Insight into the Potential of Somatostatin Vaccination with Goats as a Model: From a Perspective of the Gastrointestinal Microbiota
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
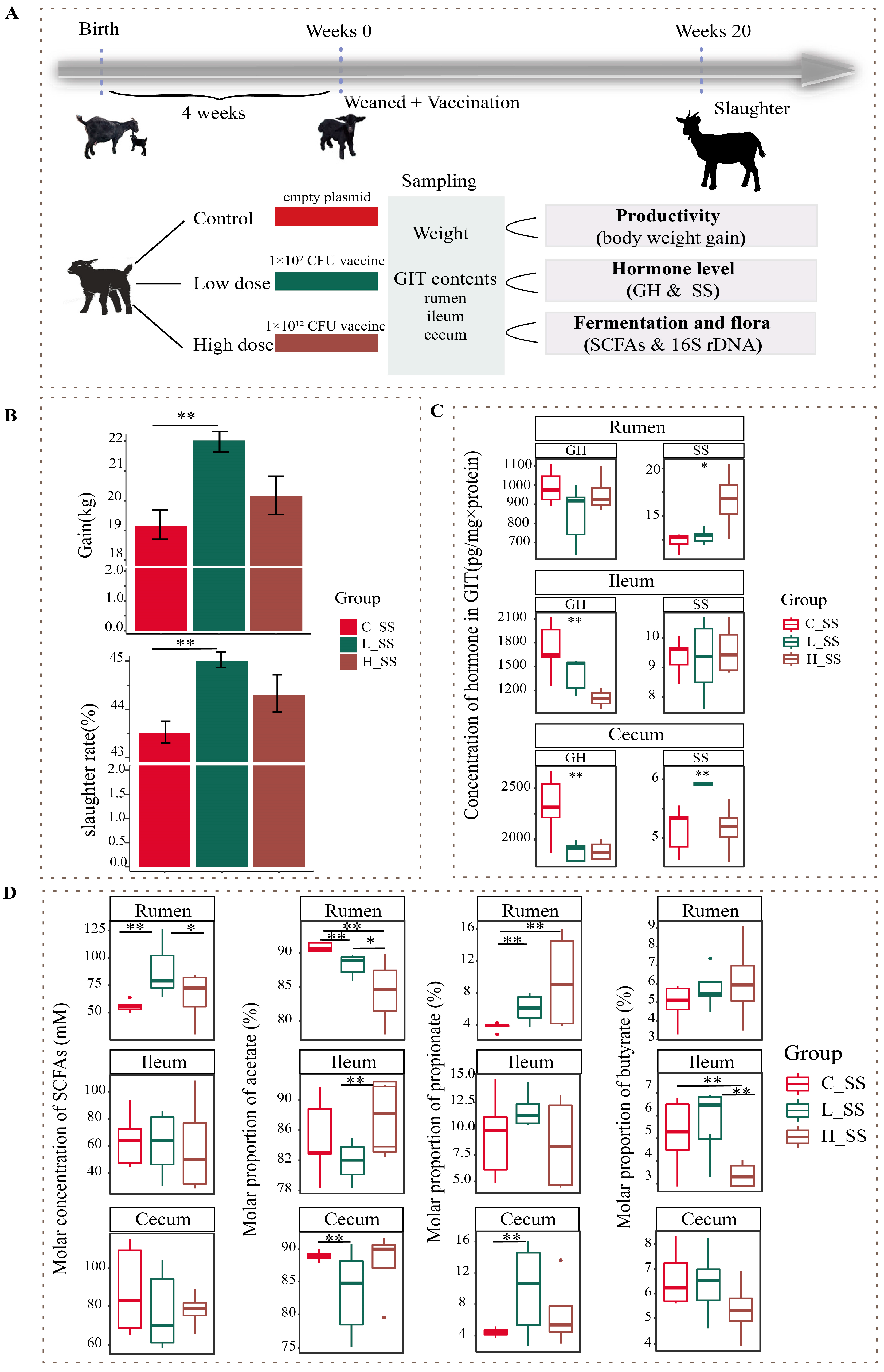
2.1. Animals and Experimental Design
2.2. Sample Collection
2.3. Determination of SS Antibody and Hormone Assays by Radioimmunoassay
2.4. Short-Chain Fatty Acids Detected by Liquid Chromatography
2.5. DNA Extraction
2.6. Amplicon Sequencing and Bioinformatics Analysis
