Scriptaid Improves Cashmere Goat Embryo Reprogramming by Affecting Donor Cell Pluripotency Molecule NANOG Expression
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Cultures
2.2. CCK-8 Toxicity Assay
2.3. RNA Extraction and qRT-PCR
2.4. Protein Extraction and Western Blot
2.5. EdU Cell Proliferation Assay
2.6. Flow Cytometry
2.7. Somatic Cell Nuclear Transfer
2.8. Statistical Analysis
3. Results
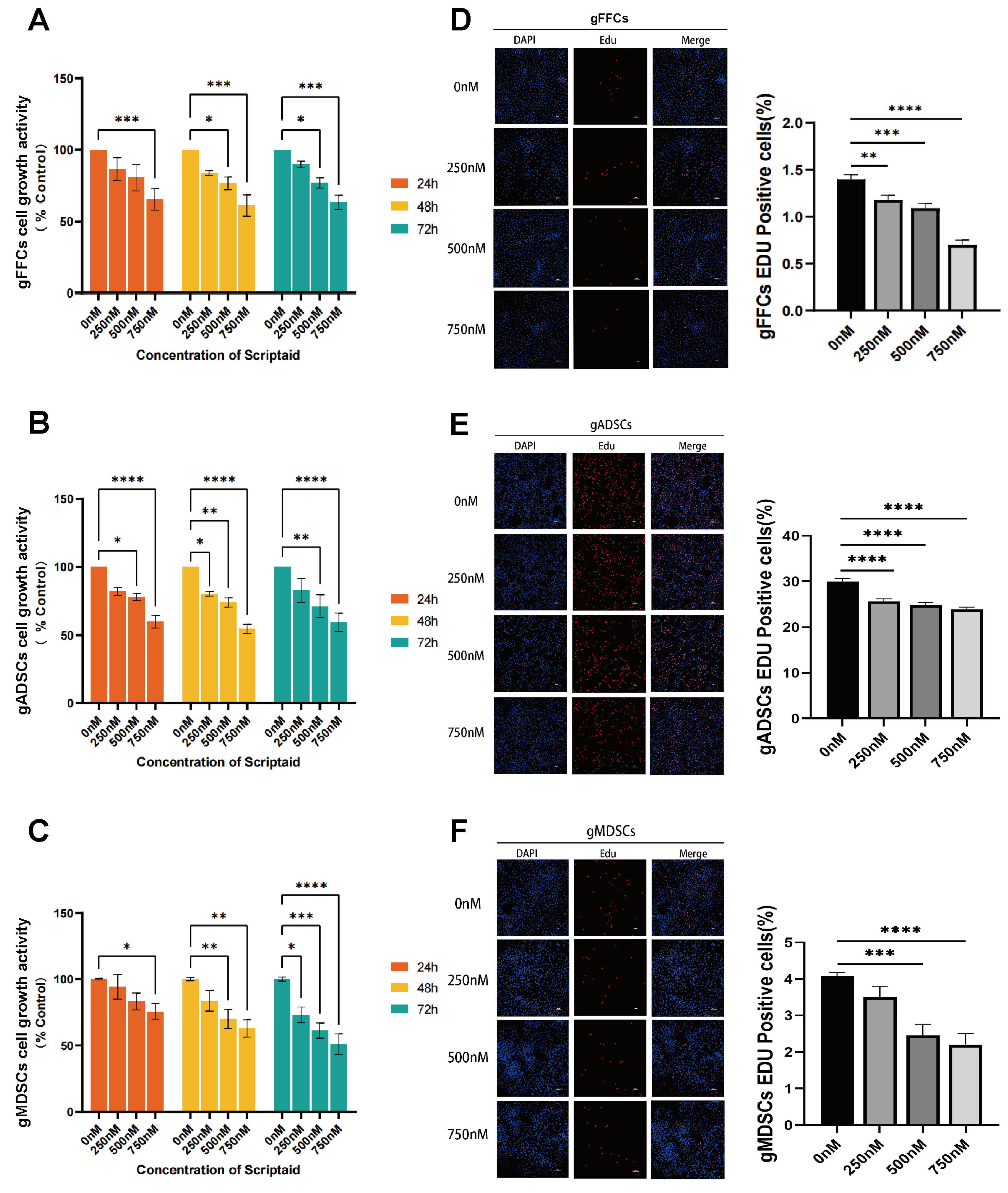
3.1. Scriptaid Suppresses Cell Proliferation in Dose- and Time-Dependent Fashion
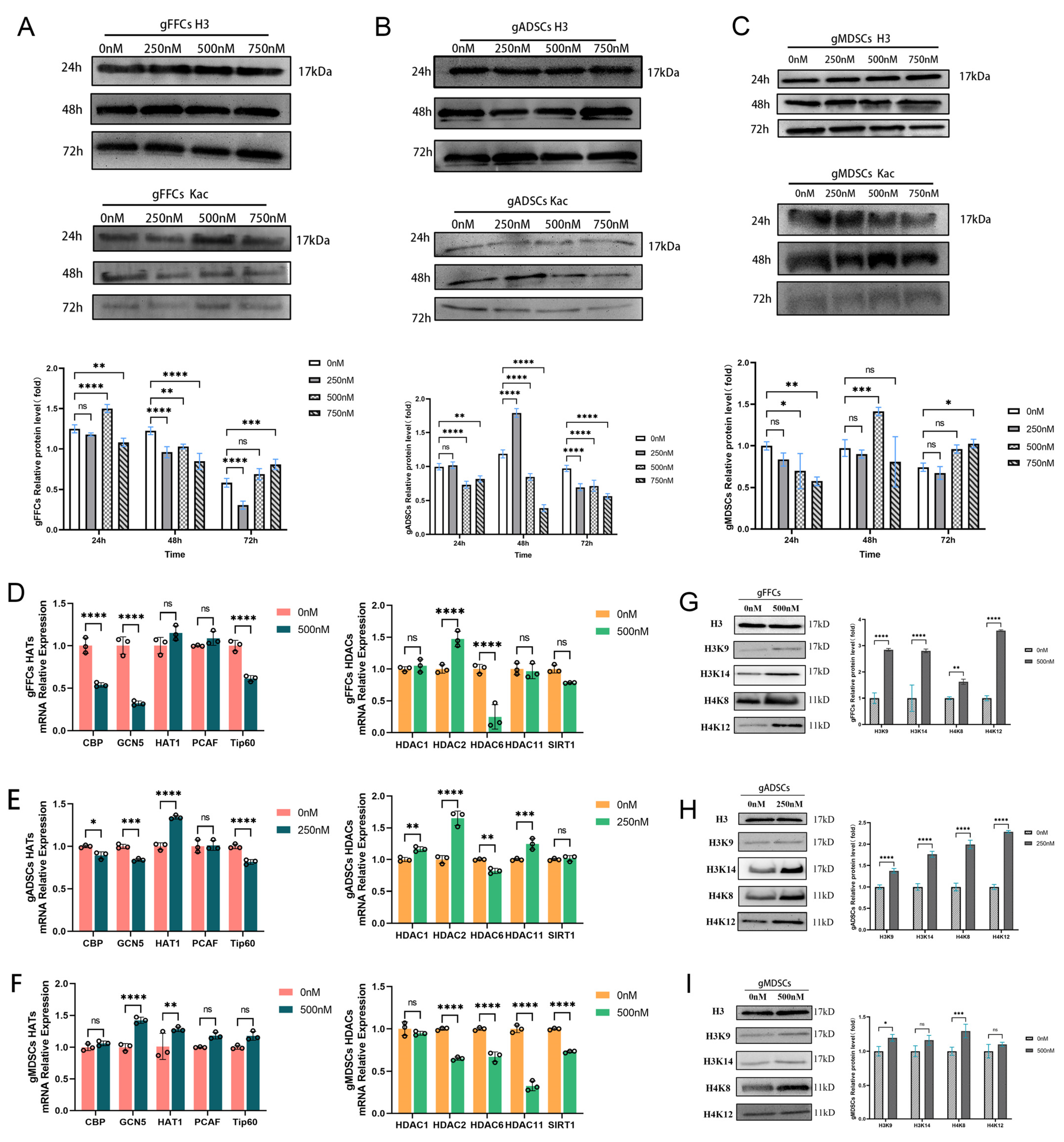
3.2. Scriptaid Regulates Alterations in Epigenetic Modifications
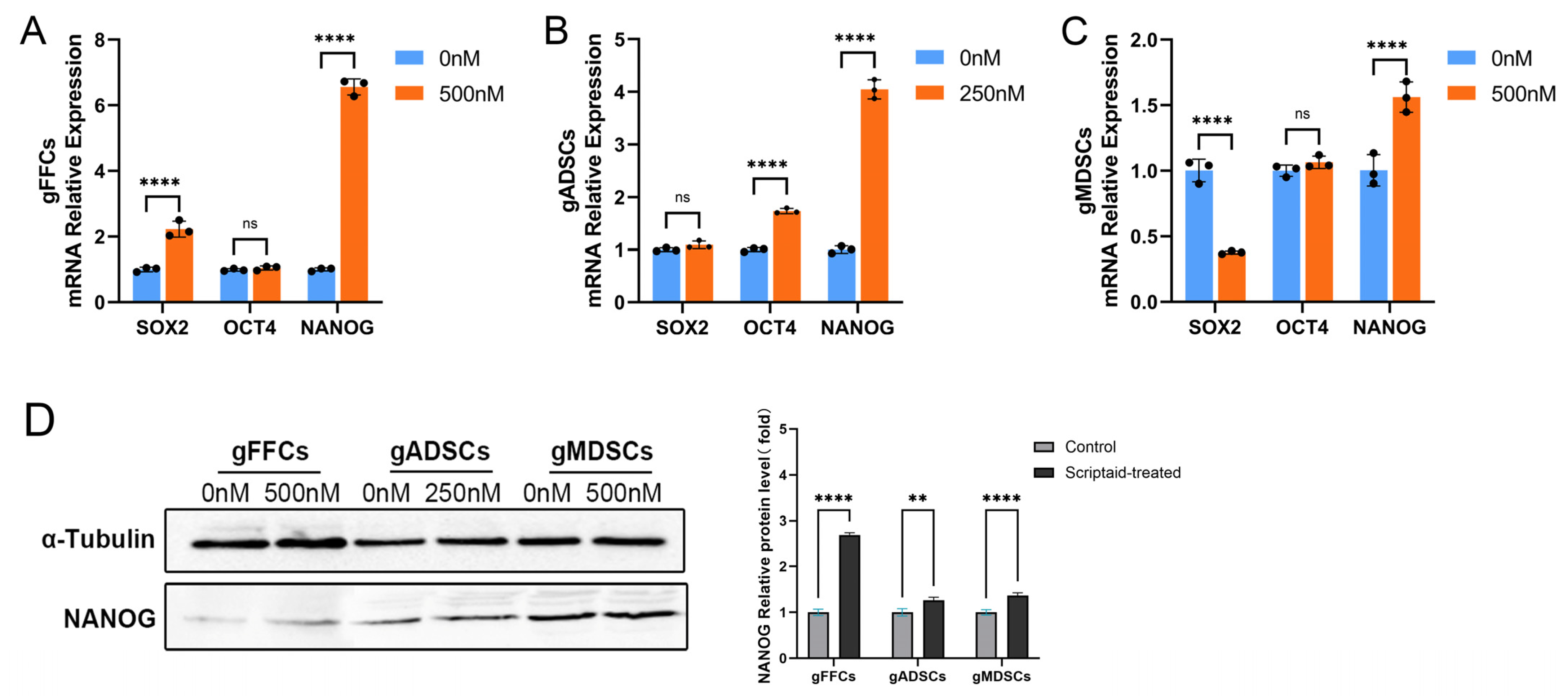
3.3. Scriptaid Perturbation of Pluripotency Molecule NANOG Expression
3.4. Scriptaid Induces Apoptosis and Blocks Cell Cycle Progression
3.5. Scriptaid Treatment of Donor Cells Increases the Rate of SCNT Embryo Oviposition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCNT | Somatic cell nuclear transfer |
| HDACi | Histone deacetylase inhibitor |
| Dux | Double homeobox |
| gFFCs | Goat fetal fibroblast cells |
| gADSCs | Goat adipose-derived stem cells |
| gMDSCs | Goat muscle-derived satellite cells |
| ZGA | Zoom genetic algorithm |
| Kac | Lysine-acetylated pan-antibody |
| HAT | Histone acetylation transferase |
| HDAC | Histone deacetylas |
| IVF | In vitro fertilization |
| iSCNT | Interspecific somatic cell nuclear transfer |
| DRB | 5,6-Dichlorobenzimidazole 1-β-D-ribofuranoside |
| TSA | Trichostatin A |
| SAHA | Suberoylanilide hydroxamic acid |
