Pulmonary Artery Banding in a Cat with Atrioventricular Canal Defect Type A with Concurrent Muscular Septal Defect
Simple Summary
Abstract
1. Introduction
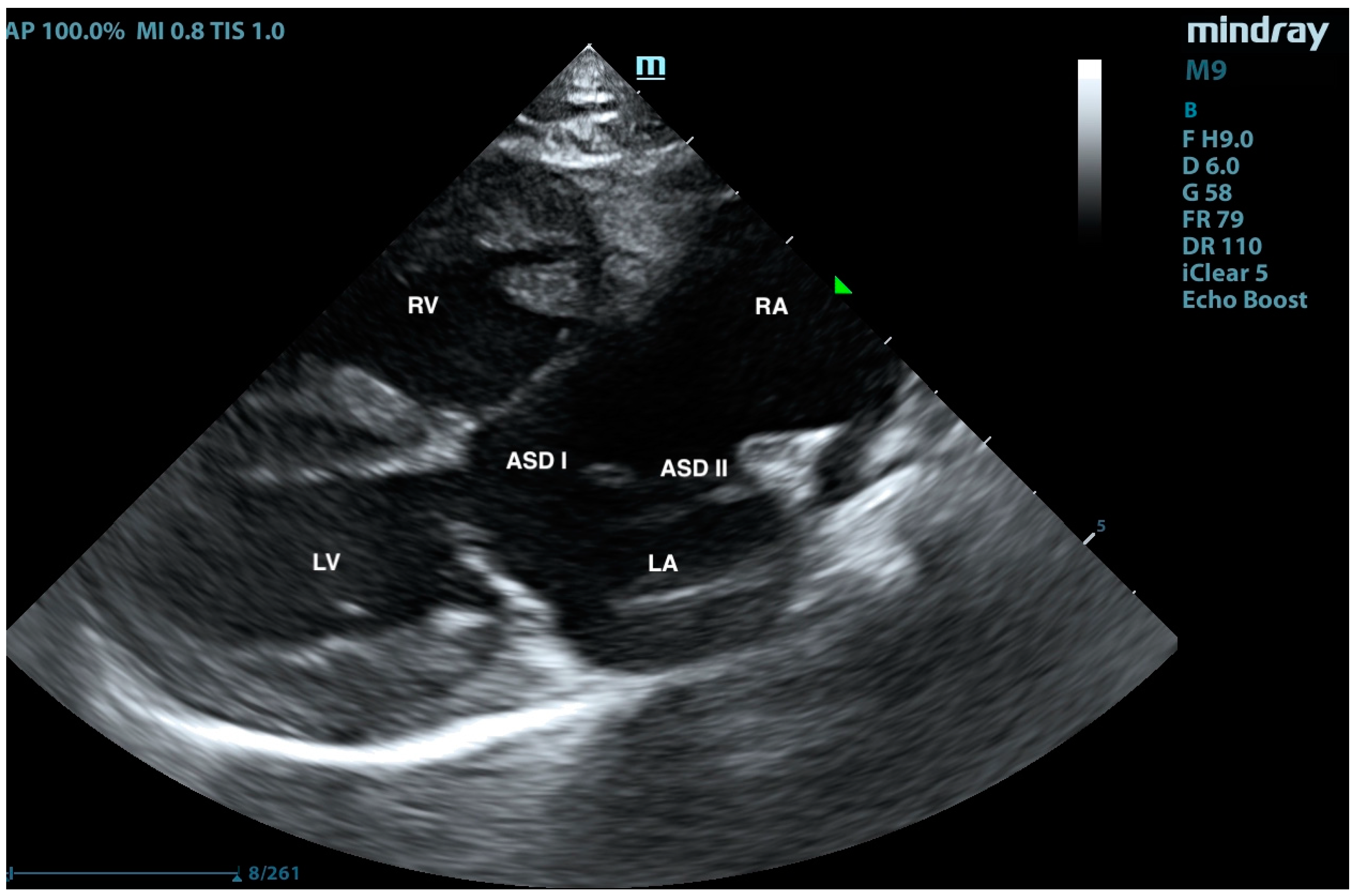
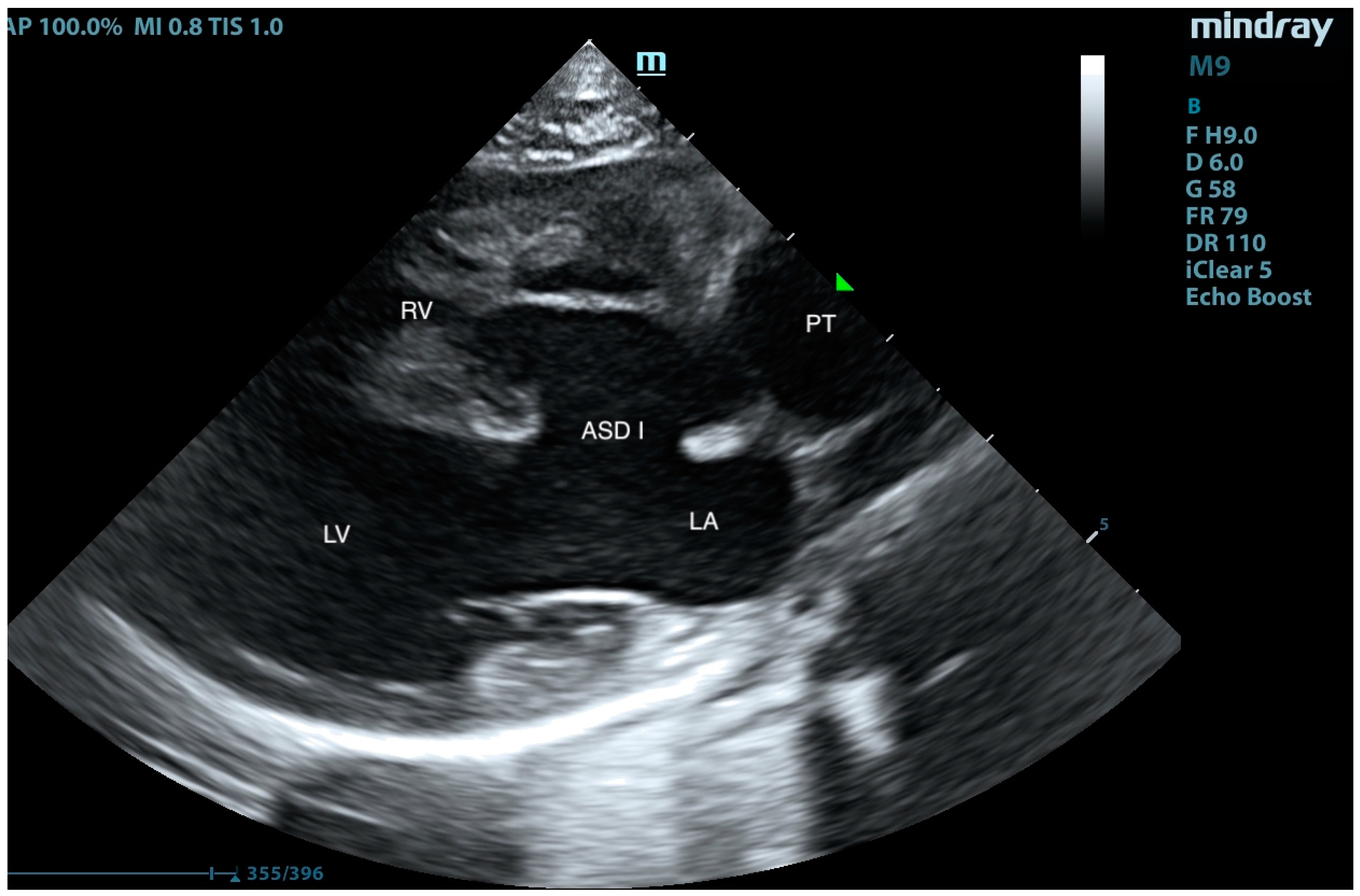
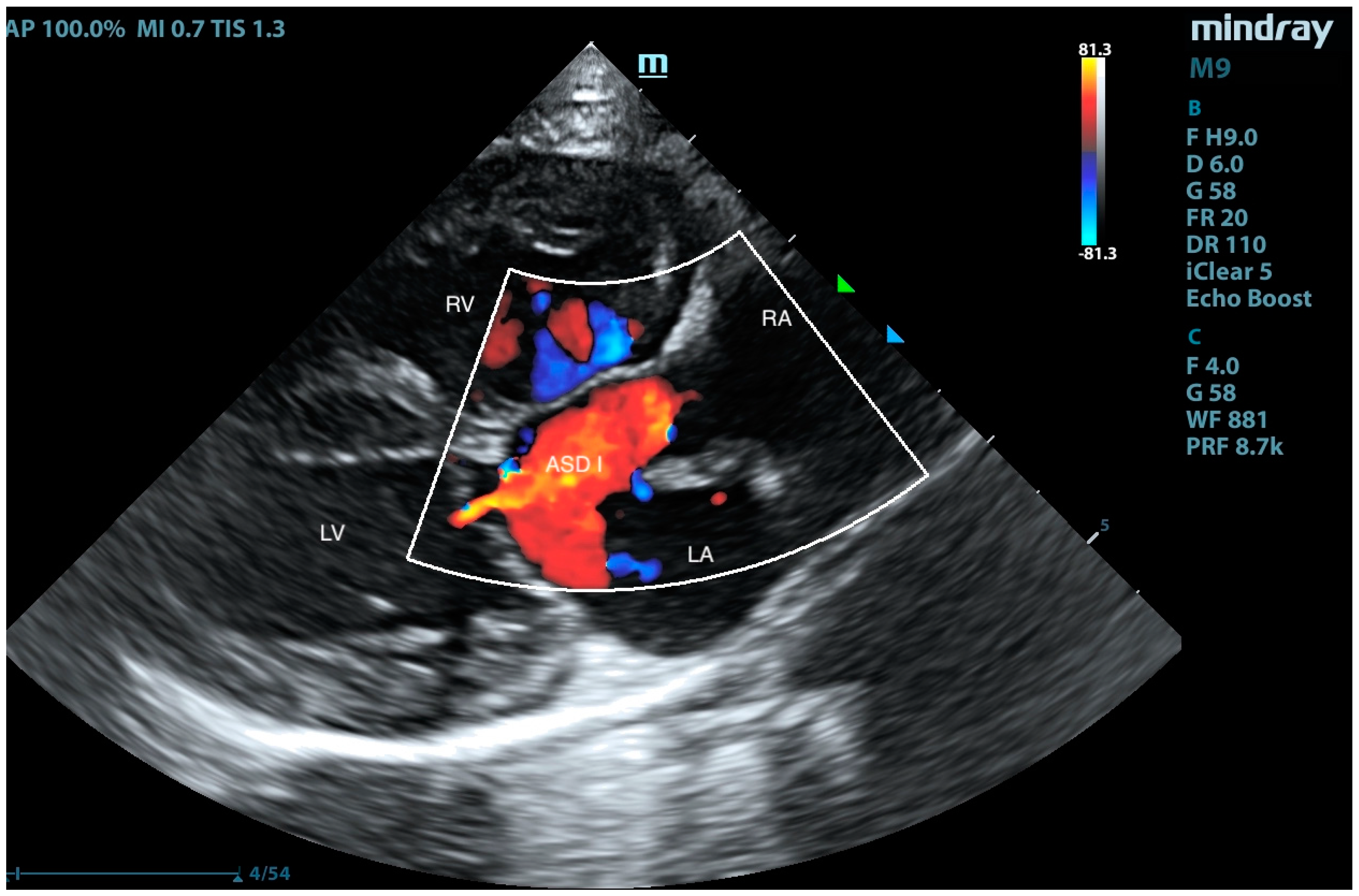
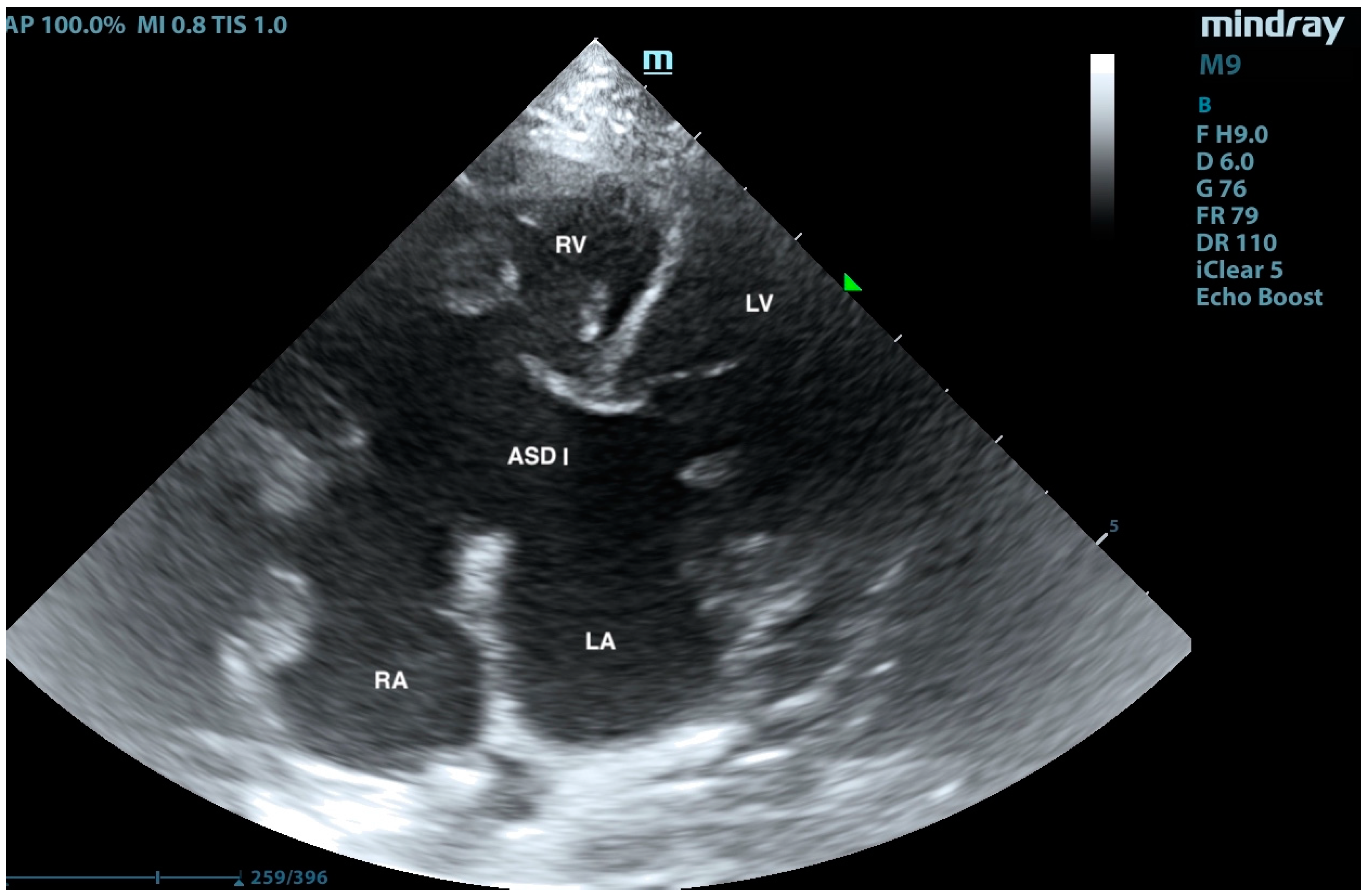
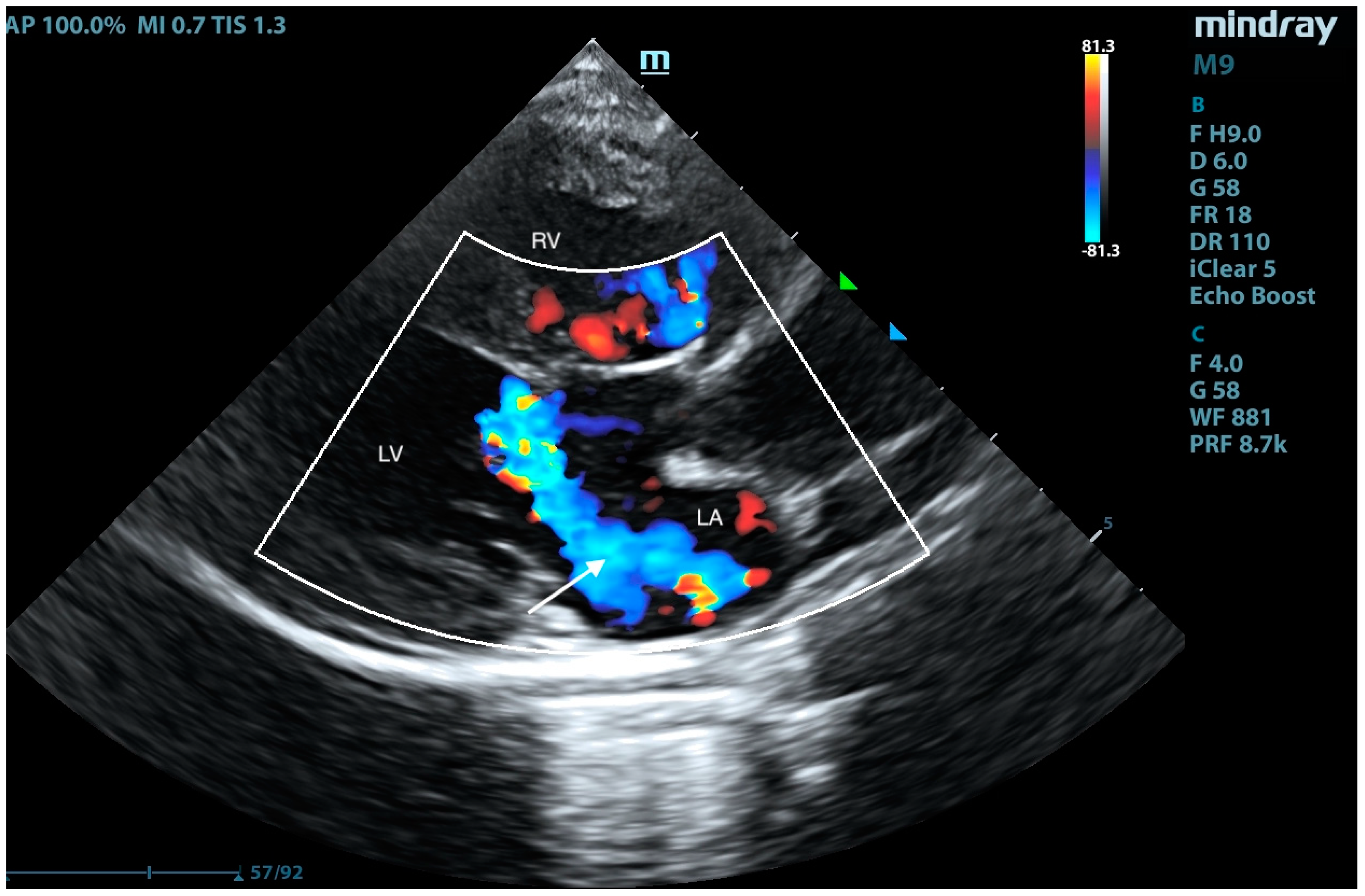
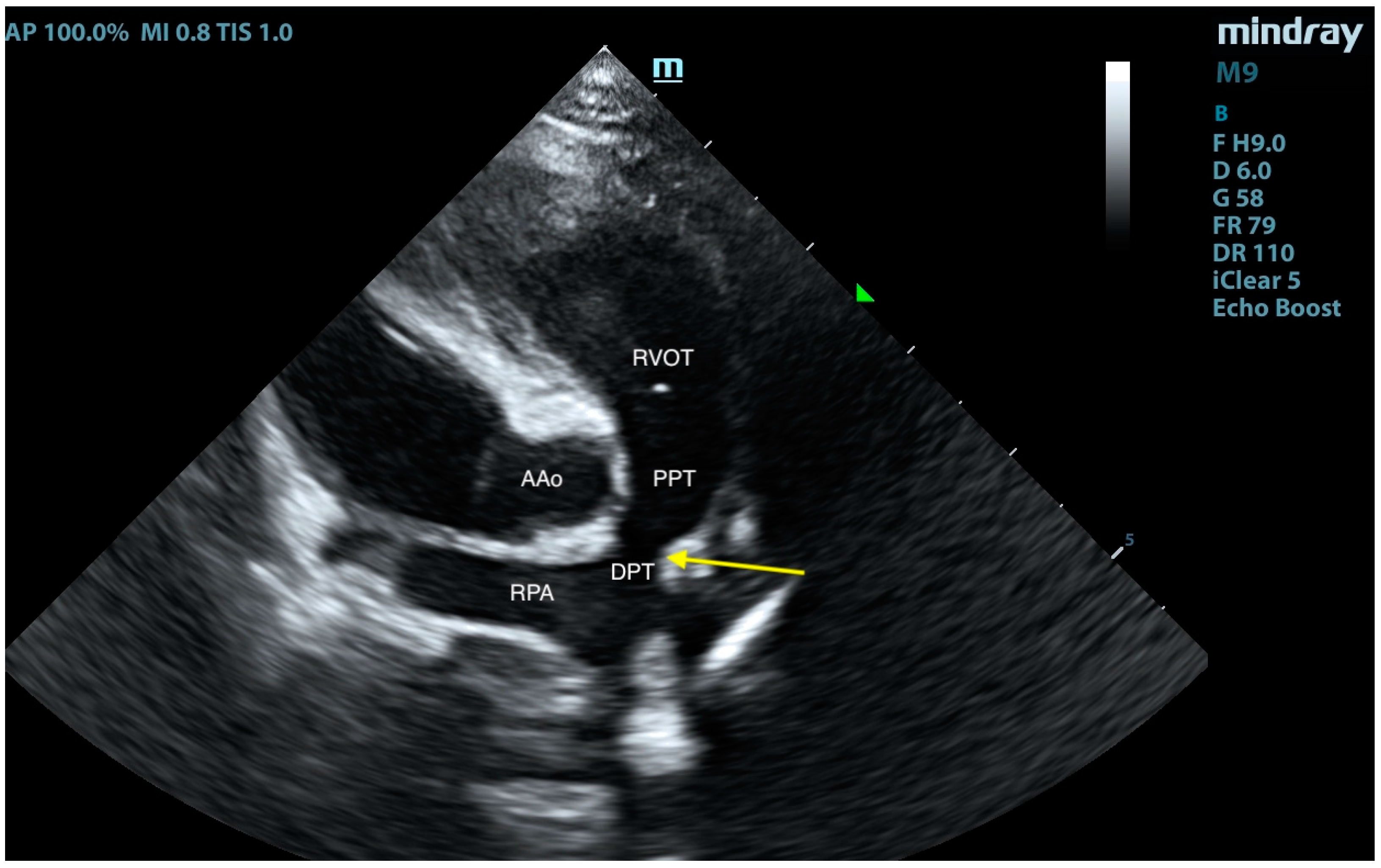
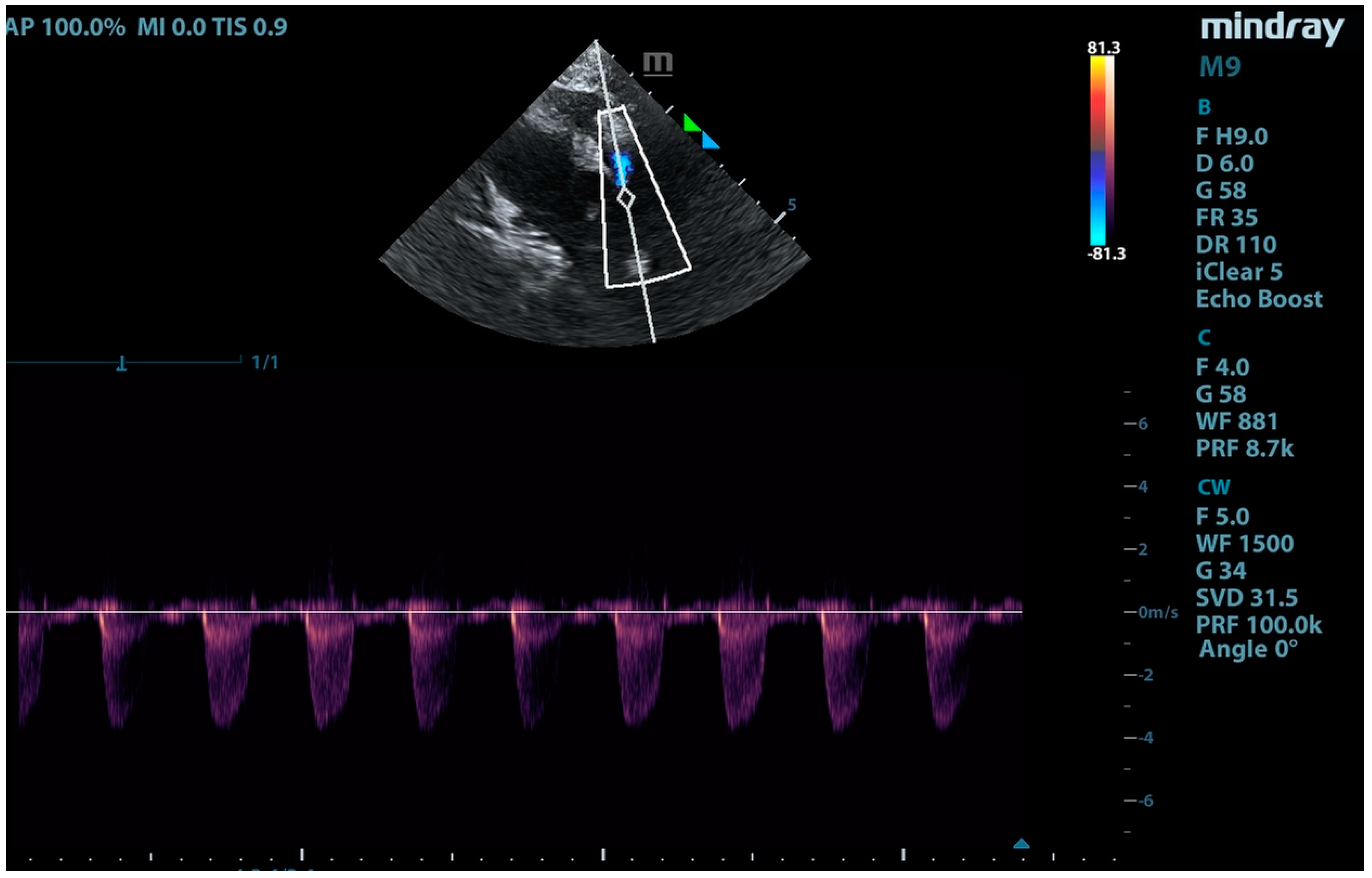
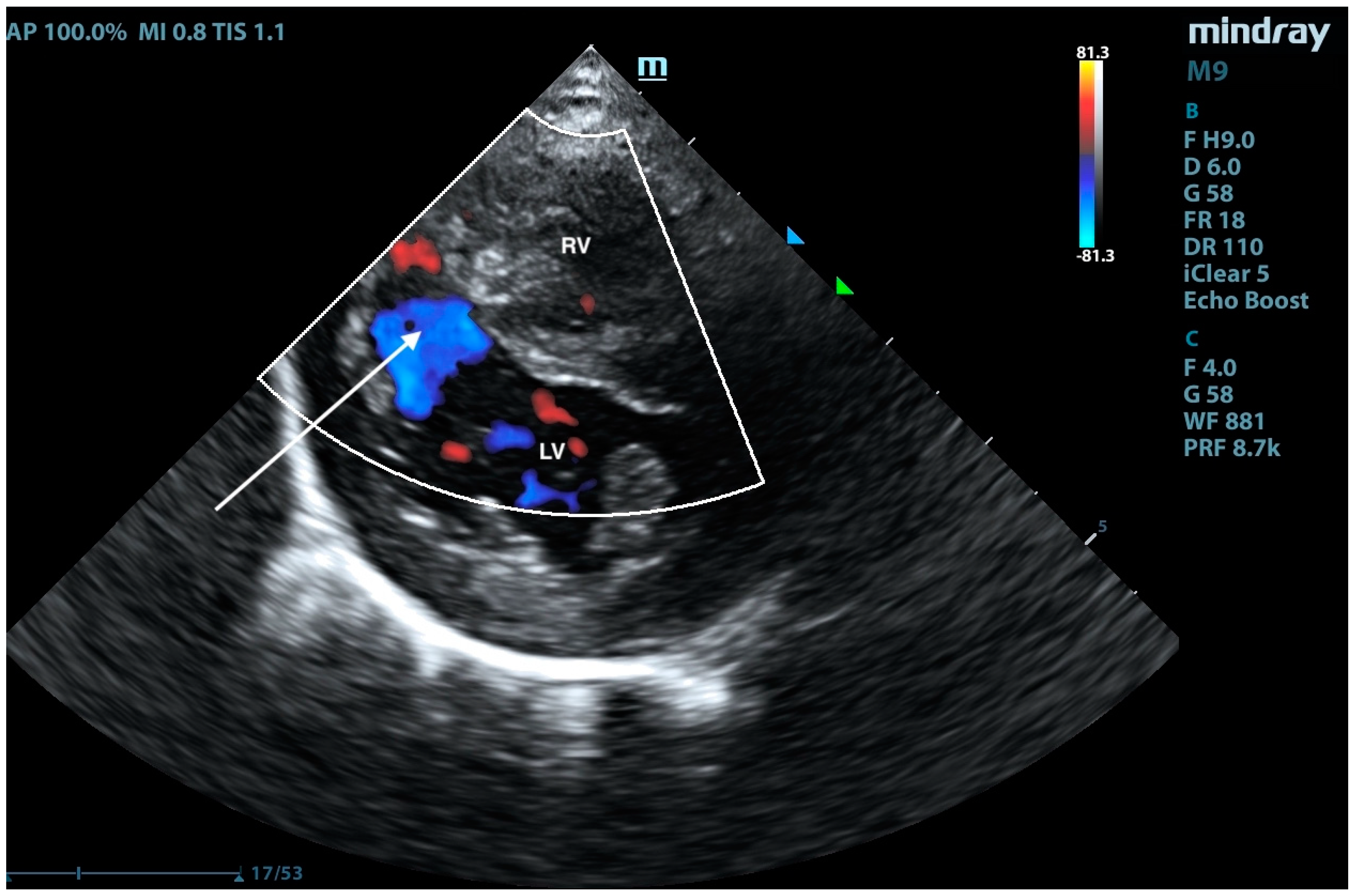
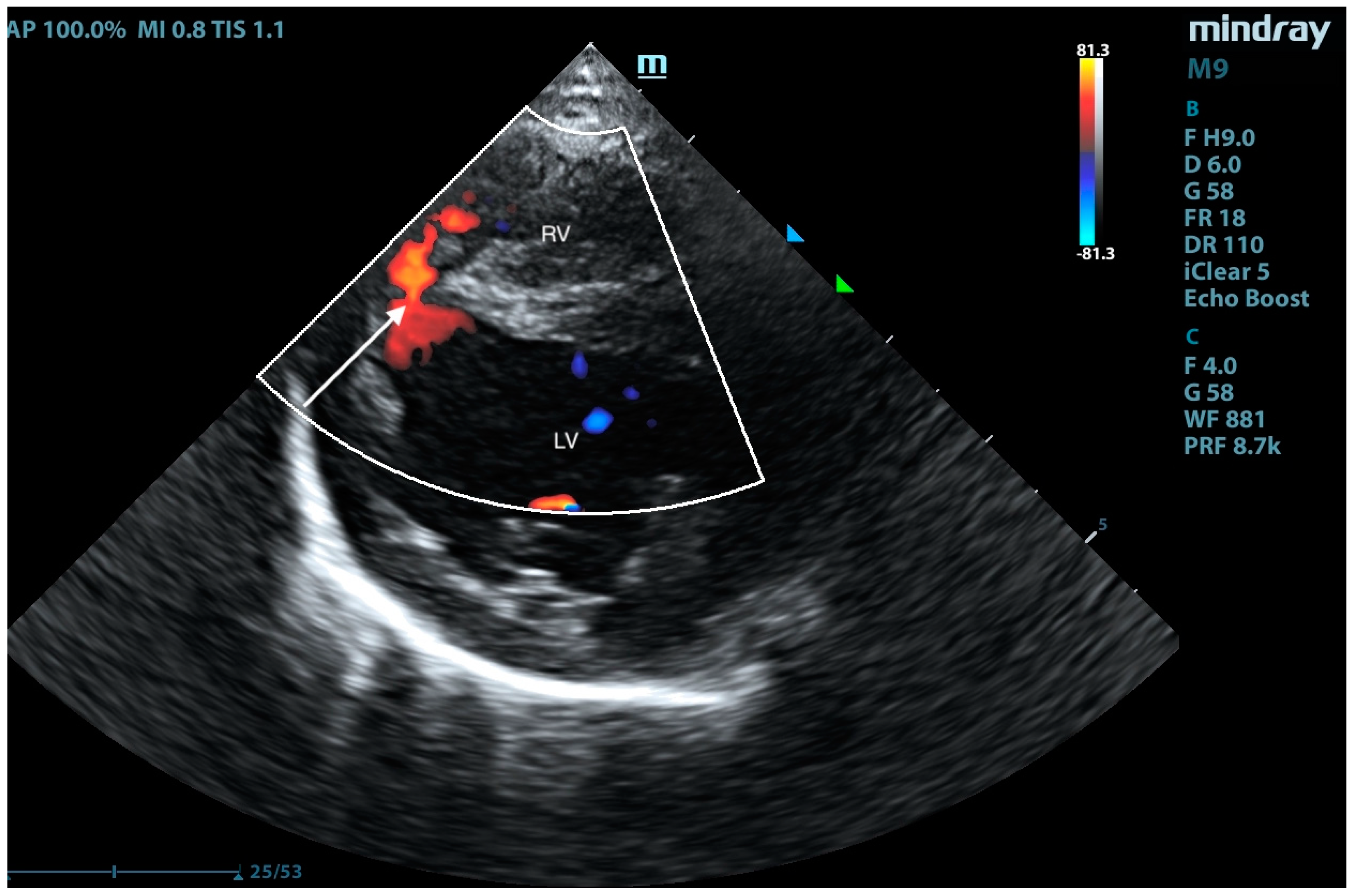
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akiyama, M.; Tanaka, R.; Maruo, K.; Yamane, Y. Surgical Correction of a Partial Atrioventricular Septal Defect with a Ventricular Septal Defect in a Dog. J. Am. Anim. Hosp. Assoc. 2005, 41, 137–143. [Google Scholar] [PubMed]
- Ratajska, A.; Kołodzińska, A.; Ciszek, B.; Wasiutyński, A. Relationship between heart development and pathogenesis of congenital heart defects in current literature. Kardiol. Pol. 2010, 68, 418–427. [Google Scholar]