2.7. Statistical Analysis and Data Visualization
3. Results
3.1. Anti-Somatostatin Antibody Response and Hormone Level as Well as Body Weight Gain Altered by Immunoneutralization SS
3.2. Fermentation Pattern of GIT Was Altered by SS Vaccine with a Dose- and Region-Dependent Manner
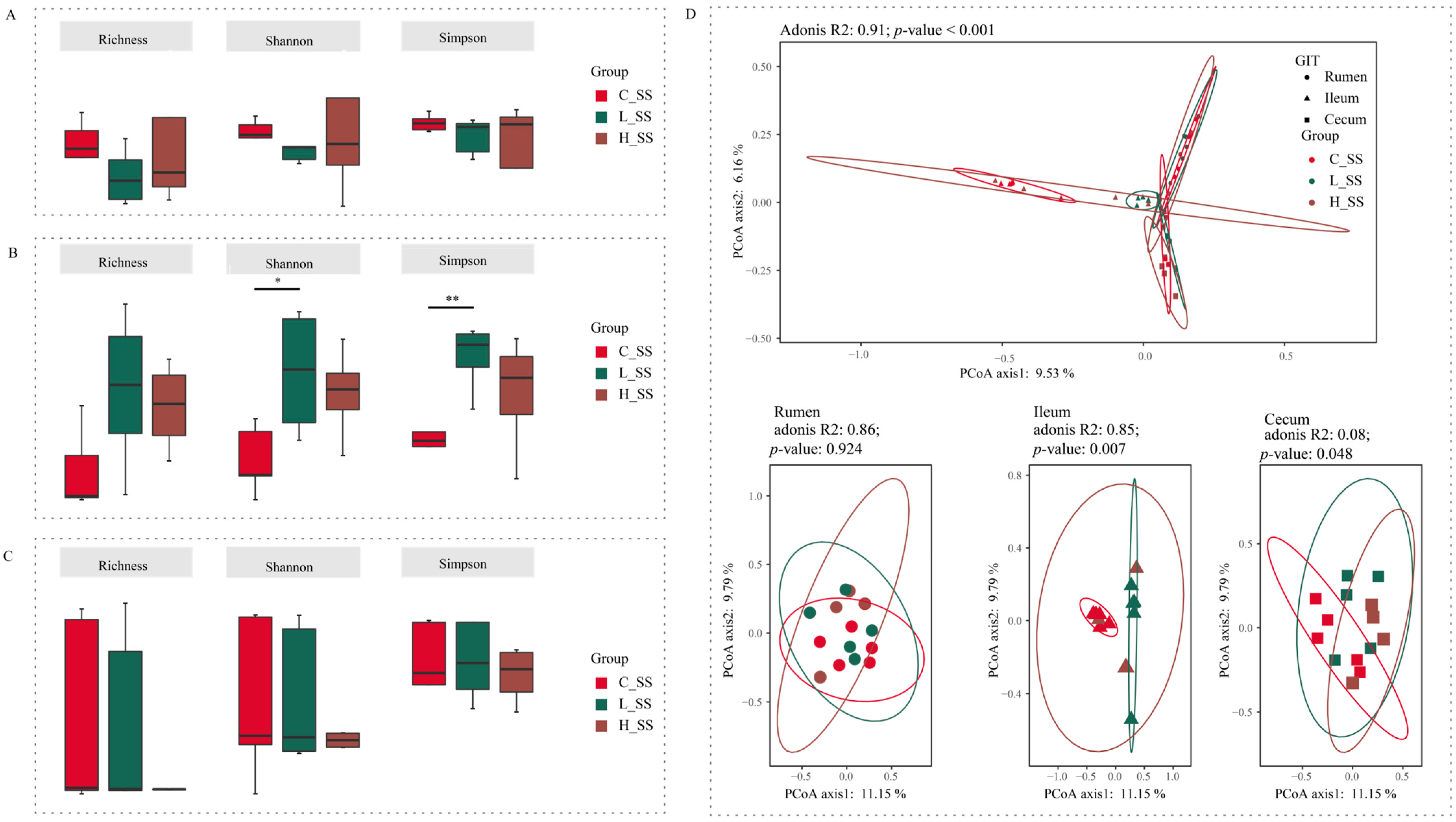
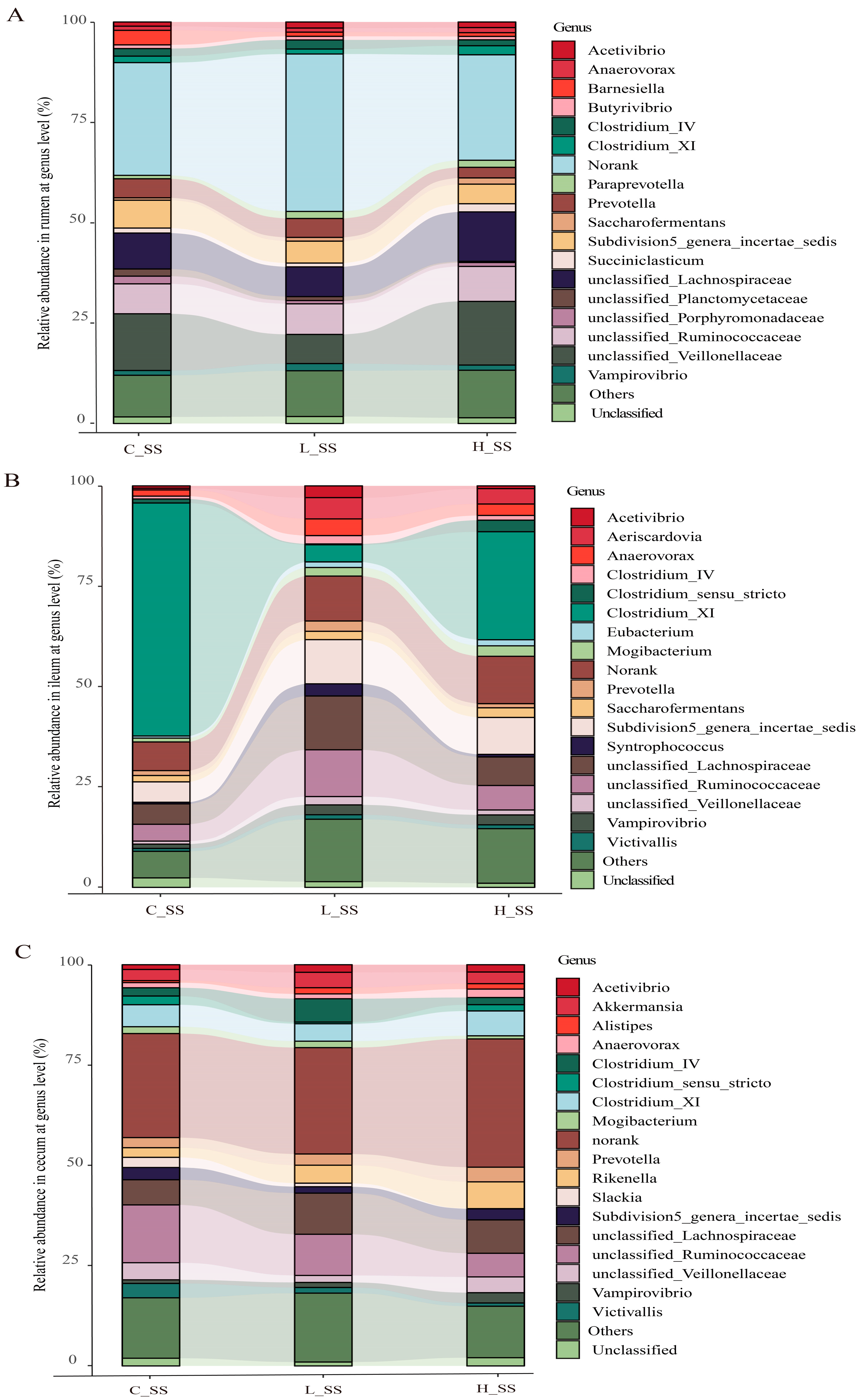
3.3. The Different Dose SS Vaccination Selected Distinct Microbial Communities in GIT