| VPA | Valproic acid |
| COCs | Cumulus–oocyte complexes |
References
- Samiec, M. Molecular Mechanisms of Somatic Cell Cloning and Other Assisted Reproductive Technologies in Mammals: Which Determinants Have Been Unraveled Thus Far?—Current Status, Further Progress and Future Challenges. Int. J. Mol. Sci. 2024, 25, 13675. [Google Scholar] [CrossRef]
- Gouveia, C.; Huyser, C.; Egli, D.; Pepper, M.S. Lessons Learned from Somatic Cell Nuclear Transfer. Int. J. Mol. Sci. 2020, 21, 2314. [Google Scholar] [CrossRef]
- Srirattana, K.; Kaneda, M.; Parnpai, R. Strategies to Improve the Efficiency of Somatic Cell Nuclear Transfer. Int. J. Mol. Sci. 2022, 23, 1969. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, L.F.; Liu, W.Q.; Qiao, Z.B.; Shen, S.J.; Zhu, Q.S.; Gao, R.; Wang, M.T.; Wang, M.Z.; Li, C.; et al. Dux-Mediated Corrections of Aberrant H3K9ac during 2-Cell Genome Activation Optimize Efficiency of Somatic Cell Nuclear Transfer. Cell Stem Cell 2021, 28, 150–163.e5. [Google Scholar] [CrossRef]
- de Macedo, M.P.; Glanzner, W.G.; Gutierrez, K.; Currin, L.; Guay, V.; Herrera, M.E.C.; da Silva, Z.; Baldassarre, H.; McGraw, S.; Bordignon, V. Simultaneous Inhibition of Histone Deacetylases and RNA Synthesis Enables Totipotency Reprogramming in Pig SCNT Embryos. Int. J. Mol. Sci. 2022, 23, 14142. [Google Scholar] [CrossRef]
- Ono, T.; Li, C.; Mizutani, E.; Terashita, Y.; Yamagata, K.; Wakayama, T. Inhibition of Class IIb Histone Deacetylase Significantly Improves Cloning Efficiency in Mice. Biol. Reprod. 2010, 83, 929–937. [Google Scholar] [CrossRef]
