Winter Ecology of the Hen Harrier, Circus cyaneus: Bridging Behavioral Insights and Conservation Requirements
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Taxonomy and Identification
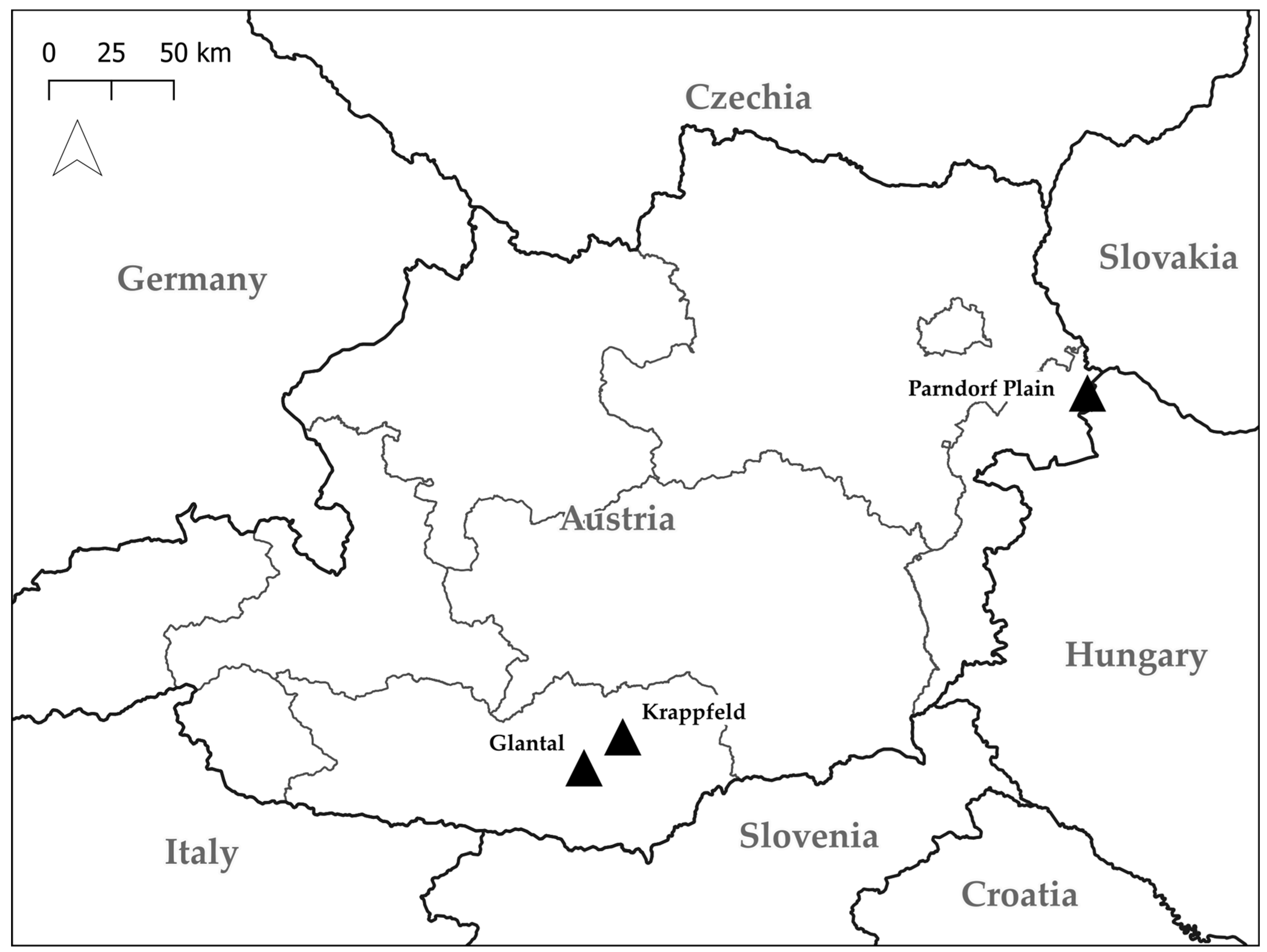
2.2. Study Areas
2.3. Prey Identification and Consumption
2.4. Time and Energy Budgets
2.5. Vole Abundance Index and Territoriality
2.6. Land-Use Change
2.7. Data Analysis
3. Results
3.1. Energy Intake: Diet, Consumption Rate, and Capture Sites
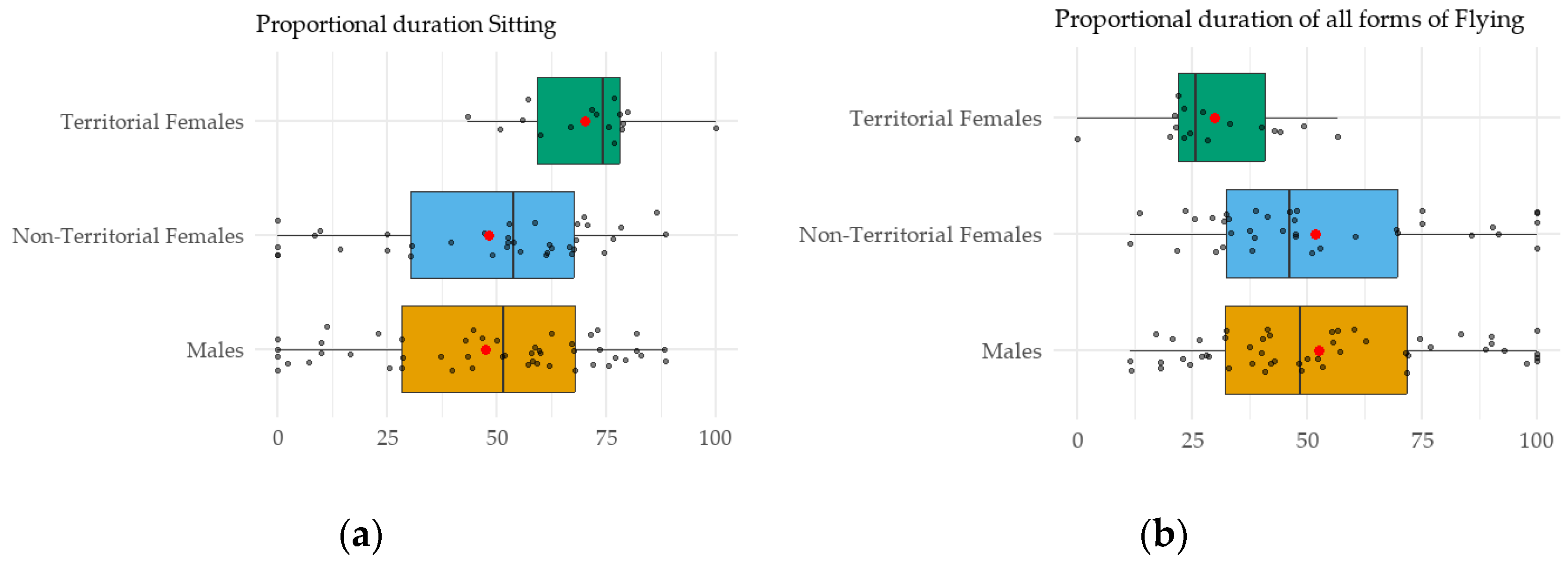
3.2. Time Budget and Energy Expenditure: Sex and Territoriality Effects
3.3. Energy Budget: Food Requirements and Foraging Efficiency
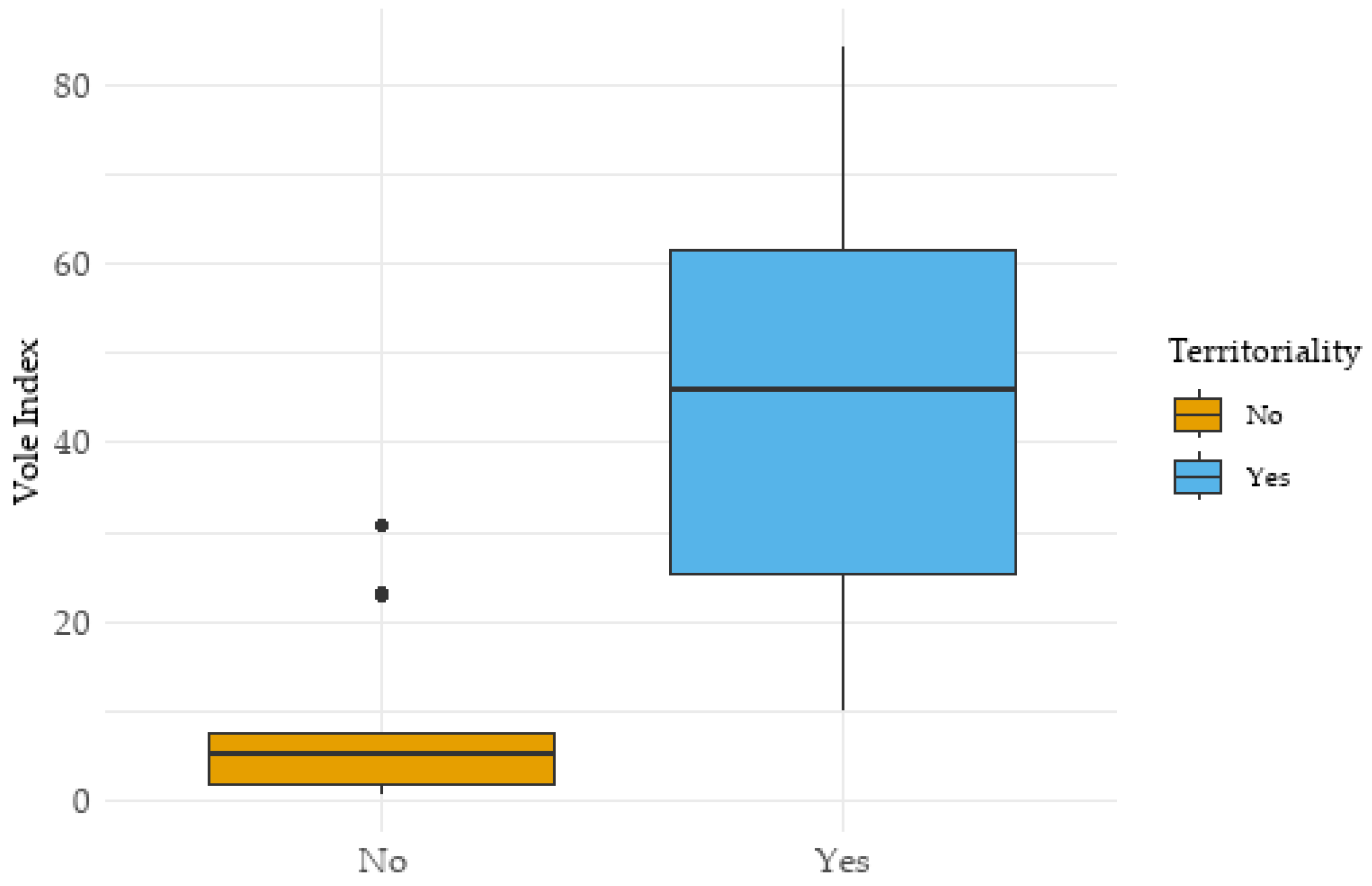
3.4. Vole Availability and Territoriality
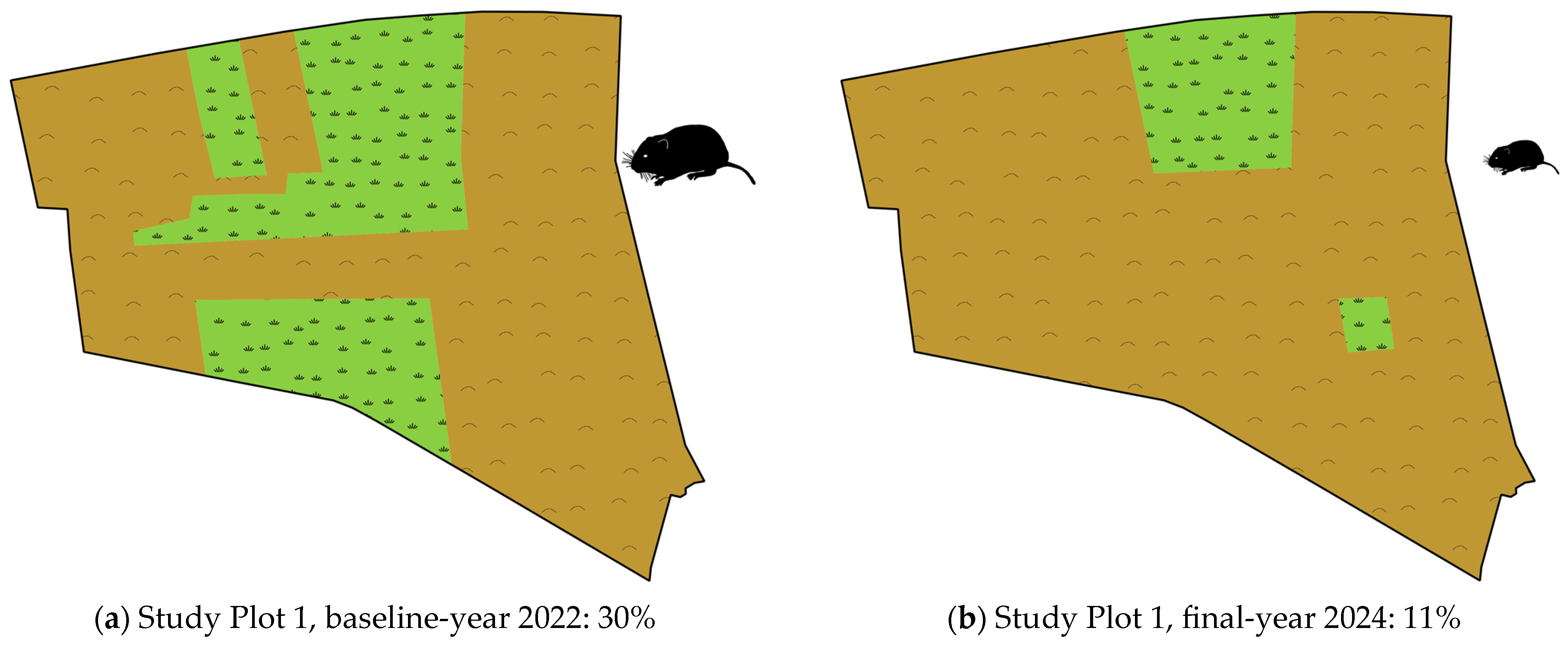
3.5. Habitat Alteration
4. Discussion
- Habitat use, prey availability, and top-down effects: Hen harriers’ winter habitat use is shaped by the interplay of prey availability and predation risk, particularly in forest-rich regions such as the inner Alps or riparian forest margins. Voles, their primary prey in Central Europe, are most abundant in meadows and fallow land [110], with availability influenced by habitat suitability, vegetation structure, and population cycles [111,112,113]. Although voles also occur in higher Alpine regions [42,114,115], hen harriers almost exclusively use open lowlands with expansive flat terrain for hunting and resting. This preference suggests that top-down effects and reduced predation risk significantly shape habitat selection at the landscape scale [116,117,118], beyond the influence of higher vole densities in lowland areas. We assume that the ‘landscape of fear’ created by potent predators such as the Eurasian goshawk, Astur gentilis, strongly influences spatial behavior [119]. Our observations include two unsuccessful goshawk attacks, several instances of hen harriers fleeing when the large predator was hunting pigeons nearby, and repeated circling of harriers above perched goshawks. Among other predators, a domestic cat, Felis catus, was the only one observed attempting to capture a harrier. While data on nocturnal threats such as wild boars, Sus scrofa, eagle owls, Bubo bubo, and red foxes, Vulpes vulpes, are scarce [120], these predators may affect roosting site selection [121,122]. Large raptors, including golden eagles, Aquila chrysaetos, Eastern imperial eagles, Aquila heliaca, and white-tailed eagles, Haliaeetus albicilla, overlap with hen harriers in winter habitats, but their specific impact remains unclear. Notably, the latter two species demonstrate significant spatial overlap with overwintering hen harriers and are expanding in Central Europe [123,124].
- Competitive interactions: In our study areas, hen harriers face various competitive pressures during winter, primarily involving resource displacement and kleptoparasitism. Common buzzards and Saker falcons regularly displace hen harriers, forcing them to abandon valuable hunting grounds and prey [125]. Particularly the ‘ubiquitous’ buzzards benefit from structured landscapes, launching their attempts from elevated perches. Large Falco species, including Saker, gyr, F. rusticolus, and peregrine, F. peregrinus, occasionally may injure hen harriers during competitive encounters or, in rare cases, even kill them intentionally [15,126]. Smaller raptors, such as the common kestrel, are regularly robbed of their prey by hen harriers but have also been observed displacing (male) harriers from favorable hunting grounds in isolated cases [127]. The relationship with carrion and hooded crows, Corvus corone and Corvus cornix (including numerous hybrids in our study areas), is dual in nature: while mobbing crows can disrupt hen harriers’ hunting efforts, they may also serve as an early warning system against predators such as the goshawk, which threaten both species.