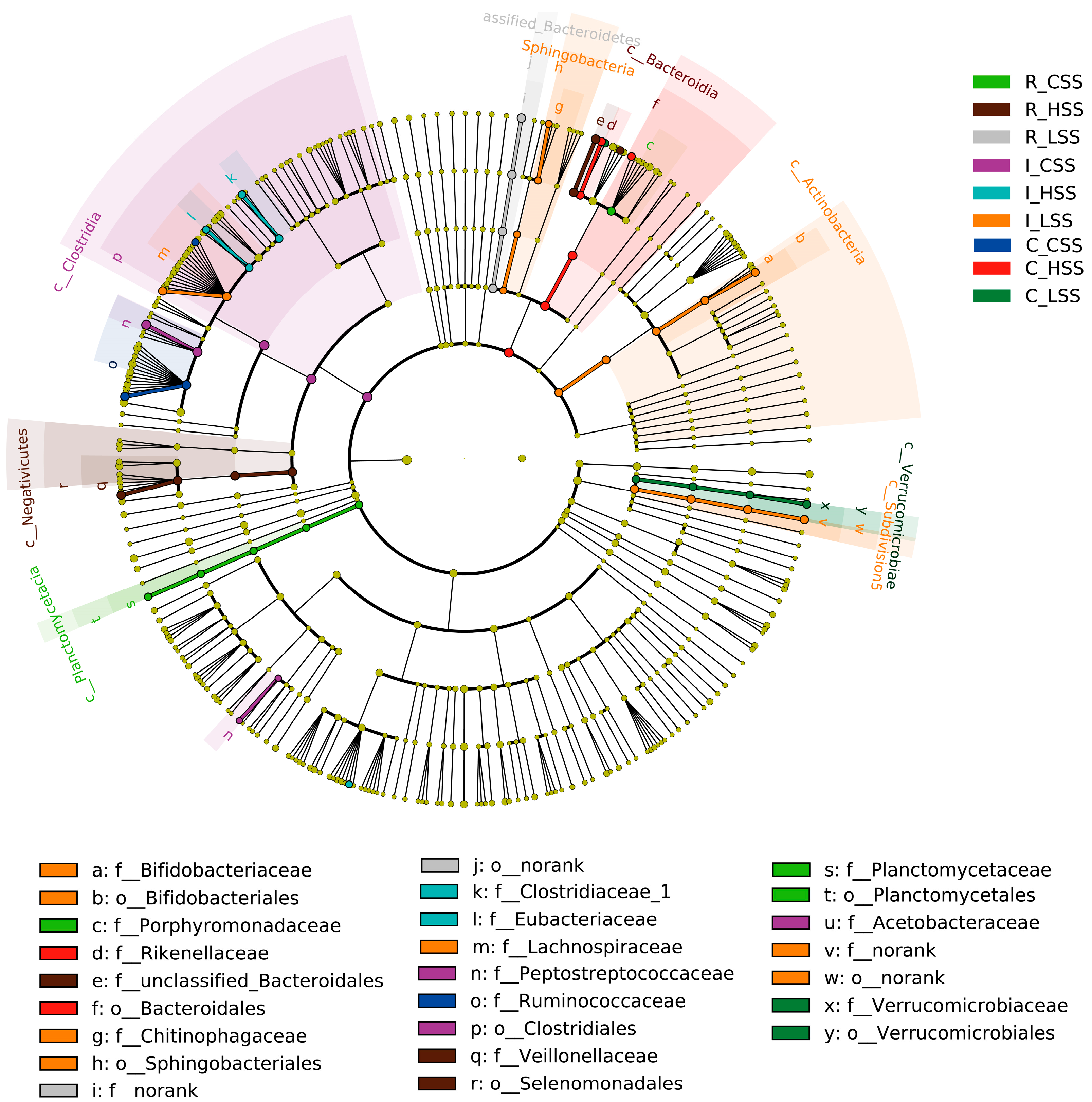
3.4. The Discriminative Microbiota Among Different-Dose SS Vaccination Groups in GIT
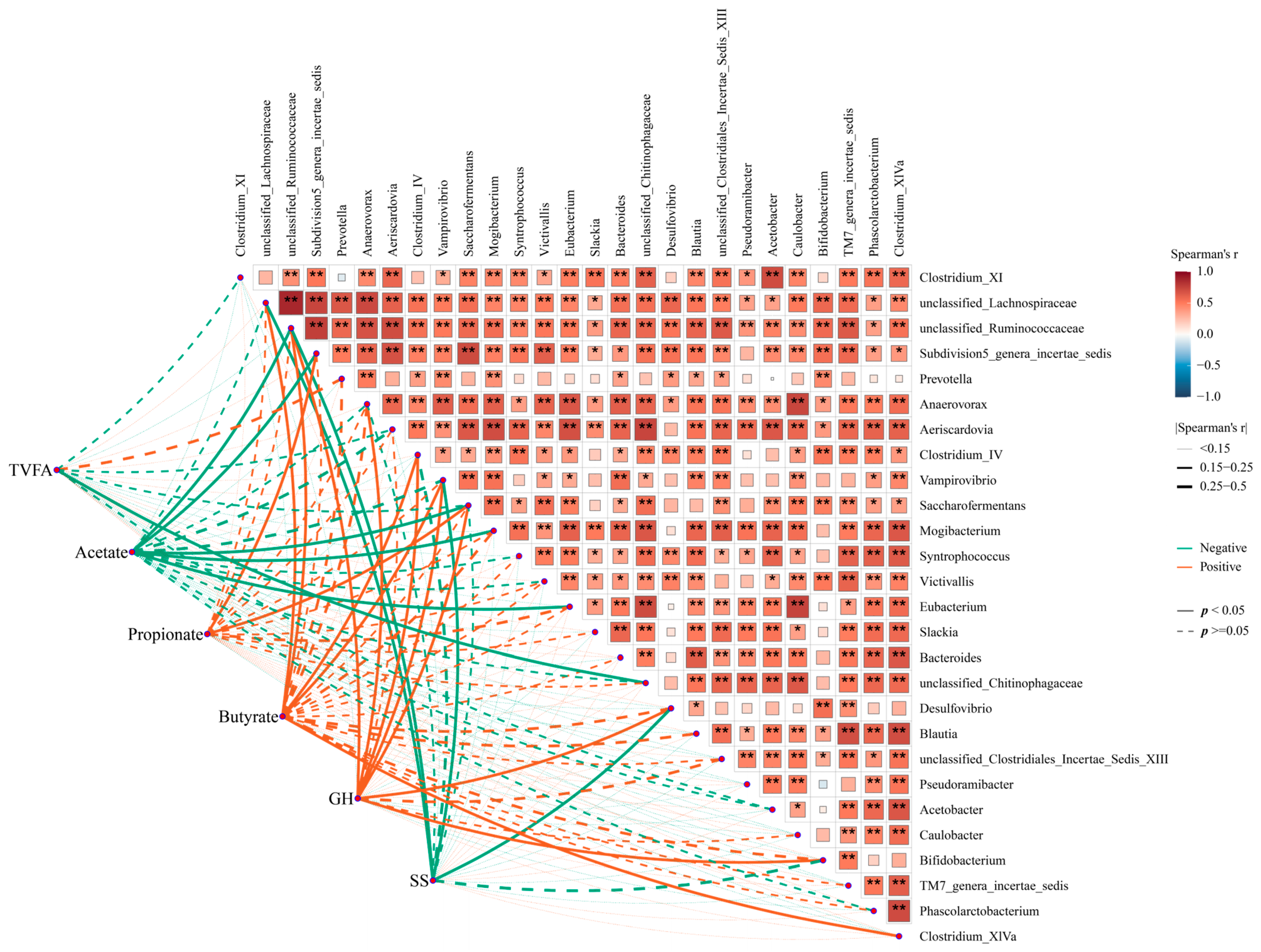
3.5. Prediction of the Functional Variation of Microbiota in GIT by SS Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.; Zhang, X.; Wang, M.; Zhou, C.; Jiao, J.; Tan, Z. Enhancing metabolic efficiency through optimizing metabolizable protein profile in a time progressive manner with weaned goats as a model: Involvement of gut microbiota. Microbiol. Spectr. 2022, 10, e0254521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, J.; Zhou, C.; Wang, M.; Tan, Z.; Jiao, J. Temporal changes in muscle characteristics during growth in the goat. Meat Sci. 2023, 200, 109145. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rodriguez, J.; Urrutia, O.; Lobon, S.; Ripoll, G.; Bertolin, J.R.; Joy, M. Insights into the role of major bioactive dietary nutrients in lamb meat quality: A review. J. Anim. Sci. Biotechnol. 2022, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, I.; Wallace, R.J.; Morais, S. The rumen microbiome: Balancing food security and environmental impacts. Nat. Rev. Microbiol. 2021, 19, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Nations, U. World Population Prospects 2022: Data Sources. (UN DESA/POP/2022/DC/NO. 9). 2022. Available online: https://www.un.org/development/desa/pd/content/World-Population-Prospects-2022 (accessed on 11 January 2024).