- Xu, W.H.; Li, Z.C.; Yu, B.; He, X.Y.; Shi, J.S.; Zhou, R.; Liu, D.W.; Wu, Z.F. Effects of and Inhibitors on Gene-Specific Methylation Reprogramming during Porcine Somatic Cell Nuclear Transfer. PLoS ONE 2013, 8, e6470510. [Google Scholar] [CrossRef]
- Ning, S.F.; Li, Q.Y.; Liang, M.M.; Yang, X.G.; Xu, H.Y.; Lu, Y.Q.; Lu, S.S.; Lu, K.H. Methylation characteristics and developmental potential of Guangxi Bama minipig cloned embryos from donor cells treated with trichostatin A and 5-aza-2′-deoxycytidine. Zygote 2013, 21, 178–186. [Google Scholar] [CrossRef]
- No, J.G.; Hur, T.Y.; Zhao, M.H.; Lee, S.; Choi, M.K.; Nam, Y.S.; Yeom, D.H.; Im, G.S.; Kim, D.H. Scriptaid improves the reprogramming of donor cells and enhances canine-porcine interspecies embryo development. Reprod. Biol. 2018, 18, 18–26. [Google Scholar] [CrossRef]
- Jafarpour, F.; Hosseini, S.M.; Hajian, M.; Forouzanfar, M.; Ostadhosseini, S.; Abedi, P.; Gholami, S.; Ghaedi, K.; Gourabi, H.; Shahverdi, A.H.; et al. Somatic cell-induced hyperacetylation, but not hypomethylation, positively and reversibly affects the efficiency of in vitro cloned blastocyst production in cattle. Cell. Reprogram. 2011, 13, 483–493. [Google Scholar] [CrossRef]
- Luo, C.; Lu, F.H.; Wang, X.L.; Wang, Z.Q.; Li, X.P.; Gong, F.Q.; Jiang, J.R.; Liu, Q.Y.; Shi, D.S. Treatment of donor cells with trichostatin A improves development and reprogramming of buffalo nucleus transfer embryos. Theriogenology 2013, 80, 878–886. [Google Scholar] [CrossRef]
