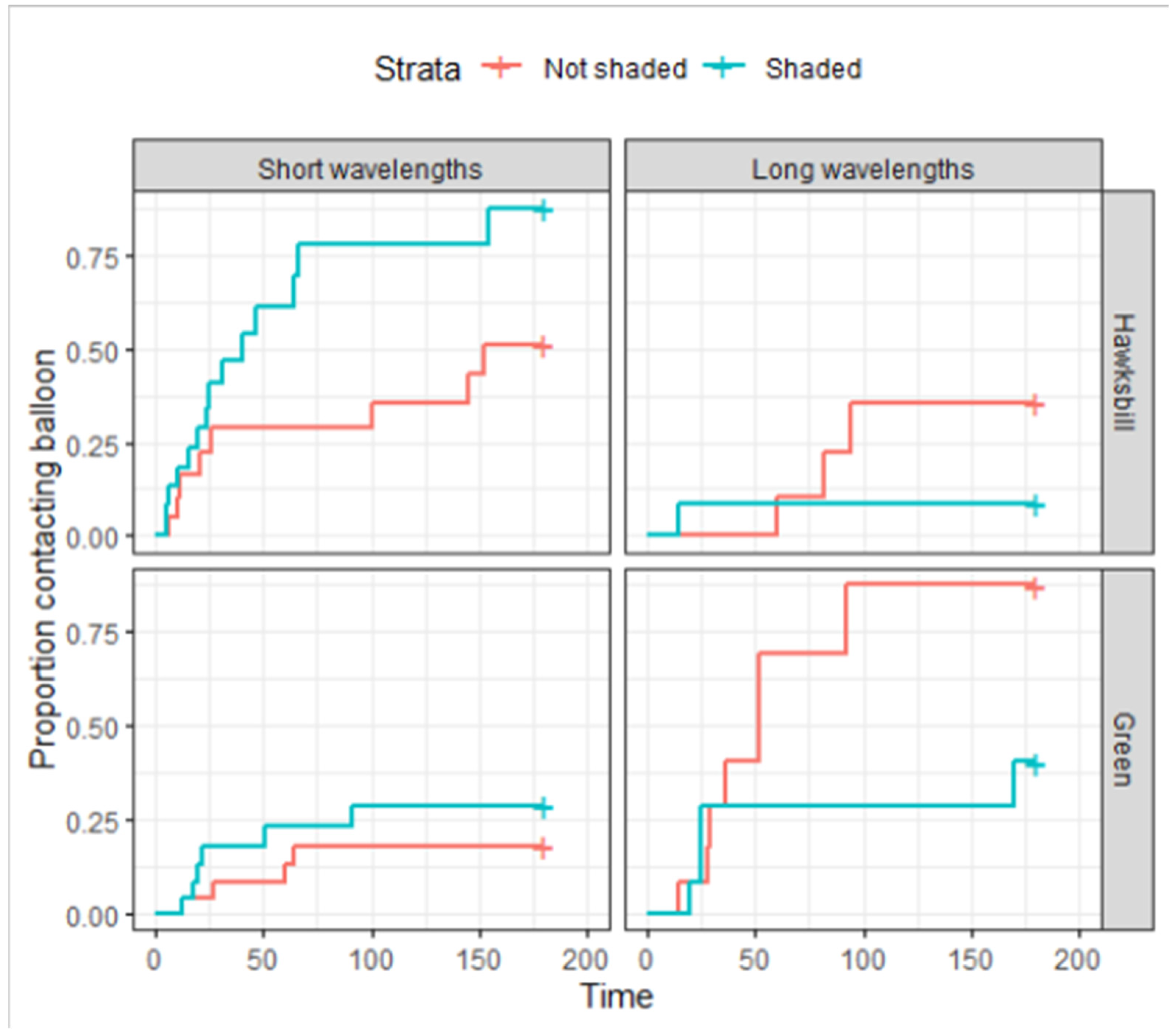
2.3.1. Analysis of the Probability of Contact with a Balloon
2.3.2. Analysis of the Time Taken for Turtles to Contact Balloons
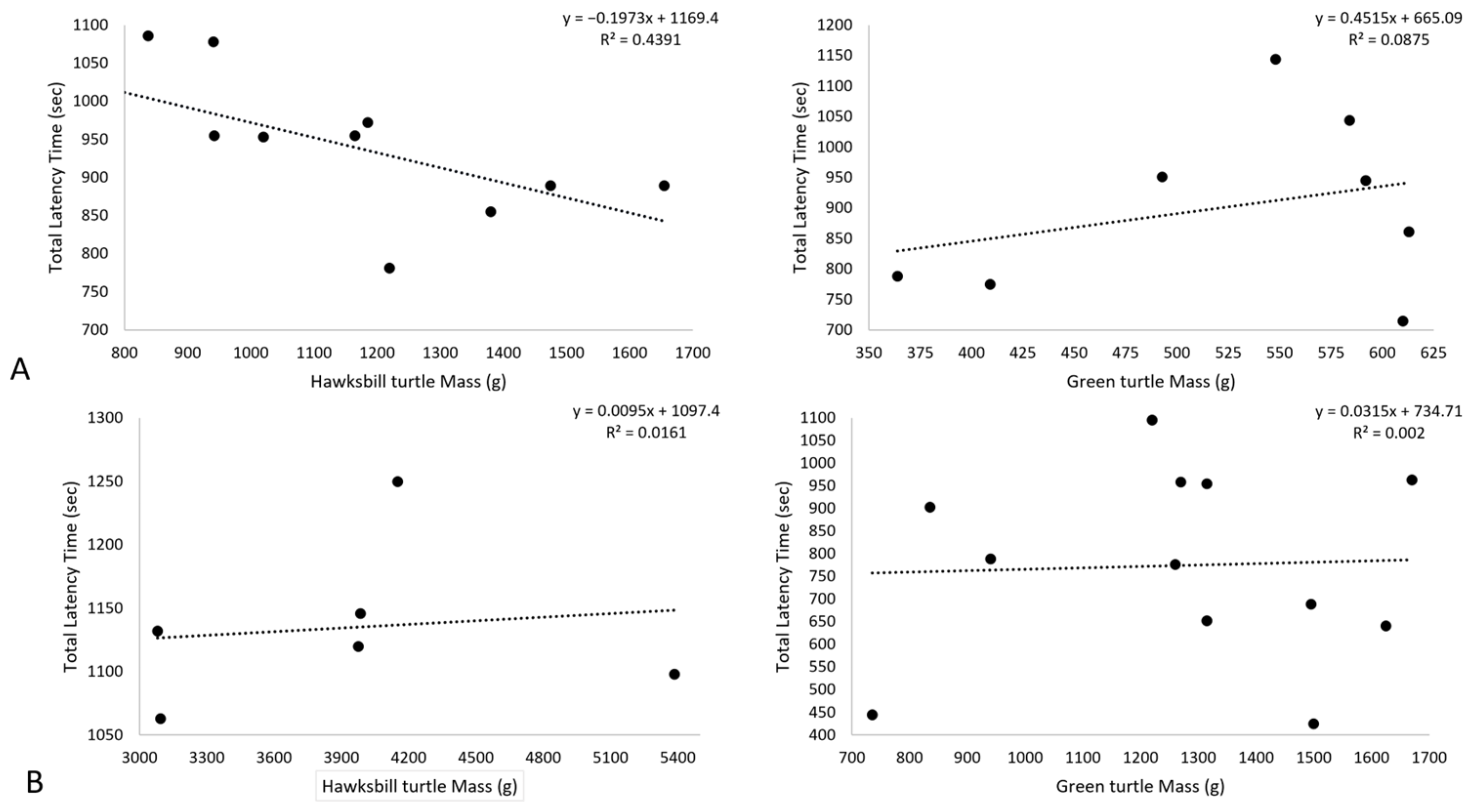
2.3.3. Mass and Contact Time, and Light Intensity Comparison
3. Results
3.1. Probability of Contact with a Balloon
3.2. Time Taken for Turtles to Contact a Balloon
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hauzman, E.; Bonci, D.M.O.; Ventura, D.F. Retinal Topographic Maps: A glimpse into the Animals’ Visual World. In Sensory Nervous System; IntechOpen: London, UK, 2018; pp. 101–126. [Google Scholar]
- Kelber, A.; Osorio, D. From spectral information to animal colour vision: Experiments and concepts. Proc. R. Soc. B 2010, 277, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Gerl, E.J.; Morris, M.R. The Causes and Consequences of Color Vision. Evol. Educ. Outreach 2008, 1, 476–486. [Google Scholar] [CrossRef]
- Oliver, L.J.; Salmon, M.; Wyneken, J.; Hueter, R.; Cronin, T.W. Retinal anatomy of hatchling sea turtles: Anatomical specializations and behavioural correlates. Mar. Freshw. Behav. Physiol. 2000, 33, 233–248. [Google Scholar] [CrossRef]
- Stevens, M.; Ruxton, G.D. Linking the evolution and form of warning coloration in nature. Proc. R. Soc. B 2012, 279, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Cheney, K.L.; Skogh, C.; Hart, N.S.; Marshall, N.J. Mimicry, colour forms and spectral sensitivity of the bluestriped fangblenny, Plagiotremus rhinorhynchos. Proc. R. Soc. B 2009, 276, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Klint, T. Influence of male nutial plumage on mate selection in the female mallard (Anas platyrhynchos). Anim. Behav. 1980, 28, 1230–1238. [Google Scholar] [CrossRef]
- Endler, J.A.; Day, L.B. Ornament colour selection, visual contrast and the shape of colour preference functions in great bowerbirds, Chlamydera nuchalis. Anim. Behav. 2006, 72, 1405–1416. [Google Scholar] [CrossRef]
- Dunn, P.O.; Armenta, J.K.; Whittingham, L.A. Natural and sexual selection act on different axes of variation in avian plumage color. Evol. Biol. 2015, 1, e1400155. [Google Scholar] [CrossRef] [PubMed]
- Belliure, J.; Fresnillo, B.; Cuervo, J.J. Male mate choice based on female coloration in a lizard: The role of a juvenile trait. Behav. Ecol. 2018, 29, 543–552. [Google Scholar] [CrossRef]