- Santamarina, G.; Espino, L.; Vila, M.; Suarez, M. Partial atrioventricular canal defect in a dog. J. Small Anim. Pract. 2002, 43, 17–21. [Google Scholar]
- Agudelo, C.F.; Jekl, V.; Hauptman, K.; Crha, M.; Kocaturk, M. A case of a complete atrioventricular canal defect in a ferret. BMC Vet. Res. 2021, 17, 45. [Google Scholar] [CrossRef]
- Graczyk, S.; Grzeczka, A.; Pasławski, R.; Pasławska, U. Complete atrioventricular canal in a dog-sounds like a final judgment but is it actually one? A case report. Vet. Res. Commun. 2024, 48, 3981–3987. [Google Scholar]
- Nakao, S.; Tanaka, R.; Hamabe, L.; Suzuki, S.; Hsu, H.; Fukushima, R.; Machida, N. Cor triatriatum sinister with incomplete atrioventricular septal defect in a cat. J. Feline Med. Surg. 2011, 13, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Goya, S.; Kanno, N.; Teshima, K.; Anndo, T.; Fujioka, T. Surgery for partial atrioventricular septal defect with pulmonary hypertension in an adult dog. J. Vet. Med. Sci. 2018, 80, 1183–1189. [Google Scholar] [PubMed]