- Lee, H.S.; Yu, X.F.; Bang, J.I.; Cho, S.J.; Deb, G.K.; Kim, B.W.; Kong, I.K. Enhanced histone acetylation in somatic cells induced by a histone deacetylase inhibitor improved inter-generic cloned leopard cat blastocysts. Theriogenology 2010, 74, 1439–1449. [Google Scholar] [CrossRef]
- Zhao, J.G.; Hao, Y.H.; Ross, J.W.; Spate, L.D.; Walters, E.M.; Samuel, M.S.; Rieke, A.; Murphy, C.N.; Prather, R.S. Histone Deacetylase Inhibitors Improve and Developmental Competence of Somatic Cell Nuclear Transfer Porcine Embryos. Cell. Reprogram. 2010, 12, 75–83. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, M.L.; Guo, T.X.; Zhao, L.H.; Guo, X.Y.; Yin, Z.B.; Cheng, L.X.; Liu, H.; Zhao, L.X.; Li, X.H.; et al. Species origin of exogenous transcription factors affects the activation of endogenous pluripotency markers and signaling pathways of porcine induced pluripotent stem cells. Front. Cell Dev. Biol. 2023, 11, 119627310. [Google Scholar] [CrossRef]
- Pan, G.J.; Thomson, J.A. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 2007, 17, 42–49. [Google Scholar] [CrossRef]
- Shan, J.J.; Shen, J.J.; Liu, L.M.; Xia, F.; Xu, C.; Duan, G.J.; Xu, Y.M.; Ma, Q.H.; Yang, Z.; Zhang, Q.Z.; et al. Nanog regulates self-renewal of cancer stem cells through the insulin-like growth factor pathway in human hepatocellular carcinoma. Hepatology 2012, 56, 1004–1014. [Google Scholar] [CrossRef]
- Akagi, S.; Matsukawa, K.; Takahashi, S. Factors Affecting the Development of Somatic Cell Nuclear Transfer Embryos in Cattle. J. Reprod. Dev. 2014, 60, 329–335. [Google Scholar] [CrossRef]