- Weber, S.P.; Weber, P.G. The effect of female color, size, dominance and early experience up mate selection in male convict cichlids, Cichlasoma Nigrofasciat um Gunther (Pisces, Chichllidae). Behaviour 1976, 56, 116–135. [Google Scholar] [CrossRef]
- Maan, M.E.; Seehausen, O.; Van Alphen, J.J.M. Female mating preferences and male coloration covary with water transparency in a Lake Victoria cichlid fish. Biol. J. Linn. Soc. 2010, 99, 398–406. [Google Scholar] [CrossRef]
- Osorio, D.; Vorobyev, M. A review of the evolution of animal colour vision and visual communication signals. Vis. Res. 2008, 48, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Kelber, A.; Vorobyev, M.; Osorio, D. Animal colour vision—Behavioural tests and physiological concepts. Biol. Rev. 2003, 78, 81–118. [Google Scholar] [CrossRef]
- Swihart, C.A.; Swihart, S.L. Colour selection and learner feeding preferences in the butterfly, Iheliconius charitonius Linn. Anim. Behav. 1970, 18, 60–64. [Google Scholar] [CrossRef]
- Gumbert, A. Color choices by bumble bees (Bombus terrestris): Innate preferences and generalization after learning. Behav. Ecol. Sociobiol. 2000, 48, 36–43. [Google Scholar] [CrossRef]
- Roth, L.S.V.; Kelber, A. Nocturnal colour vision in geckos. R. Soc. 2004, 271, 5485–5487. [Google Scholar] [CrossRef] [PubMed]
- Young, M.; Salmon, M.; Forward, R. Visual wavelength discrimination by the loggerhead turtle, Caretta caretta. Biol. Bullitin 2012, 222, 46–55. [Google Scholar] [CrossRef]
- Zaccardi, G.; Kelber, A.; Sison-Mangus, M.P.; Briscoe, A.D. Color discrimination in the red range with only one long-wavelength sensitive opsin. J. Exp. Biol. 2012, 209, 1944–1955. [Google Scholar] [CrossRef]
- Hall, R.J.; Robson, S.K.A.; Ellen, A. Colour vision of green turtle (Chelonia mydas) hatchlings: Do they still prefer blue under water? PeerJ 2018, 6, e5572. [Google Scholar] [CrossRef]
- Mrosovsky, N.; Carr, A. Preference for light of short wavelengths in hatchling green sea turtles, Chelonia mydas, tested on their natural nesting beaches. Behaviour 1967, 28, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Mrosovsky, N.; Shettleworth, S.J. Wavelength preferences and brightness cues in the water finding behaviour of Sea Turtles. Behaviour 1968, 32, 211–257. [Google Scholar] [CrossRef]
