Effect of Chicken AvBD11 on the Cytokines in the Erythrocytes of Chickens Infected with the Avian Influenza Virus of the Subtype H9N2
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Cloning of AvBD11
2.2. Production of Recombinant AvBD11 Protein
2.3. Cell Culture and Virus Strains
2.4. Quantitative PCR Analysis
2.5. Experimental Animals
2.6. Statistical Analysis
3. Results
3.1. Recombinant Construction Plasmid and Protein Expression
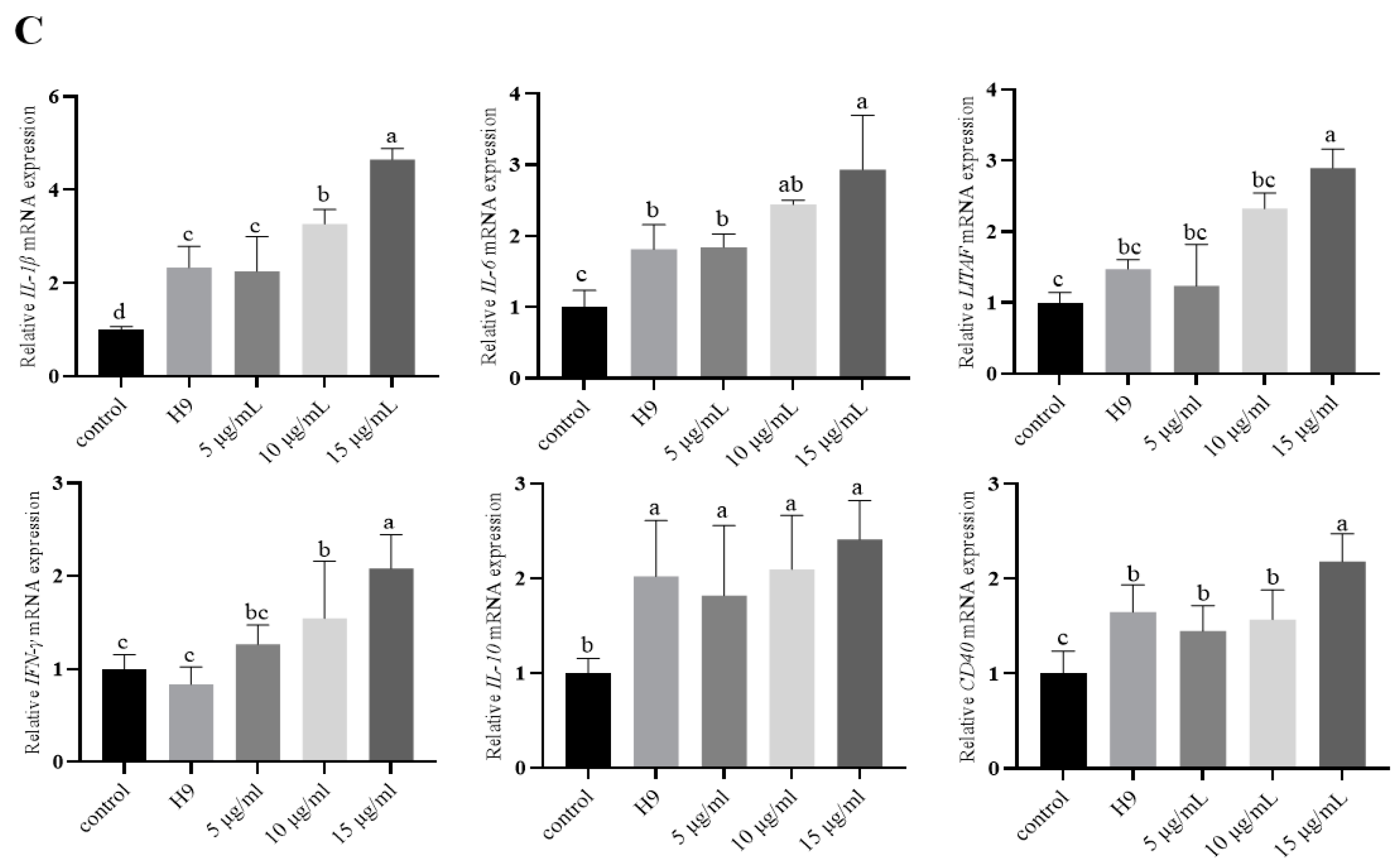
3.2. Effect of AvBD11 on the Expression of Several Cytokines in H9N2-Infected Chicken Erythrocytes
3.3. Effect of AvBD11 on the Expression of Various Cytokines in Erythrocytes from H9N2-Infected Chickens In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LITAF | Lipopolysaccharide-induced TNF factor |
| IL-10 | Interleukin-10 |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1β |
| IFN-γ | Interferon-γ |
References
- Homme, P.J.; Easterday, B.C. Avian influenza virus infections. I. Characteristics of influenza A/Turkey/Wisconsin/1966 virus. Avian Dis. 1970, 14, 66–74. [Google Scholar] [CrossRef]
- Iqbal, M.; Yaqub, T.; Mukhtar, N.; Shabbir, M.; McCauley, J. Infectivity and transmissibility of H9N2 avian influenza virus in chickens and wild terrestrial birds. Vet. Res. 2013, 44, 100. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Xu, D.; Yang, X.; Zhang, J.; Wang, S.; Shi, H.; Liu, X. Genetic and biological characterization of H9N2 avian influenza viruses isolated in China from 2011 to 2014. PLoS ONE 2018, 13, e0199260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Tang, Y.; Liu, X.; Peng, D.; Liu, W.; Liu, H.; Lu, S.; Liu, X. Characterization of H9N2 influenza viruses isolated from vaccinated flocks in an integrated broiler chicken operation in eastern China during a 5 year period (1998–2002). J. Gen. Virol. 2008, 89, 3102–3112. [Google Scholar] [CrossRef] [PubMed]
- RahimiRad, S.; Alizadeh, A.; Alizadeh, E.; Hosseini, S.M. The avian influenza H9N2 at avian-human interface: A possible risk for the future pandemics. J. Res. Med. Sci. 2016, 21, 51. [Google Scholar] [CrossRef]
- Shafee, T.M.A.; Lay, F.; Phan, T.; Anderson, M.; Hulett, M. Convergent evolution of defensin sequence, structure and function. Cell. Mol. Life Sci. 2017, 74, 663–682. [Google Scholar] [CrossRef]
- Selsted, M.; Ouellette, A. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 2005, 6, 551–557. [Google Scholar] [CrossRef]