- Rahimi, J.; Fillol, E.; Mutua, J.Y.; Cinardi, G.; Robinson, T.P.; Notenbaert, A.M.O.; Ericksen, P.J.; Graham, M.W.; Butterbach-Bahl, K. A shift from cattle to camel and goat farming can sustain milk production with lower inputs and emissions in north sub-Saharan Africa’s drylands. Nat. Food 2022, 3, 523–531. [Google Scholar] [CrossRef]
- Tester, M.; Langridge, P. Breeding Technologies to Increase Crop Production in a Changing World. Science 2010, 327, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Lupu, F.; Terwilliger, J.D.; Lee, K.; Segre, G.V.; Efstratiadis, A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev. Biol. 2001, 229, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Kubrak, O.; Koyama, T.; Ahrentløv, N.; Jensen, L.; Malita, A.; Naseem, M.T.; Lassen, M.; Nagy, S.; Texada, M.J.; Halberg, K.V.; et al. The gut hormone allatostatin C/Somatostatin regulates food intake and metabolic homeostasis under nutrient stress. Nat. Commun. 2022, 13, 692. [Google Scholar] [CrossRef]
- Han, Y.-G.; Han, Y.-Q.; Wu, J.-Y.; Guo, J.; Na, R.-S.; Zeng, Y.; Ee, G.-X.; Zhao, Y.-J.; Huang, Y.-F. Immunisation of somatostatin DNA vaccine in ewes can affect serum somatostatin and prolactin levels in offspring lambs through maternal related hormones. Pak. J. Zool. 2020, 52, 801–804. [Google Scholar] [CrossRef]
- Han, Y.-G.; Peng, X.-L.; Li, K.; Zhao, Y.-H.; Jiang, X.-P.; Ee, G.-X.; Zhao, Y.-J.; Ye, J.-H.; Xu, L.; Zhao, Q.-T.; et al. Evaluation of the fusion type CpG adjuvant for the enhancement of somatostatin DNA vaccine in ram lambs. Pak. J. Zool. 2019, 51, 413–419. [Google Scholar] [CrossRef]
- Coate, K.C.; Kliewer, S.A.; Mangelsdorf, D.J. SnapShot: Hormones of the gastrointestinal tract. Cell 2014, 159, 1478–1478.e1. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Farzi, A. Neuropeptides and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014, 817, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-G.; Ye, J.-H.; Zhao, Q.-T.; Huang, Y.-J.; Li, K.; Xu, L. Oral SS-14 DNA vaccine is more potent than oral SS-28 DNA vaccine in promoting rat lactation. Pak. J. Zool. 2019, 51, 1711–1719. [Google Scholar] [CrossRef]
- Lustig, Y.; Gonen, T.; Meltzer, L.; Gilboa, M.; Indenbaum, V.; Cohen, C.; Amit, S.; Jaber, H.; Doolman, R.; Asraf, K.; et al. Superior immunogenicity and effectiveness of the third compared to the second BNT162b2 vaccine dose. Nat. Immunol. 2022, 23, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.; Fukushima, W.; Nakagama, Y.; Kido, Y.; Kase, T.; Kondo, K.; Kaku, N.; Matsumoto, K.; Suita, A.; Mukai, E.; et al. Factors impacting antibody kinetics, including fever and vaccination intervals, in SARS-CoV-2-naïve adults receiving the first four mRNA COVID-19 vaccine doses. Sci. Rep. 2024, 14, 7217. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.G.; Liang, A.X.; Han, L.; Guo, A.; Jiang, X.; Yang, L. Efficacy and safety of an oral somatostatin DNA vaccine without antibiotic resistance gene in promoting growth of piglets. Scand. J. Immunol. 2014, 79, 244–250. [Google Scholar] [CrossRef]
- Jiao, J.; Zhou, C.; Guan, L.L.; McSweeney, C.S.; Tang, S.; Wang, M.; Tan, Z. Shifts in host mucosal innate immune function are associated with ruminal microbial succession in supplemental feeding and grazing goats at different ages. Front. Microbiol. 2017, 8, 1655. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Zhou, C.; Tan, Z.; Jiao, J. Spatial and temporal organization of jejunal microbiota in goats during animal development process. J. Appl. Microbiol. 2020, 131, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Wu, J.; Jiao, J.; He, Z.; Tan, Z.; Han, X. Rumen-protected glucose supplementation in transition dairy cows shifts fermentation patterns and enhances mucosal immunity. Anim. Nutr. 2021, 7, 1182–1188. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Han, X.; Tan, Z.; Jiao, J. Effects of rumen-protected glucose on ileal microbiota and genes involved in ileal epithelial metabolism and immune homeostasis in transition dairy cows. Anim. Feed. Sci. Technol. 2019, 254, 114199. [Google Scholar] [CrossRef]