- Anthropogenic threats apart from agricultural intensification: Firstly, open landscapes are reduced in size through ecosystem conversion, including permanent soil sealing from expanding settlements or the establishment of new industrial areas [128,129]. Secondly, infrastructure development and recreational activities contribute to ecosystem degradation, fragmentation, and disturbances, thereby reducing habitat quality [130,131,132,133]. Thirdly, rodenticides pose a significant risk to non-target organisms due to their high toxicity, environmental persistence, and bioaccumulative properties. Second-generation anticoagulant rodenticides may negatively affect predators both through reduced vole numbers following rodenticide campaigns and through direct mortality as well as sublethal fitness effects [134,135,136]. The accumulation of rodenticides in bioindicators such as raptors has recently been confirmed in Austria [137]. However, neither the exact extent of these threats, nor their cumulative impact on hen harriers at larger spatial scales, has been quantified.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McClure, C.J.W.; Westrip, J.R.S.; Johnson, J.A.; Schulwitz, S.E.; Virani, M.Z.; Davies, R.; Symes, A.; Wheatley, H.; Thorstrom, R.; Amar, A.; et al. State of the world’s raptors: Distributions, threats, and conservation recommendations. Biol. Conserv. 2018, 227, 390–402. [Google Scholar] [CrossRef]
- Newton, I. Population Ecology of Raptors; T. & A.D. Poyser: London, UK, 1990. [Google Scholar]
- Sergio, F.; Newton, I.; Marchesi, L.; Pedrini, P. Ecologically justified charisma: Preservation of top predators delivers biodiversity conservation. J. Appl. Ecol. 2006, 43, 1049–1055. [Google Scholar] [CrossRef]
- Donázar, J.A.; Cortés-Avizanda, A.; Fargallo, J.A.; Margalida, A.; Moleón, M.; Morales-Reyes, Z.; Moreno-Opo, R.; Pérez-García, J.M.; Sánchez-Zapata, J.A.; Zuberogoitia, I.; et al. Roles of raptors in a changing world: From flagships to providers of key ecosystem services. Ardeola 2016, 63, 181–234. [Google Scholar] [CrossRef]
- Blumstein, D.T.; Fernández-Juricic, E. The emergence of conservation behavior. Conserv. Biol. 2004, 18, 1175–1177. [Google Scholar] [CrossRef]
- Berger-Tal, O.; Polak, T.; Oron, A.; Lubin, Y.; Kotler, B.P.; Saltz, D. Integrating animal behavior and conservation biology: A conceptual framework. Behav. Ecol. 2011, 22, 236–239. [Google Scholar] [CrossRef]
- Berger-Tal, O.; Saltz, D. (Eds.) Conservation Behavior: Applying Behavioral Ecology to Wildlife Conservation and Management; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Simmons, R.E. Harriers of the World: Their Behaviour and Ecology; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Watson, D. The Hen Harrier; Bloomsbury Natural History: London, UK, 2017. [Google Scholar]
- Balfour, E.; Cadbury, C.J. Polygyny, spacing and sex ratio among hen harriers Circus cyaneus in Orkney, Scotland. Ornis Scand. 1979, 10, 133–141. [Google Scholar] [CrossRef]
- Panuccio, M.; Melone, U.; Agostini, N. (Eds.) Migration Strategies of Birds of Prey in the Western Palearctic; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Newton, I. Migration mortality in birds. Ibis 2025, 167, 106–123. [Google Scholar] [CrossRef]
- Jankowiak, Ł.; Polakowski, M.; Kułakowski, T.; Świętochowski, P.; Tumiel, T.; Broniszewska, M.; Takács, V. Habitat and weather requirements of diurnal raptors wintering in river valleys. Biologia 2015, 70, 1136–1142. [Google Scholar] [CrossRef]
- Kostrzewa, R.; Kostrzewa, A. Winter weather, spring and summer density, and subsequent breeding success of Eurasian kestrels, common buzzards, and northern goshawks. The Auk 1991, 108, 342–347. [Google Scholar]
- Glutz von Blotzheim, U.N. (Ed.) Handbuch der Vögel Mitteleuropas, Band 4: Falconiformes—Greifvögel; 2. Aufl.; AULA: Wiesbaden, Germany, 1989. [Google Scholar]
- Dobler, G. Territorial behaviour of the hen harrier in winter. Br. Birds 2021, 114, 133–147. [Google Scholar]
- Fernández-Bellon, D.; Lusby, J.; Bos, J.; Schaub, T.; McCarthy, A.; Caravaggi, A.; Irwin, S.; O’Halloran, J. Expert knowledge assessment of threats and conservation strategies for breeding hen harrier and short-eared owl across Europe. Bird Conserv. Int. 2021, 31, 268–285. [Google Scholar] [CrossRef]
- Bos, J.; Schaub, T.; Klaassen, R.; Kuiper, M. (Eds.) Abstracts Proceedings of the International Hen Harrier and Short-Eared Owl Meeting 2019, Groningen, The Netherlands, 22 March 2019; Vogelbescherming Nederland: Groningen, The Netherlands, 2019. [Google Scholar]