- Kittleson, M.D.; Kienle, R.D. Small Animal Cardiovascular Medicine; Mosby: St. Louis, MO, USA, 1998. [Google Scholar]
- Schrope, D.P. Atrioventricular septal defects: Natural history, echocardiographic, electrocardiographic, and radiographic findings in 26 cats. J. Vet. Cardiol. 2013, 15, 233–242. [Google Scholar]
- Rastelli, G.C.; Kirklin, J.W.; Titus, J.L. Anatomic observations on complete form of persistent common atrioventricular canal with special reference to atrioventricular valves. Mayo Clin. Proc. 1966, 41, 296–308. [Google Scholar]
- Amberger, C.N.; Boujon, C.E.; Amberger, C.; Boujon, C. Atrioventricular Canal Defect (incomplete form of endocardial cushion defect) and Ostium Secundum Type Interatrial Septal defect in a dog. J. Vet. Cardiol. 2002, 4, 37–41. [Google Scholar]
- Block, C.L.; Glassman, M.M. Pulmonary artery banding in a kitten with a partial atrioventricular septal defect. J. Vet. Cardiol. 2019, 24, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.S.; Pariaut, R.; Alcaraz, A.; Gelzer, A.R.; Malik, N.; Renaud-Farrell, S.; Charter, M.E.; Fox, P.R.; Moïse, N.S. Complete atrioven-tricular canal defect in a foal: Clinical and pathological features. J. Vet. Cardiol. 2005, 7, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Kutasi, O.; Vörös, K.; Biksi, I.; Szenci, O.; Sötonyi, P. Common atrioventricular canal in a newborn foal--case report and review of the literature. Acta Vet. Hung. 2007, 55, 51–65. [Google Scholar] [CrossRef]
- Atkins, C.; Strugnell, B.; Fingerhood, S. Complete atrioventricular septal defect with double orifice mitral valve in a calf with congestive heart failure. Vet. Rec. Case Rep. 2024, 13, e1038. [Google Scholar] [CrossRef]
- Chow, K.S.; Beijerink, N.J.; Ettinger, S.; Fliegner, R.; Beatty, J.; Barrs, V. Use of sildenafil citrate in a cat with Eisenmenger syndrome and an atrial septal defect. JFMS Open Rep. 2015, 1, 2055116915579680. [Google Scholar]