- Chen, P.; Xu, H.; Tang, H.; Zhao, F.; Yang, C.; Kwok, L.; Cong, C.; Wu, Y.; Zhang, W.; Zhou, X.; et al. Modulation of gut mucosal microbiota as a mechanism of probiotics-based adjunctive therapy for ulcerative colitis. Microb. Biotechnol. 2020, 13, 2032–2043. [Google Scholar] [CrossRef]
- Esther, C.R., Jr.; Muhlebach, M.S.; Ehre, C.; Hill, D.B.; Wolfgang, M.C.; Kesimer, M.; Ramsey, K.A.; Markovetz, M.R.; Garbarine, I.C.; Forest, M.G.; et al. Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis. Sci. Transl. Med. 2019, 11, eaav3488. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Oksanen, J.A.I. Vegan: Ecological Diversity. 2016. Available online: https://www.researchgate.net/publication/260135884_Vegan_ecological_diversity (accessed on 11 January 2024).
- Douglas, G.; Maffei, V.; Zaneveld, J.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Ginestet, C. ggplot2: Elegant graphics for data analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2011, 174, 245. [Google Scholar] [CrossRef]
- Huang, H. linkET: Everything Is Linkable. R Package Version 0.0.3. 2021. Available online: https://github.com/Hy4m/linkET#:~:text=linkET.%20The%20goal%20of%20linkET%20is (accessed on 11 January 2024).
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Na, R.; Jiang, X.; Wu, J.; Han, Y.; Zeng, Y.; Ee, G.; Liang, A.; Yang, L.; Zhao, Y.; et al. Effect of a novel somatostatin-14 DNA vaccine fused to tPA signal peptide and CpG adjuvant on goat lactation and milk composition. Small Rumin. Res. 2020, 187, 106107. [Google Scholar] [CrossRef]
- Liang, A.; Cao, S.; Han, L.; Yao, Y.; Moaeen, M.; Yang, L. Construction and evaluation of the eukaryotic expression plasmid encoding two copies of somatostatin genes fused with hepatitis B surface antigen gene S. Vaccine 2008, 26, 2935–2941. [Google Scholar] [CrossRef]
- Uhl, P.; Lowengrub, J.; Komarova, N.; Wodarz, D. Spatial dynamics of feedback and feedforward regulation in cell lineages. PLoS Comput. Biol. 2022, 18, e1010039. [Google Scholar] [CrossRef] [PubMed]
- Corleto, V. Somatostatin and the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes 2010, 17, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ma, X. Dietary Amino Acids and the Gut-Microbiome-Immune Axis: Physiological Metabolism and Therapeutic Prospects. Compr. Rev. Food Sci. Food Saf. 2019, 18, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.Z.; Wu, J.; Zhou, C.S.; He, Z.X.; Tan, Z.L.; Wang, M. Ecological niches and assembly dynamics of diverse microbial consortia in the gastrointestine of goat kids. ISME J. 2024, 18, wrae002. [Google Scholar] [CrossRef]
- Stewart, R.D.; Auffret, M.D.; Warr, A.; Walker, A.W.; Roehe, R.; Watson, M. Compendium of 4941 rumen metagenome-assembled genomes for rumen microbiome biology and enzyme discovery. Nat. Biotechnol. 2019, 37, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Cabral, L.; Persinoti, G.F.; Paixao, D.A.A.; Martins, M.P.; Morais, M.A.B.; Chinaglia, M.; Domingues, M.N.; Sforca, M.L.; Pirolla, R.A.S.; Generoso, W.C.; et al. Gut microbiome of the largest living rodent harbors unprecedented enzymatic systems to degrade plant polysaccharides. Nat. Commun. 2022, 13, 629. [Google Scholar] [CrossRef] [PubMed]