- O’Donoghue, B.G. Hen harrier Circus cyaneus ecology and conservation during the non-breeding season in Ireland. Bird Study 2020, 67, 344–359. [Google Scholar] [CrossRef]
- Arroyo, B.E.; Garcia, J.T. Diet composition influences annual breeding success of Montagu’s harriers Circus pygargus feeding on diverse prey. Bird Study 2006, 53, 73–78. [Google Scholar] [CrossRef]
- Amar, A.; Redpath, S.; Thirgood, S. Evidence for food limitation in the declining hen harrier population on the Orkney Islands, Scotland. Biol. Conserv. 2003, 111, 377–384. [Google Scholar] [CrossRef]
- Temeles, E.J. The effects of prey availability and capture success on the foraging and territory economics of a predatory bird, Circus hudsonius. J. Ornithol. 2022, 163, 535–546. [Google Scholar] [CrossRef]
- Picozzi, N. Sex ratio, survival and territorial behaviour of polygynous hen harriers Circus c. cyaneus in Orkney. Ibis 1984, 126, 356–365. [Google Scholar] [CrossRef]
- Millon, A.; Bourrioux, J.-L.; Riols, C.; Bretagnolle, V. Comparative breeding biology of hen harrier and Montagu’s harrier: An 8-year study in north-eastern France. Ibis 2002, 144, 94–105. [Google Scholar] [CrossRef]
- Arroyo, B.; García, J.T.; Bretagnolle, V. Conservation of the Montagu’s harrier (Circus pygargus) in agricultural areas. Anim. Conserv. 2002, 5, 283–290. [Google Scholar] [CrossRef]
- Greggor, A.L.; Blumstein, D.T.; Wong, B.B.M.; Berger-Tal, O. Using animal behavior in conservation management: A series of systematic reviews and maps. Environ. Evid. 2019, 8, 23. [Google Scholar] [CrossRef]
- Daan, S.; Altenburg, W.; Boedeltje, G. Timing of vole hunting in aerial predators. Mammal Rev. 1982, 12, 169–181. [Google Scholar] [CrossRef][Green Version]
- Temeles, E.J. Reversed sexual size dimorphism: Effect on resource defense and foraging behaviors of nonbreeding northern harriers. Auk 1986, 103, 70–78. [Google Scholar] [CrossRef]
- Temeles, E.J. The relative importance of prey availability and intruder pressure in feeding territory size regulation by harriers, Circus cyaneus. Oecologia 1987, 74, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Temeles, E.J. Northern harriers on feeding territories respond more aggressively to neighbors than to floaters. Behav. Ecol. Sociobiol. 1990, 26, 57–63. [Google Scholar] [CrossRef]
- McClure, C.J.W.; Vargas, F.H.; Amar, A.; Concepcion, C.B.; MacColl, C.; Sumasgutner, P. Conservation letter: Monitoring raptor populations—A call for increased global collaboration and survey standardization. J. Raptor Res. 2023, 57, 106–113. [Google Scholar] [CrossRef]
- Forsman, D. Flight Identification of Raptors of Europe, North Africa and the Middle East; Helm Identification Guides: London, UK, 2016. [Google Scholar]
- Dobler, G. Field Identification of the Hen Harrier, Montagu’s Harrier and Pallid Harrier; Zeiss Edition on Field Ornithology; Carl Zeiss AG: Oberkochen, Germany, 2022; Volume 3. [Google Scholar]
- Lontkowski, J. Die Unterscheidung von Korn- (Circus cyaneus), Wiesen- (Circus pygargus) und Steppenweihe (Circus macrourus). Limicola 1995, 9, 233–275. [Google Scholar]
- Dobler, G. Hen Harrier (Circus cyaneus): Aging and Sexing; Zeiss Edition on Field Ornithology; DWJ-Verlags-GmbH: Blaufelden, Germany, 2020; Volume 1. [Google Scholar]
- Dobler, G. Hunting and Territorial Behaviour of the Hen Harrier in Winter; Zeiss Edition on Field Ornithology; Carl Zeiss AG: Oberkochen, Germany, 2021; Volume 2. [Google Scholar]
- Laber, J. Zum Wintervorkommen der Kornweihe (Circus cyaneus) im Seewinkel/Burgenland. Egretta 1995, 38, 13–21. [Google Scholar]
- Sachslehner, L. Artenschutzprojekt für gefährdete Vogelarten in NÖ 2022–2024, Modul 3: Korn- und Wiesenweihe. Endbericht 2024; Bericht im Auftrag des Landes Niederösterreich; Büro für Naturschutzpraxis und Forschung: Wien, Austria, 2024. [Google Scholar]
- Teufelbauer, N.; Seaman, B.; Hohenegger, J.A.; Nemeth, E.; Karner-Ranner, E.; Probst, R.; Berger, A.; Lugerbauer, L.; Berg, H.-M.; Laßnig-Wlad, C. (Eds.) Österreichischer Brutvogelatlas 2013–2028; Verlag des Naturhistorischen Museums Wien: Vienna, Austria, 2023. [Google Scholar]