- Wang, X.Y.; Qu, J.D.; Li, J.; He, H.B.; Liu, Z.H.; Huan, Y.J. Epigenetic Reprogramming During Somatic Cell Nuclear Transfer: Recent Progress and Future Directions. Front. Genet. 2020, 11, 20510. [Google Scholar] [CrossRef]
- Klinger, B.; Schnieke, A. 25th Anniversary of Cloning by Somatic-Cell Nuclear Transfer Twenty-five years after Dolly: How far have we come? Reproduction 2021, 162, F1–F10. [Google Scholar] [CrossRef]
- Ongaratto, F.L.; Rodriguez-Villamil, P.; Bertolini, M.; Carlson, D.F. Influence of oocyte selection, activation with a zinc chelator and inhibition of histone deacetylases on cloned porcine embryo and chemically activated oocytes development. Zygote 2020, 28, 286–290. [Google Scholar] [CrossRef]
- Fleming, C.L.; Natoli, A.; Schreuders, J.; Devlin, M.; Yoganantharajah, P.; Gibert, Y.; Leslie, K.G.; New, E.J.; Ashton, T.D.; Pfeffer, F.M. Highly fluorescent and HDAC6 selective scriptaid analogues. Eur. J. Med. Chem. 2019, 162, 321–333. [Google Scholar] [CrossRef]
- Akagi, S.; Matsukawa, K.; Mizutani, E.; Fukunari, K.; Kaneda, M.; Watanabe, S.; Takahashi, S. Treatment with a Histone Deacetylase Inhibitor after Nuclear Transfer Improves the Preimplantation Development of Cloned Bovine Embryos. J. Reprod. Dev. 2011, 57, 120–126. [Google Scholar] [CrossRef]
- Masui, S.; Nakatake, Y.; Toyooka, Y.; Shimosato, D.; Yagi, R.; Takahashi, K.; Okochi, H.; Okuda, A.; Matoba, R.; Sharov, A.A.; et al. Pluripotency governed by via regulation of expression in mouse embryonic stem cells. Nat. Cell Biol. 2007, 9, U625–U626. [Google Scholar] [CrossRef]