- Calusinska, M.; Marynowska, M.; Bertucci, M.; Untereiner, B.; Klimek, D.; Goux, X.; Sillam-Dussès, D.; Gawron, P.; Halder, R.; Wilmes, P.; et al. Integrative omics analysis of the termite gut system adaptation to Miscanthus diet identifies lignocellulose degradation enzymes. Commun. Biol. 2020, 3, 275. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ma, Y.; Ding, S.; Fang, J.; Liu, G. Regulation of dietary fiber on intestinal microorganisms and its effects on animal health. Anim. Nutr. 2023, 14, 356–369. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2018, 59, S130–S152. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-Q.; Zhang, T.; Yang, W.-G.; Zhong, H.-L.; Xiao, P.; Liu, L.-W.; Wang, Y.-W.; Chen, H.; Kong, R.; Wang, G.; et al. Gut microbiota patterns associated with somatostatin in patients undergoing pancreaticoduodenectomy: A prospective study. Cell Death Discov. 2020, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, S.; Zhang, M.; Wang, P.; Liang, G.; Gan, Z.; Gao, X. Unlocking the potential of rosa roxburghii tratt polyphenol: A novel approach to treating acute lung injury from a perspective of the lung-gut axis. Front. Microbiol. 2024, 15, 1351295. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Kuang, X.; Yan, H.; Ren, P.; Yang, X.; Liu, H.; Liu, Q.; Yang, H.; Kang, X.; Shen, X.; et al. A novel synbiotic alleviates autoimmune hepatitis by modulating the gut microbiota-liver axis and inhibiting the hepatic TLR4/NF-kB/NLRP3 signaling pathway. mSystems 2023, 8, e0112722. [Google Scholar] [CrossRef]
- Chang, Y.Q.; Moon, S.K.; Wang, Y.Q.; Xie, L.M.; Cho, H.S.; Kim, S.K. Supplemental effects of different production methods of pine needle additives on growth performance, intestinal environment, meat quality and serum of broiler chickens. Anim. Biosci. 2024, 37, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Di, Y.; Song, Y.; Xu, K.; Wang, Q.; Zhang, L.; Liu, Q.; Zhang, M.; Liu, X.; Wang, Y. Chicoric Acid Alleviates Colitis via Targeting the Gut Microbiota Accompanied by Maintaining Intestinal Barrier Integrity and Inhibiting Inflammatory Responses. J. Agric. Food Chem. 2024, 72, 6276–6288. [Google Scholar] [CrossRef] [PubMed]
- Abdugheni, R.; Wang, W.Z.; Wang, Y.J.; Du, M.; Liu, F.; Zhou, N.; Jiang, C.; Wang, C.; Wu, L.; Ma, J.; et al. Metabolite profiling of human-originated Lachnospiraceae at the strain level. iMeta 2022, 1, e58. [Google Scholar] [CrossRef] [PubMed]
- Jami, M.; Ghanbari, M.; Kneifel, W.; Domig, K.J. Phylogenetic diversity and biological activity of culturable Actinobacteria isolated from freshwater fish gut microbiota. Microbiol. Res. 2015, 175, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Chen, J.; Zhang, S.; Wei, B.; Han, Y.; Zhao, Z. Insight into the Potential of Somatostatin Vaccination with Goats as a Model: From a Perspective of the Gastrointestinal Microbiota. Animals 2025, 15, 728. https://doi.org/10.3390/ani15050728
Zhang X, Chen J, Zhang S, Wei B, Han Y, Zhao Z. Insight into the Potential of Somatostatin Vaccination with Goats as a Model: From a Perspective of the Gastrointestinal Microbiota. Animals. 2025; 15(5):728. https://doi.org/10.3390/ani15050728
Chicago/Turabian StyleZhang, Xiaoli, Juncai Chen, Siqi Zhang, Bingni Wei, Yanguo Han, and Zhongquan Zhao. 2025. "Insight into the Potential of Somatostatin Vaccination with Goats as a Model: From a Perspective of the Gastrointestinal Microbiota" Animals 15, no. 5: 728. https://doi.org/10.3390/ani15050728
APA StyleZhang, X., Chen, J., Zhang, S., Wei, B., Han, Y., & Zhao, Z. (2025). Insight into the Potential of Somatostatin Vaccination with Goats as a Model: From a Perspective of the Gastrointestinal Microbiota. Animals, 15(5), 728. https://doi.org/10.3390/ani15050728