- Bělka, T.; Horal, D.; Mrlík, V.; Navrátil, P.; Krejčí, J.; Bartes, P. Breeding of the hen harrier (Circus cyaneus) in the Czech Republic in 2019. Crex 2020, 38, 9–23. [Google Scholar]
- Keller, V.; Herrando, S.; Voríšek, P.; Franch, M.; Kipson, M.; Milanesi, P.; Martí, D.; Anton, M.; Klvanová, A.; Kalyakin, M.V.; et al. European Breeding Bird Atlas 2: Distribution, Abundance and Change; Lynx Edicions/European Bird Census Council (EBCC): Barcelona, Spain, 2020. [Google Scholar]
- Spitzenberger, F. (Ed.) Die Säugetierfauna Österreichs; Grüne Reihe des Bundesministeriums für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft; Austria Medien Service: Graz, Austria, 2001. [Google Scholar]
- Jacob, J.; Manson, P.; Barfknecht, R.; Fredricks, T. Common vole (Microtus arvalis) ecology and management: Implications for risk assessment of plant protection products. Pest Manag. Sci. 2014, 70, 869–878. [Google Scholar] [CrossRef]
- Kauer, L.; Imholt, C.; Jacob, J.; Kuehn, R. Assessing the effects of land-use intensity on small mammal community composition and genetic variation in Myodes glareolus and Microtus arvalis across grassland and forest habitats. Landsc. Ecol. 2024, 40, 1. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L. The long-term dynamics of shrew communities: Is there a downward trend? Life 2024, 14, 1393. [Google Scholar] [CrossRef]
- Janova, E.; Heroldova, M. Response of small mammals to variable agricultural landscapes in Central Europe. Mamm. Biol. 2016, 81, 488–493. [Google Scholar] [CrossRef]
- Fischer, C.; Schröder, B. Predicting spatial and temporal habitat use of rodents in a highly intensive agricultural area. Agric. Ecosyst. Environ. 2014, 189, 145–153. [Google Scholar] [CrossRef]
- Greenwood, P.J. Timing of activity of the bank vole Clethrionomys glareolus and the wood mouse Apodemus sylvaticus in a deciduous woodland. Oikos 1978, 31, 123–127. [Google Scholar] [CrossRef]
- Viviano, A.; Scarfò, M.; Mori, E. Temporal partitioning between forest-dwelling small rodents in a Mediterranean deciduous woodland. Animals 2022, 12, 279. [Google Scholar] [CrossRef] [PubMed]
- Probst, R.; Probst, R. High frequency of Apodemus mice boosts inverse activity pattern of bank voles, Clethrionomys glareolus, through non-aggressive intraguild competition. Animals 2023, 13, 981. [Google Scholar] [CrossRef]
- Probst, R.; Probst, R. Seasonal changes in nycthemeral availability of sympatric temperate mixed forest rodents: The predators’ perspective. Life 2024, 14, 45. [Google Scholar] [CrossRef]
- Brockmann, H.J.; Barnard, C.J. Kleptoparasitism in birds. Anim. Behav. 1979, 27, 487–514. [Google Scholar] [CrossRef]
- Aschoff, J.; Pohl, H. Der Ruheumsatz von Vögeln als Funktion der Tageszeit und der Körpergröße. J. Ornithol. 1970, 111, 38–47. [Google Scholar] [CrossRef]
- Wolf, L.L.; Hainsworth, F.R. Time and energy budgets of territorial hummingbirds. Ecology 1971, 52, 980–988. [Google Scholar] [CrossRef]
- Wakeley, J.S. Activity budgets, energy expenditures, and energy intakes of nesting ferruginous hawks. Auk 1978, 95, 667–676. [Google Scholar] [CrossRef]
- Wakeley, J.S. Hunting methods and factors affecting their use by ferruginous hawks. Condor 1978, 80, 327–333. [Google Scholar] [CrossRef]
- Furness, R.W.; Trinder, M.; MacArthur, D.; Douse, A. A theoretical approach to estimating bird risk of collision with wind turbines where empirical flight activity data are lacking. Energy Power Eng. 2016, 8, 183–194. [Google Scholar] [CrossRef][Green Version]
- Górecki, A. Energy values of body in small mammals. Acta Theriol. 1965, 10, 333–352. [Google Scholar] [CrossRef]
- Halle, S. Avian predation upon a mixed community of common voles (Microtus arvalis) and wood mice (Apodemus sylvaticus). Oecologia 1988, 75, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ebeling, M.; Hahne, J. Relevance of body weight effects for the population development of common voles and its significance in regulatory risk assessment of pesticides in the European Union. Environ. Sci. Eur. 2019, 31, 54. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L. Selective predation on common voles by tawny owls and long-eared owls in winter and spring. Turk. J. Zool. 2014, 38, 242–249. [Google Scholar] [CrossRef]