- Simmet, K.; Zakhartchenko, V.; Philippou-Massier, J.; Blum, H.; Klymiuk, N.; Wolf, E. OCT4/POU5F1 is required for NANOG expression in bovine blastocysts. Proc. Natl. Acad. Sci. USA 2018, 115, 2770–2775. [Google Scholar] [CrossRef]
- Klein, D.C.; Hainer, S.J. Chromatin regulation and dynamics in stem cells. Curr. Top. Dev. Biol. 2020, 138, 1–71. [Google Scholar] [CrossRef]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019, 176, 98–112.e14. [Google Scholar] [CrossRef]
- Kelly, R.D.W.; Stengel, K.R.; Chandru, A.; Johnson, L.C.; Hiebert, S.W.; Cowley, S.M. Histone deacetylases maintain expression of the pluripotent gene network via recruitment of RNA polymerase II to coding and noncoding loci. Genome Res. 2024, 34, 34–46. [Google Scholar] [CrossRef]
- Gong, S.; Li, Q.; Jeter, C.R.; Fan, Q.; Tang, D.G.; Liu, B. Regulation of NANOG in cancer cells. Mol. Carcinog. 2015, 54, 679–687. [Google Scholar] [CrossRef]
- Lai, S.W.; Bamodu, O.A.; Tsai, W.C.; Chang, Y.M.; Lee, W.H.; Yeh, C.T.; Chao, T.Y. The therapeutic targeting of the FGFR1/Src/NF-κB signaling axis inhibits pancreatic ductal adenocarcinoma stemness and oncogenicity (vol 35, pg 663, 2018). Clin. Exp. Metastas. 2019, 36, 67. [Google Scholar] [CrossRef]
- Yao, R.S.; Han, D.Y.; Sun, X.Y.; Xie, Y.; Wu, Q.Y.; Fu, C.L.; Yao, Y.; Li, H.J.; Li, Z.Y.; Xu, K.L. Scriptaid inhibits cell survival, cell cycle, and promotes apoptosis in multiple myeloma via epigenetic regulation of p21. Exp. Hematol. 2018, 60, 63–72. [Google Scholar] [CrossRef]