- Witherington, B.E.; Bjorndal, K.A. Influences of artificial lighting on the seaward orientation of hatchling loggerhead turtles (Caretta caretta). Biol. Conserv. 1991, 55, 139–149. [Google Scholar] [CrossRef]
- Mrosovsky, N. The water-finding ability of sea turtles. Behav. Stud. Physiol. Specul. 1972, 5, 202–225. [Google Scholar] [CrossRef] [PubMed]
- Schuyler, Q.A.; Wilcox, C.; Townsend, K.; Hardesty, B.D.; Marshall, N.J. Mistaken identity? Visual similarities of marine debris to natural prey items of sea turtles. BMC Ecol. 2014, 14, 14. [Google Scholar] [CrossRef]
- Mathger, L.M.; Litherland, L.; Fritsches, K.A. An anatomical study of the visual capabilities of the green turtle, Chelonia mydas. Am. Soc. Ichthyol. Herpetol. 2007, 1, 169–179. [Google Scholar] [CrossRef]
- Kophamel, S.; Rudd, D.; Ward, L.C.; Shum, E.; Ariel, E.; Mendez, D.; Starling, J.; Mellers, R.; Burchell, R.K.; Munns, S.L. Haematological and biochemical reference intervals for wild green turtles (Chelonia mydas): A Bayesian approach for small sample sizes. Convservation Physiol. 2022, 10, coac043. [Google Scholar] [CrossRef]
- Bissonette, J.A. Small sample size problems in wildlife ecology: A contingent analytical approach. Wildl. Biololgy 1999, 5, 65–71. [Google Scholar] [CrossRef]
- Levenson, D.H.; Eckert, S.A.; Crognale, M.A.; Deegan, J.F.; Jacobs, G.H. Photopic spectral sensitivity of green and loggerhead sea turtles. Copia 2004, 4, 908–914. [Google Scholar] [CrossRef]
- Bartol, S.M.; Musick, J.A. Sensory Biology of Sea Turtles; CRC Press LLC.: Boca Raton, FL, USA, 2002; Volume 2. [Google Scholar]
- Wilby, D.; Roberts, N.W. Optical influence of oil droplets on cone photoreceptor sensitivity. J. Exp. Biol. 2017, 220, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Fritsches, K.A. Australian Loggerhead sea turtle hatchlings do not avoid yellow. Mar. Freshw. Behav. Physiol. 2012, 45, 79–89. [Google Scholar] [CrossRef]
- Hu, M.; Hu, Z.; Yang, Z.; Zheng, Z.; He, D.; Zhang, X. Guinea pigs reared in a monochromatic environment exhibit changes in cone density and opsin expression. Exp. Eye Res. 2011, 6, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, G.H.; Fenwick, J.A.; Williams, G.A. Cone-based vision of rats for ultraviolet and visible lights. J. Exp. Biol. 2001, 204, 2439–2446. [Google Scholar] [CrossRef] [PubMed]
- Warrant, E.J.; Johnsen, S. Vision and the light environment. Curr. Biol. 2013, 23, R990–R994. [Google Scholar] [CrossRef] [PubMed]