- Jacob, J. Body weight dynamics of common voles in agro-ecosystems. Mammalia 2003, 67, 559–566. [Google Scholar] [CrossRef]
- Raymond, M.; Robitaille, J.-F.; Lauzon, P.; Vaudry, R. Prey-dependent profitability of foraging behaviour of male and female ermine, Mustela erminea. Oikos 1990, 58, 323–328. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L. Sexual body size dimorphism in small mammals: A case study from Lithuania. Biology 2024, 13, 1032. [Google Scholar] [CrossRef]
- Dare, P. The Life of Buzzards; Whittles Publishing Ltd.: Dunbeath, UK, 2015. [Google Scholar]
- Delattre, P.; Giraudoux, P.; Baudry, J.; Quéré, J.P.; Fichet, E. Effect of landscape structure on common vole (Microtus arvalis) distribution and abundance at several space scales. Landsc. Ecol. 1996, 11, 279–288. [Google Scholar] [CrossRef]
- Delattre, P.; Giraudoux, P.; Damange, J.-P.; Quéré, J.-P. Recherche d’un indicateur de la cinétique démographique des populations du campagnol des champs (Microtus arvalis). Rev. D’écologie 1990, 45, 375–384. [Google Scholar] [CrossRef]
- Hollander, M.; Chicken, E.; Wolfe, D.A. Nonparametric Statistical Methods, 3rd ed.; Wiley Series in Probability and Statistics; Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Kloke, J.; McKean, J.W. Nonparametric Statistical Methods Using R, 1st ed.; Chapman & Hall/CRC: New York, NY, USA, 2014. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression, 3rd ed.; Wiley Series in Probability and Statistics; Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Masman, D.; Daan, S.; Dijkstra, C. Time allocation in the kestrel (Falco tinnunculus), and the principle of energy minimization. J. Anim. Ecol. 1988, 57, 411–432. [Google Scholar] [CrossRef]
- McCarthy, A.; Smiddy, P.; Nagle, T.; Mee, A.; Irwin, S.; Caravaggi, A.; O’Halloran, J. Landscape and temporal influences on the winter diet of a threatened diurnal raptor, the hen harrier Circus cyaneus. Bird Study 2021, 68, 408–421. [Google Scholar] [CrossRef]
- Toffoli, R. L’alimentazione invernale dell’albanella reale (Circus cyaneus) in Piemonte (Aves, Accipitriformes). Riv. Piem. St. Nat. 1994, 15, 155–161. [Google Scholar]
- Van Boekel, W.; Berghuis, P. Overwinterende blauwe kiekendieven (Circus cyaneus) in De Onlanden. De Takkeling 2014, 22, 214–224. [Google Scholar]
- Van Manen, W. Demografie en voedsel van overwinterende blauwe kiekendieven (Circus cyaneus) in Drenthe. Limosa 1996, 69, 9–12. [Google Scholar]
- Bergmann, F. Zum Auftreten der Kornweihe (Circus cyaneus) am südlichen Oberrhein. Naturschutz Südl. Oberrh. 1998, 2, 195–204. [Google Scholar]
- Einstein, J. Zug, Überwinterung und Verhalten der Kornweihe (Circus cyaneus) am Federsee (Süddeutschland, Oberschwaben). Orn. Jb. Bad.-Württ. 2000, 16, 13–32. [Google Scholar]
- De Boer, P.; Voskamp, P.; Van Rijn, S. Overwinterende blauwe kiekendieven in het Limburgse heuvelland: Vormen hamsterreservaten een ecologische val? Limosa 2013, 86, 169–179. [Google Scholar]
- Noga, M. The winter diet of the hen harrier (Circus cyaneus) in the Záhorí region. Crex 2014, 34, 113–117. [Google Scholar]
- Redpath, S.M.; Clarke, R.; Madders, M.; Thirgood, S.J. Assessing raptor diet: Comparing pellets, prey remains, and observational data at hen harrier nests. Condor 2001, 103, 184–188. [Google Scholar] [CrossRef]
- Risdiyanto, I.; Santosa, Y.; Santoso, N.; Sunkar, A. Modeling trophic cascades to identify key mammalian species for ecosystem stability. Ecologies 2024, 5, 585–609. [Google Scholar] [CrossRef]
- Bro, E.; Arroyo, B.; Migot, P. Conflict between grey partridge Perdix perdix hunting and hen harrier Circus cyaneus protection in France: A review. Wildl. Biol. 2006, 12, 233–247. [Google Scholar] [CrossRef]
- DeLong, J.P.; Coblentz, K.E.; La Sorte, F.A.; Uiterwaal, S.F. The global diet diversity spectrum in avian apex predators. Proc. R. Soc. B 2024, 291, 20242156. [Google Scholar] [CrossRef]
- Niethammer, J.; Krapp, F. Handbuch der Säugetiere Europas: Cricetidae, Arvicolidae, Zapodidae, Spalacidae, Hystricidae, Capromyidae; Akademische Verl.: Wiesbaden, Germany, 1982. [Google Scholar]