- Hyun, H.; Lee, S.E.; Son, Y.J.; Shin, M.Y.; Park, Y.G.; Kim, E.Y.; Park, S.P. Cell Synchronization by Rapamycin Improves the Developmental Competence of Porcine SCNT Embryos. Cell. Reprogram. 2016, 18, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, M.J.; Naushad, S.M.; Lavanya, A.; Srinivas, C.; Devi, T.A.; Sampathkumar, S.; Dharan, D.B.; Bhadra, M.P. Scriptaid cause histone deacetylase inhibition and cell cycle arrest in HeLa cancer cells: A study on structural and functional aspects. Gene 2017, 627, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Rissi, V.B.; Glanzner, W.G.; Mujica, L.K.S.; Antoniazzi, A.Q.; Gonçalves, P.B.D.; Bordignon, V. Effect of Cell Cycle Interactions and Inhibition of Histone Deacetylases on Development of Porcine Embryos Produced by Nuclear Transfer. Cell. Reprogram. 2016, 18, 8–16. [Google Scholar] [CrossRef]
- Zhao, J.G.; Ross, J.W.; Hao, Y.H.; Spate, L.D.; Walters, E.M.; Samuel, M.S.; Rieke, A.; Murphy, C.N.; Prather, R.S. Significant Improvement in Cloning Efficiency of an Inbred Miniature Pig by Histone Deacetylase Inhibitor Treatment after Somatic Cell Nuclear Transfer. Biol. Reprod. 2009, 81, 525–530. [Google Scholar] [CrossRef]
- Chai, Z.; Wu, J.; Qi, Z.C.; Liu, Y.; Lv, Y.J.; Zhang, Y.T.; Yu, Z.R.; Jiang, C.Q.; Liu, Z.H. Molecular characterizations and functional roles of NANOG in early development of porcine embryos. Gene 2024, 892, 14785610. [Google Scholar] [CrossRef]