- Veilleux, C.C.; Lewis, R.J. Effects of habitat light intensity on mammalian eye shape. Anat. Rec. 2011, 294, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Thèry, M. Forest light and its influence on habitat selection. Plant Ecol. 2001, 153, 251–261. [Google Scholar] [CrossRef]
- Gislen, A.; Gislen, L. On the optical theory of underwater vision in humans. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2004, 11, 2061–2064. [Google Scholar] [CrossRef]
- Doron, M.; Babin, M.; Mangin, A.; Hembise, O. Estimation of light penetration, and horizontal and vertical visibility in oceanic and coastal waters from surface reflectance. J. Geophys. Res. Ocean. 2007, 112. [Google Scholar] [CrossRef]
- Duntley, S.Q. Light in the Sea. J. Opt. Soc. Am. 1963, 53, 215–233. [Google Scholar] [CrossRef]
- Boyle, M.C.; Limpus, C.J. The stomach contents of post-hatchling green and loggerhead sea turtles in the southwest Pacific: An insight into habitat association. Mar. Biol. 2008, 155, 233–241. [Google Scholar] [CrossRef]
- Brandis, R.G.; Mortimer, J.A.; Reilly, B.K. In-water observations of the diving behaviour of immature Hawksbill Turtles, Eretmochelys imbricata, on a coral reef at D’Arros Island, Republic of Seychelles. Chelonian Conversat. Biol. 2010, 9, 26–32. [Google Scholar] [CrossRef]
- Leon, Y.M.; Bjorndal, K.A. Selective feeding in the hawksbill turtles, an important predator in coral reef ecosystems. Mar. Ecol. Prog. Ser. 2002, 245, 249–258. [Google Scholar] [CrossRef]
- Hailman, J.P.; Jaeger, R.G. On Criteria for Color Preferences in Turtles. J. Herpetol. 1971, 5, 83–85. [Google Scholar] [CrossRef]

| Behaviour | Explanation |
|---|---|
| Resting | For green turtles, front flippers were placed flat on the carapace and back flippers were either out to the sides or tucked under the plastron. Green turtles rested while floating on the surface of the water. |
| For hawksbill turtles, front and back flippers were either positioned away from the body in a star shape or tucked under the plastron. Hawksbill turtles rested on the floor of the pen. | |
| Diving | Turtle is completely submerged below the surface and resurfacing quickly. |
| Swimming (Wall Hugging) | Turtle swimming with plastron against the walls of the compartment, often lapping the full length of the compartment, or going from the wall opposite the balloon to the halfway area and then lapping back, remaining in the opposite half of the compartment from the balloon. |