- Bělka, T.; Bělkova, J. Winter habitat use and migration of hen harriers based on GPS-tracking. In Proceedings of the International Hen Harrier and Short-Eared Owl Meeting 2019, Groningen, The Netherlands, 20–22 March 2019; Bos, J., Schaub, T., Klaassen, R., Kuiper, M., Eds.; Vogelbescherming Nederland: Groningen, The Netherlands, 2019; p. 32. [Google Scholar]
- Redpath, S.; Amar, A.; Madders, M.; Leckie, F.; Thirgood, S. Hen harrier foraging success in relation to land use in Scotland. Anim. Conserv. 2002, 5, 113–118. [Google Scholar] [CrossRef]
- Warkentin, I.G.; West, N.H. Ecological energetics of wintering merlins Falco columbarius. Physiol. Zool. 1990, 63, 308–333. [Google Scholar] [CrossRef]
- Kirkwood, J.K. Bioenergetics and Growth in the Kestrel (Falco tinnunculus). Ph.D. Thesis, University of Bristol, Bristol, UK, 1981. [Google Scholar]
- Daan, S.; Masman, D.; Strijkstra, A.; Verhulst, S. Intraspecific allometry of basal metabolic rate: Relations with body size, temperature, composition, and circadian phase in the Kestrel, Falco tinnunculus. J. Biol. Rhythm. 1989, 4, 155–171. [Google Scholar] [CrossRef]
- Schipper, W.J.A.; Buurma, L.S.; Bossenbroek, P. Comparative study of hunting behaviour of wintering hen harriers Circus cyaneus and marsh harriers Circus aeruginosus. Ardea 1975, 63, 1–29. [Google Scholar]
- Vincheuski, D. Differences in hunting between wintering males and females of hen harrier in Belarus. In Proceedings of the International Hen Harrier and Short-Eared Owl Meeting, Groningen, The Netherlands, 22 March 2019; Bos, J., Schaub, T., Klaassen, R., Kuiper, M., Eds.; Vogelbescherming Nederland: Groningen, The Netherlands, 2019; p. 31. [Google Scholar]
- Marquiss, M. Habitat and diet of male and female hen harriers in Scotland in winter. Br. Birds 1980, 73, 555–560. [Google Scholar]
- Habel, J.C.; Dengler, J.; Janišová, M.; Török, P.; Wellstein, C.; Wiezik, M. European grassland ecosystems: Threatened hotspots of biodiversity. Biodivers. Conserv. 2013, 22, 2131–2138. [Google Scholar] [CrossRef]
- Donald, P.F.; Green, R.E.; Heath, M.F. Agricultural intensification and the collapse of Europe’s farmland bird populations. Proc. R. Soc. B 2001, 268, 25–29. [Google Scholar] [CrossRef]
- Bundesministerium für Land- und Forstwirtschaft, Regionen und Wasserwirtschaft. Grüner Bericht 2024: Die Situation der österreichischen Land- und Forstwirtschaft im Jahr 2023; 65. Auflage; Bundesministerium für Land- und Forstwirtschaft, Regionen und Wasserwirtschaft: Wien, Austria, 2024. [Google Scholar]
- Gregory, R.D.; Eaton, M.A.; Burfield, I.J.; Grice, P.V.; Howard, C.; Klvaňová, A.; Noble, D.; Šilarová, E.; Staneva, A.; Stephens, P.A.; et al. Drivers of the changing abundance of European birds at two spatial scales. Philos. Trans. R. Soc. B 2023, 378, 20220198. [Google Scholar] [CrossRef]
- Aschwanden, J.; Birrer, S.; Jenni, L. Are ecological compensation areas attractive hunting sites for common kestrels (Falco tinnunculus) and long-eared owls (Asio otus)? J. Ornithol. 2005, 146, 279–286. [Google Scholar] [CrossRef]
- Reifeltsammer, S. Kulturlandschaftsgenese seit 1950 und deren Auswirkungen auf das Biodiversitätspotenzial am Beispiel der Gemeinde St. Georgen am Längsee. Carinthia II 2020, 210./130., 97–116. [Google Scholar]
- Broughton, R.K.; Shore, R.F.; Heard, M.S.; Amy, S.R.; Meek, W.R.; Redhead, J.W.; Turk, A.; Pywell, R.F. Agri-environment scheme enhances small mammal diversity and abundance at the farm-scale. Agric. Ecosyst. Environ. 2014, 192, 122–129. [Google Scholar] [CrossRef]
- Wiersma, P.; Bos, J. Birdfields for hen harriers and short-eared owls. In Proceedings of the International Hen Harrier and Short-Eared Owl Meeting, Groningen, The Netherlands, 22 March 2019; Bos, J., Schaub, T., Klaassen, R., Kuiper, M., Eds.; Vogelbescherming Nederland: Groningen, The Netherlands, 2019; pp. 46–47. [Google Scholar]
- Canonne, C.; Chiffard, J.; Curtet, L.; Besnard, A. Response of grassland birds to local features strongly depends on landscape context. Agric. Ecosyst. Environ. 2024, 365, 108905. [Google Scholar] [CrossRef]
- Cardador, L.; Carrete, M.; Mañosa, S. Can intensive agricultural landscapes favour some raptor species? The marsh harrier in north-eastern Spain. Anim. Conserv. 2011, 14, 382–390. [Google Scholar] [CrossRef]