- Brown, A.J.; Davison, E.; Sleeper, M.M. Clinical efficacy of sildenafil in treatment of pulmonary arterial hypertension in dogs. J. Vet. Intern. Med. 2010, 24, 850–854. [Google Scholar] [CrossRef]
- Corno, A.F. Pulmonary artery banding. Swiss Med. Wkly. 2005, 135, 515–519. [Google Scholar]
- Angeli, E.; Pace Napoleone, C.; Turci, S.; Oppido, G.; Gargiulo, G. Pulmonary artery banding. Multimed. Man. Cardiothorac. Surg. 2012, 2012, mms010. [Google Scholar] [CrossRef] [PubMed]
- Devlin, P.J.; Jegatheeswaran, A.; McCrindle, B.W.; Karamlou, T.; Blackstone, E.H.; Williams, W.G.; DeCampli, W.M.; Mertens, L.; Fackoury, C.T.; Eghtesady, P.; et al. Pulmonary artery banding in complete atrioventricular septal defect. J. Thorac. Cardiovasc. Surg. 2020, 159, 1493–1503. [Google Scholar] [CrossRef]
- Sutherland, B.J.; Pierce, K.V.; Gagnon, A.L.; Scansen, B.A.; Orton, E.C. Dilatable pulmonary artery banding for ventricular septal defect: Surgical technique and case report of three cats. J. Vet. Cardiol. 2019, 25, 32–40. [Google Scholar] [CrossRef]