| Swimming (Corner) | Turtle is facing away from balloon and actively swimming into a corner at the opposite end of the compartment from the balloon. Often, the turtle is pushing its head out of the water. |
| Splashing | Turtle slapping the surface of the water while quickly swimming around the compartment, usually while swimming along the walls or into a corner. Seen as a possible sign of agitation. |
| Balloon Touch (Flipper) | Turtle approaching a balloon close enough to hit with a front flipper. These touches were seen when a turtle was turning around to swim away from a balloon and not as a deliberate touch. |
| Balloon Touch (Carapace) | Turtle hitting a balloon with any part of the carapace, most often the back end, while turning around and swimming away from a balloon and therefore not regarded as a deliberate touch. |
| Carapace Turn | Turtle making a sharp turn in front of balloon exposing the majority of the carapace at a balloon. This behaviour was often repeated with turtle lapping the half of the compartment opposite to balloon and swimming quickly. |
| Balloon Touch (Beak) | Turtle touching a balloon with beak/mouth. Any form of beak to balloon contact was considered a deliberate touch including mouth open, mouth closed, from above or below the balloon. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drake, J.; Khayat, M.F.; Jones, R.; Ariel, E. Colour Preference of Post Hatchling Hawksbill (Eretmochelys imbricata) and Green (Chelonia mydas) Sea Turtles in Captivity. Animals 2025, 15, 628. https://doi.org/10.3390/ani15050628
Drake J, Khayat MF, Jones R, Ariel E. Colour Preference of Post Hatchling Hawksbill (Eretmochelys imbricata) and Green (Chelonia mydas) Sea Turtles in Captivity. Animals. 2025; 15(5):628. https://doi.org/10.3390/ani15050628
Chicago/Turabian StyleDrake, Jordan, Mohammed F. Khayat, Rhondda Jones, and Ellen Ariel. 2025. "Colour Preference of Post Hatchling Hawksbill (Eretmochelys imbricata) and Green (Chelonia mydas) Sea Turtles in Captivity" Animals 15, no. 5: 628. https://doi.org/10.3390/ani15050628
APA StyleDrake, J., Khayat, M. F., Jones, R., & Ariel, E. (2025). Colour Preference of Post Hatchling Hawksbill (Eretmochelys imbricata) and Green (Chelonia mydas) Sea Turtles in Captivity. Animals, 15(5), 628. https://doi.org/10.3390/ani15050628