- Cheng, Y.; Prickett, M.; Gutowska, W.; Kuo, R.; Belov, K.; Burt, D. Evolution of the avian β-defensin and cathelicidin genes. BMC Evol. Biol. 2015, 15, 188. [Google Scholar] [CrossRef]
- Sugiarto, H.; Yu, P. Avian antimicrobial peptides: The defense role of beta-defensins. Biochem. Biophys. Res. Commun. 2004, 323, 721–727. [Google Scholar] [CrossRef]
- Dijk, V.; Hedegaard, C.; Haagsman, H.; Heegaard, P. The potential for immunoglobulins and host defense peptides (HDPs) to reduce the use of antibiotics in animal production. Vet. Res. 2018, 49, 68. [Google Scholar] [CrossRef]
- Guyot, N.; Meudal, H.; Trapp, S.; Iochmann, S.; Silvestre, A.; Jousset, G.; Labas, V.; Reverdiau, P.; Loth, K.; Hervé, V.; et al. Structure, function, and evolution of Gga-AvBD11, the archetype of the structural avian-double-β-defensin family. Proc. Natl. Acad. Sci. USA 2020, 117, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Mann, K. Proteomic analysis of the chicken egg vitelline membrane. Proteomics 2008, 8, 2322–2332. [Google Scholar] [CrossRef]
- Quiñones-Mateu, M.; Lederman, M.; Feng, Z.; Chakraborty, B.; Weber, J.; Rangel, H.; Marotta, M.; Mirza, M.; Jiang, B.; Kiser, P.; et al. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS 2003, 17, F39–F48. [Google Scholar] [CrossRef]
- Othumpanga, S.; Noti, J. β-Defensin-1 Regulates Influenza Virus Infection in Human Bronchial Epithelial Cells through the STAT3 Signaling Pathway. Pathogens 2023, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Ruchala, P.; Lehrer, R.; Ross, C.; Rowland, R.; Blecha, F. Antimicrobial host defense peptides in an arteriviral infection: Differential peptide expression and virus inactivation. Viral Immunol. 2009, 22, 235–242. [Google Scholar] [CrossRef]
- Leonard, W.; Lin, J. Strategies to therapeutically modulate cytokine action. Nat. Rev. Drug Discov. 2023, 22, 827–854. [Google Scholar] [CrossRef] [PubMed]
- Siegel, I.; Liu, T.; Gleicher, N. The red-cell immune system. Lancet 1981, 2, 556–559. [Google Scholar] [CrossRef]
- Zhou, J.; Qiao, M.; Jahejo, A.; Han, X.; Wang, P.; Wang, Y.; Ren, J.; Niu, S.; Zhao, Y.; Zhang, D.; et al. Effect of avian influenza virus subtype H9N2 on the expression of complement-associated genes in chicken erythrocytes. Br. Poult. Sci. 2023, 64, 467–475. [Google Scholar] [CrossRef]
- Niu, S.; Jahejo, A.; Jia, F.; Li, X.; Ning, G.; Zhang, D.; Ma, H.; Hao, W.; Gao, W.; Zhao, Y.; et al. Transcripts of antibacterial peptides in chicken erythrocytes infected with Marek’s disease virus. BMC Vet. Res. 2018, 14, 363. [Google Scholar] [CrossRef]