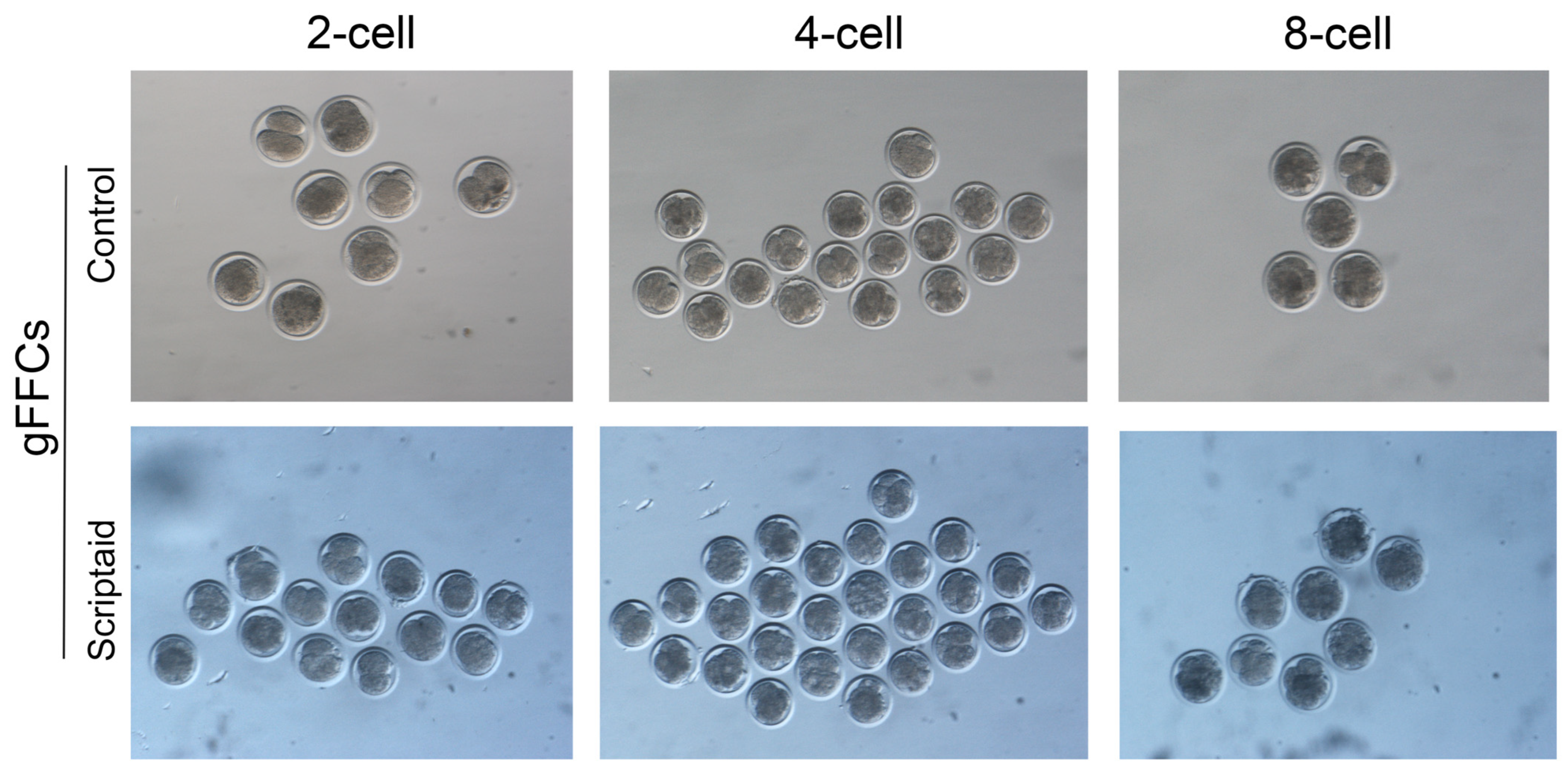
| Treatment | No. Constructed Embryo | Cleavage Rate (%) | 2-Cell Rate (%) | 4-Cell Rate (%) | 8-Cell Rate (%) |
|---|---|---|---|---|---|
| Control | 324 | 48.14 | 50 a | 30.76 a | 19.23 a |
| Scriptaid | 350 | 64.58 | 37.93 b | 35.12 b | 24.14 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhe, X.; Ma, H.; Zhang, W.; Ding, R.; Hao, F.; Gao, Y.; Uri, G.; Jiri, G.; Jiri, G.; Liu, D. Scriptaid Improves Cashmere Goat Embryo Reprogramming by Affecting Donor Cell Pluripotency Molecule NANOG Expression. Animals 2025, 15, 1022. https://doi.org/10.3390/ani15071022
Zhe X, Ma H, Zhang W, Ding R, Hao F, Gao Y, Uri G, Jiri G, Jiri G, Liu D. Scriptaid Improves Cashmere Goat Embryo Reprogramming by Affecting Donor Cell Pluripotency Molecule NANOG Expression. Animals. 2025; 15(7):1022. https://doi.org/10.3390/ani15071022
Chicago/Turabian StyleZhe, Xiaoshu, Hairui Ma, Wenqi Zhang, Rui Ding, Fei Hao, Yuan Gao, Gumara Uri, Gellegen Jiri, Garangtu Jiri, and Dongjun Liu. 2025. "Scriptaid Improves Cashmere Goat Embryo Reprogramming by Affecting Donor Cell Pluripotency Molecule NANOG Expression" Animals 15, no. 7: 1022. https://doi.org/10.3390/ani15071022
APA StyleZhe, X., Ma, H., Zhang, W., Ding, R., Hao, F., Gao, Y., Uri, G., Jiri, G., Jiri, G., & Liu, D. (2025). Scriptaid Improves Cashmere Goat Embryo Reprogramming by Affecting Donor Cell Pluripotency Molecule NANOG Expression. Animals, 15(7), 1022. https://doi.org/10.3390/ani15071022