- Andreatta, D.; Bazzi, G.; Nardelli, R.; Siddi, L.; Cecere, J.G.; Chamberlain, D.; Morganti, M.; Rubolini, D.; Assandri, G. Remote sensing reveals scale-specific effects of forage crop mowing and landscape structure on a declining farmland bird. J. Appl. Ecol. 2025, 62, 502–515. [Google Scholar] [CrossRef]
- Lugonja, T.N.; Pouwels, R.; Arok, M.; Radišić, D.; Ćosić, N.; Ćirović, D.; Wamelink, G.W.W. Combining local monitoring data and scientific models to prioritize conservation for European ground squirrel and safeguard grassland habitats. Landsc. Ecol. 2025, 40, 21. [Google Scholar] [CrossRef]
- Zulka, K.P.; Huchler, K.; Schön, B.; Wrbka, T.; Kudrnovsky, H.; Schindler, S. Österreichische Hotspots der Biodiversität zur systematischen Naturschutzplanung; Report REP-0945; Umweltbundesamt: Wien, Austria, 2024. [Google Scholar]
- McClure, C.J.W.; Carignan, A.; Buij, R. Lack of Standardization in the Use of Road Counts for Surveying Raptors. Condor 2021, 123, duaa061. [Google Scholar] [CrossRef]
- Zimova, M.; Newey, S.; Denny, B.; Pedersen, S.; Scott Mills, L. Scottish mountain hares do not respond behaviorally to camouflage mismatch. Oikos 2024, 2024, e10834. [Google Scholar] [CrossRef]
- Pasquet, A.; Torre, I.; Díaz, M. Indirect human influences in fear landscapes: Varying effects of moonlight on small mammal activity along man-made gradients of vegetation structure. Life 2023, 13, 681. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, R.; Schlaich, A.E.; Bouten, W.; Both, C.; Koks, B.J. Eerste resultaten van het jaarrond volgen van Blauwe Kiekendieven broedend in het Oost-Groningse akkerland. Limosa 2014, 87, 135–148. [Google Scholar]
- Frühauf, J.; Bieringer, G. Der Einfluss von ÖPUL 2000 auf die winterliche Raumnutzung von Greifvögeln und anderen Vogelarten in der Ackerbauregion Ostösterreichs; Bericht im Auftrag des Bundesministeriums für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft; BirdLife Österreich: Wien, Austria, 2003. [Google Scholar]
- Andreassen, H.P.; Sundell, J.; Ecke, F.; Halle, S.; Haapakoski, M.; Henttonen, H.; Huitu, O.; Jacob, J.; Johnsen, K.; Koskela, E.; et al. Population cycles and outbreaks of small rodents: Ten essential questions we still need to solve. Oecologia 2021, 195, 601–622. [Google Scholar] [CrossRef]
- Wymenga, E.; Beemster, N.; Bos, D.; Bekkema, M. Recurring outbreaks of common vole (Microtus arvalis) in grasslands in the low-lying parts of the Netherlands. Lutra 2021, 64, 81–101. [Google Scholar]
- Soininen, E.M.; Neby, M. Small rodent population cycles and plants—After 70 years, where do we go? Biol. Rev. 2023, 98, brv.13021. [Google Scholar] [CrossRef]
- Ferrari, G.; Scaravelli, D.; Mustoni, A.; Armanini, M.; Zibordi, F.; Devineau, O.; Cagnacci, F.; Grasso, D.A.; Ossi, F. A comparison of small rodent assemblages after a 20-year interval in the Alps. Animals 2023, 13, 1407. [Google Scholar] [CrossRef]
- Reiter, G.; Winding, N. Verbreitung und Ökologie alpiner Kleinsäuger (Insectivora, Rodentia) an der Südseite der Hohen Tauern, Österreich. Wiss. Mitteilungen Natl. Hohe Tauern 1997, 3, 97–135. [Google Scholar]
- Kovinka, T.; Sharikov, A.; Massalskaya, T.; Volkov, S. Structure and heterogeneity of habitat determine diet of predators despite prey abundance: Similar response in long-eared, short-eared owls and common kestrels. Avian Res. 2023, 14, 100072. [Google Scholar] [CrossRef]
- Natsukawa, H.; Yuasa, H.; Fujisaki, M.; Kobayashi, T.; Maruyama, H.; Masukawa, K.; Nunokawa, K.; Saito, H.; Sato, G.; Sutton, L.J.; et al. Importance of the interplay between land cover and topography in modeling habitat selection. Ecol. Indic. 2024, 169, 112896. [Google Scholar] [CrossRef]
- Roth, T.C.; Lima, S.L. The predatory behavior of wintering Accipiter hawks: Temporal patterns in activity of predators and prey. Oecologia 2007, 152, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Kratzer, D.; Kratzer, R. Zur Jagdmethode des Habichts (Accipiter gentilis) auf Kornweihen (Circus cyaneus). Orn. Schnellmitt. Bad.-Württ. 1994, 42, 58–59. [Google Scholar]
- Schüpbach, T. Kornweihen (Circus cyaneus) als Beute von Füchsen (Vulpes vulpes)? Orn. Beob. 1996, 93, 181–184. [Google Scholar]