- Khan, A.; Jahejo, A.; Qiao, M.; Han, X.; Cheng, Q.; Mangi, R.; Qadir, M.; Zhang, D.; Bi, Y.; Wang, Y.; et al. NF-кB pathway genes expression in chicken erythrocytes infected with avian influenza virus subtype H9N2. Br. Poult. Sci. 2021, 62, 666–671. [Google Scholar] [CrossRef]
- Yang, W.; Liu, X.; Wang, X. The immune system of chicken and its response to H9N2 avian influenza virus. Vet. Q. 2023, 43, 1–14. [Google Scholar] [CrossRef]
- Albert, v.; Rodrigo, G.; Geoffrey, B.; Catherine, S.; Henk, P.; Anne-Christine, L. Evolutionary diversification of defensins and cathelicidins in birds and primates. Mol. Immunol. 2023, 157, 53–69. [Google Scholar] [CrossRef]
- Constantinou, A.; Polak-Witka, K.; Tomazou, M.; Oulas, A.; Kanti, V.; Schwarzer, R.; Helmuth, J.; Edelmann, A.; Blume-Peytavi, U.; Spyrou, G.; et al. Dysbiosis and Enhanced Beta-Defensin Production in Hair Follicles of Patients with Lichen Planopilaris and Frontal Fibrosing Alopecia. Biomedicines 2021, 9, 266. [Google Scholar] [CrossRef]
- Dinarello, C. Biologic basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.; Lee, A.; Shin, H.; Song, H.; Park, J.; Kang, T.; Lee, S.; Yang, S. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Mendoza, J.; Escalante, N.; Jude, K.; Bellon, J.; Su, L.; Horton, T.; Tsutsumi, N.; Berardinelli, S.; Haltiwanger, R.; Piehler, J.; et al. Structure of the IFNγ receptor complex guides design of biased agonists. Nature 2019, 567, 56–60. [Google Scholar] [CrossRef]
- Ivashkiv, L. IFNγ: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef]
- Li, J.; Lin, W.; Chen, H.; Xu, Z.; Ye, Y.; Chen, M. Dual-target IL-12-containing nanoparticles enhance T cell functions for cancer immunotherapy. Cell. Immunol. 2020, 349, 104042. [Google Scholar] [CrossRef]
- Sun, J.; Yan, Y.; Jiang, J.; Lu, P. DNA immunization against very virulent infectious bursal disease virus with VP2-4-3 gene and chicken IL-6 gene. J. Vet. Med. Ser. B Infect. Dis. Vet. Public Health 2005, 52, 1–7. [Google Scholar] [CrossRef]
- Yang, M.; Wang, C.; Yang, S. IL-6 ameliorates acute lung injury in influenza virus infection. Sci. Rep. 2017, 7, 43829. [Google Scholar] [CrossRef] [PubMed]
- Kak, G.; Raza, M.; Tiwari, B. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Dinarello, C.; Mantovani, A. The interleukin-1 family: Back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C. An expanding role for interleukin-1 blockade from gout to cancer. Mol. Med. 2014, 20 (Suppl. S1), S43–S58. [Google Scholar] [CrossRef]