- Malekzadeh-Milani, S.; Jalal, Z.; Tamisier, D.; Boudjemline, Y. Dilatable pulmonary artery band: Safety and efficacy of balloon dilatation. Catheter. Cardiovasc. Interv. 2016, 88, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Wakao, Y.; Uechi, M.; Muto, M.; Kageyama, T.; Tanaka, K.; Kawabata, M.; Tagahashi, M. A case report of surgical treatment of a dog with atrioventricular septal defect (incomplete form of endocardial cushion defect). J. Vet. Med. Sci. 1994, 56, 981–984. [Google Scholar] [PubMed]
- Monnet, E.; Orton, E.C.; Gaynor, J.; Boon, J.; Peterson, D.; Guadagnoli, M. Diagnosis and surgical repair of partial atrioventricular septal defects in two dogs. J. Am. Vet. Med. Assoc. 1997, 211, 569–572. [Google Scholar] [PubMed]
- Cichocki, B.N.; Dugat, D.R.; Baumwart, R.D. Pulmonary artery banding in a cat with a perimembranous ventricular septal defect and left-sided congestive heart failure. J. Am. Vet. Med. Assoc. 2019, 254, 723–727. [Google Scholar]
- Summerfield, N.J.; Holt, D.E. Patent Ductus Arteriosus Ligation and Pulmonary Artery Banding in a Kitten. J. Am. Anim. Hosp. Assoc. 2005, 41, 133–136. [Google Scholar]



| Echocardiographic Measurement | Units | Preoperative | Postoperative |
|---|---|---|---|
| LAD | cm | 0.85 | 0.97 |
| AoD | cm | 1.26 | 1.29 |
| LAD/AoD | 1/1 | 1.48 | 1.32 |
| RVIDd | cm | 1.08 | 0.30 |
| LVIDd | cm | 0.50 | 0.48 |
| LVIDs | cm | 2.15 | 2.14 |
| LVPWd | cm | 0.64 | 0.40 |
| LVPWs | cm | 0.57 | 0.56 |
| IVSd | cm | 1.40 | 1.20 |
| IVSs | cm | 0.64 | 0.60 |
| LVEF | % | 67 | 77 |
| FS | % | 35 | 43 |
| LVOT Vmax | m/s | 1.08 | 1.04 |
| LVOT PGmax | mmHg | 4.70 | 4.38 |
| RVOT Vmax | m/s | 1.01 | 3.81 |
| RVOT PGmax | mmHg | 4.14 | 58.07 |
| TAPSE | mm | 87 | 43 |
| MAPSE | mm | 93 | 56 |
| Qp:Qs | - | 2.89 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szaluś-Jordanow, O.; Zdeb, K.; Mądry, W.; Buczyński, M.; Świerk, A.; Nowek, Z.; Moroz-Fik, A.; Czopowicz, M. Pulmonary Artery Banding in a Cat with Atrioventricular Canal Defect Type A with Concurrent Muscular Septal Defect. Animals 2025, 15, 1044. https://doi.org/10.3390/ani15071044
Szaluś-Jordanow O, Zdeb K, Mądry W, Buczyński M, Świerk A, Nowek Z, Moroz-Fik A, Czopowicz M. Pulmonary Artery Banding in a Cat with Atrioventricular Canal Defect Type A with Concurrent Muscular Septal Defect. Animals. 2025; 15(7):1044. https://doi.org/10.3390/ani15071044
Chicago/Turabian StyleSzaluś-Jordanow, Olga, Krzysztof Zdeb, Wojciech Mądry, Michał Buczyński, Anna Świerk, Zofia Nowek, Agata Moroz-Fik, and Michał Czopowicz. 2025. "Pulmonary Artery Banding in a Cat with Atrioventricular Canal Defect Type A with Concurrent Muscular Septal Defect" Animals 15, no. 7: 1044. https://doi.org/10.3390/ani15071044
APA StyleSzaluś-Jordanow, O., Zdeb, K., Mądry, W., Buczyński, M., Świerk, A., Nowek, Z., Moroz-Fik, A., & Czopowicz, M. (2025). Pulmonary Artery Banding in a Cat with Atrioventricular Canal Defect Type A with Concurrent Muscular Septal Defect. Animals, 15(7), 1044. https://doi.org/10.3390/ani15071044