- Hong, Y.; Lee, J.; Vu, T.; Lee, S.; Lillehoj, H.; Hong, Y. Chicken avian β-defensin 8 modulates immune response via the mitogen-activated protein kinase signaling pathways in a chicken macrophage cell line. Poult. Sci. 2020, 99, 4174–4182. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, Y.; Yin, Q.; Liang, H.; She, R. Chicken intestine defensins activated murine peripheral blood mononuclear cells through the TLR4-NF-kappaB pathway. Vet. Immunol. Immunopathol. 2010, 133, 59–65. [Google Scholar] [CrossRef]




| Gene | Primer Sequence (5′–3′) | GenBank Accession Number |
|---|---|---|
| LITAF | F:CTGAGGCATTTGGAAGCAGC | NM_204267.2 |
| R:GACAGGGTAGGGGTGAGGAT | ||
| IL-10 | F:AGGAGCAAAGCCATCAAGCA | NM_001004414.4 |
| R:ACCGAACGTTAAGCTGCCAT | ||
| IL-6 | F:GCTTGGTTAACCCTGGCTCT | NM_204628.2 |
| R:AAAGTGCAGAGTGTCCGACC | ||
| IL-1β | F:GCCTGCAGAAGAAGCCTCG | XM_015297469.3 |
| R:GTGACGGGCTCAAAAACCTC | ||
| CD40 | F:TCCAAAACTGAGCCATGCCA | NM_204665.3 |
| R:GTGTGCACCAGGCAGTAGAT | ||
| IFN-γ | F:CCACACCTTCCTCCAAGACA | NM_205149.2 |
| R:GCCTGTGAGGTTGTGGATGT | ||
| β-actin | F:ATTGTCCACCGCAAATGCTTC | NM_205518.2 |
| R:AAATAAAGCCATGCCAATCTCGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Luo, S.-Q.; Xiang, W.-J.; Meng, Z.-X.; Wang, Y.; Ren, J.-L.; Zhao, Y.-J.; Fan, R.-W.; Niu, S.; Tian, W.-X. Effect of Chicken AvBD11 on the Cytokines in the Erythrocytes of Chickens Infected with the Avian Influenza Virus of the Subtype H9N2. Animals 2025, 15, 1023. https://doi.org/10.3390/ani15071023
Yu J, Luo S-Q, Xiang W-J, Meng Z-X, Wang Y, Ren J-L, Zhao Y-J, Fan R-W, Niu S, Tian W-X. Effect of Chicken AvBD11 on the Cytokines in the Erythrocytes of Chickens Infected with the Avian Influenza Virus of the Subtype H9N2. Animals. 2025; 15(7):1023. https://doi.org/10.3390/ani15071023
Chicago/Turabian StyleYu, Jie, Sheng-Qing Luo, Wen-Jun Xiang, Zi-Xuan Meng, Ying Wang, Jian-Le Ren, Yu-Jun Zhao, Rui-Wen Fan, Sheng Niu, and Wen-Xia Tian. 2025. "Effect of Chicken AvBD11 on the Cytokines in the Erythrocytes of Chickens Infected with the Avian Influenza Virus of the Subtype H9N2" Animals 15, no. 7: 1023. https://doi.org/10.3390/ani15071023
APA StyleYu, J., Luo, S.-Q., Xiang, W.-J., Meng, Z.-X., Wang, Y., Ren, J.-L., Zhao, Y.-J., Fan, R.-W., Niu, S., & Tian, W.-X. (2025). Effect of Chicken AvBD11 on the Cytokines in the Erythrocytes of Chickens Infected with the Avian Influenza Virus of the Subtype H9N2. Animals, 15(7), 1023. https://doi.org/10.3390/ani15071023