- Morris, A.J.; Gilroy, J.J. Close to the edge: Predation risks for two declining farmland passerines. Ibis 2008, 150, 168–177. [Google Scholar] [CrossRef]
- Gatter, W. Vogelzug und Vogelbestände in Mitteleuropa: 30 Jahre Beobachtung des Tagzugs am Randecker Maar; Aula: Wiebelsheim, Germany, 2010. [Google Scholar]
- Schmidt, M.; Hohenegger, J.A. Status, breeding ecology and conservation of the imperial eagle in Austria. Raptor Conserv. 2023, 2, 278–280. [Google Scholar] [CrossRef]
- Probst, R.; Schmidt, M.; McGrady, M.; Pichler, C. GPS tracking reveals the white-tailed eagle Haliaeetus albicilla as an ambassador for the Natura 2000 network. Diversity 2024, 16, 145. [Google Scholar] [CrossRef]
- Puzović, S. Nest occupation and prey grabbing by saker falcon (Falco cherrug) on power lines in the province of Vojvodina (Serbia). Arch. Biol. Sci. 2008, 60, 271–277. [Google Scholar] [CrossRef]
- Zuberogoitia, I.; Arroyo, B.; O’Donoghue, B.; Zabala, J.; Martínez, J.A.; Martínez, J.E.; Murphy, S.G. Standing out from the crowd: Are patagial wing tags a potential predator attraction for harriers (Circus spp.)? J. Ornithol. 2012, 153, 985–989. [Google Scholar] [CrossRef]
- Randler, C. Turmfalke (Falco tinnunculus) attackiert Kornweihe (Circus cyaneus). Ornithol. Mitteilungen 1993, 45, 90. [Google Scholar]
- Säurich, A.; Möller, M.; Gerighausen, H. A novel remote sensing-based approach to determine loss of agricultural soils due to soil sealing—A case study in Germany. Environ. Monit. Assess. 2024, 196, 510. [Google Scholar] [CrossRef]
- Bridges, E.M.; Oldeman, L.R. Global assessment of human-induced soil degradation. Arid. Soil Res. Rehabil. 1999, 13, 319–325. [Google Scholar] [CrossRef]
- Forman, R.T.T.; Alexander, L.E. Roads and their major ecological effects. Annu. Rev. Ecol. Syst. 1998, 29, 207–231. [Google Scholar] [CrossRef]
- Monz, C.A.; Pickering, C.M.; Hadwen, W.L. Recent advances in recreation ecology and the implications of different relationships between recreation use and ecological impacts. Front. Ecol. Environ. 2013, 11, 441–446. [Google Scholar] [CrossRef]
- Biasotto, L.D.; Kindel, A. Power lines and impacts on biodiversity: A systematic review. Environ. Impact Assess. Rev. 2018, 71, 110–119. [Google Scholar] [CrossRef]
- D’Amico, M.; Catry, I.; Martins, R.C.; Ascensão, F.; Barrientos, R.; Moreira, F. Bird on the wire: Landscape planning considering costs and benefits for bird populations coexisting with power lines. Ambio 2018, 47, 650–656. [Google Scholar] [CrossRef]
- Spadetto, L.; Gómez-Ramírez, P.; Zamora-Marín, J.M.; León-Ortega, M.; Díaz-García, S.; Tecles, F.; Fenoll, J.; Cava, J.; Calvo, J.F.; García-Fernández, A.J. Active monitoring of long-eared owl (Asio otus) nestlings reveals widespread exposure to anticoagulant rodenticides across different agricultural landscapes. Sci. Total Environ. 2024, 918, 170492. [Google Scholar] [CrossRef]
- Roos, S.; Campbell, S.T.; Hartley, G.; Shore, R.F.; Walker, L.A.; Wilson, J.D. Annual abundance of common kestrels (Falco tinnunculus) is negatively associated with second generation anticoagulant rodenticides. Ecotoxicology 2021, 30, 560–574. [Google Scholar] [CrossRef]
- Ozaki, S.; Movalli, P.; Cincinelli, A.; Alygizakis, N.; Badry, A.; Carter, H.; Chaplow, J.S.; Claßen, D.; Dekker, R.W.R.J.; Dodd, B.; et al. Significant turning point: Common buzzard (Buteo buteo) exposure to second-generation anticoagulant rodenticides in the United Kingdom. Environ. Sci. Technol. 2024, 58, 6093–6104. [Google Scholar] [CrossRef]
- Hauzenberger, I.; Lenz, K.; Loishandl-Weisz, H.; Steinbichl, P.; Offenthaler, I. Erste österreichische Fallstudie zu Rodentiziden Wirkstoffen in der Umwelt; Report REP-0733; Umweltbundesamt: Wien, Austria, 2020. [Google Scholar]
- Natsukawa, H.; Tavecchia, G.; Frías, Ó.; Sergio, F.; Hiraldo, F.; Blanco, G. Immigration hides the decline caused by an anthropogenic trap and drives the spectacular increase of a mobile predator. Oecologia 2025, 207, 15. [Google Scholar] [CrossRef]
- Roilo, S.; Hofmeester, T.R.; Frauendorf, M.; Widén, A.; Cord, A.F. The untapped potential of camera traps for farmland biodiversity monitoring: Current practice and outstanding agroecological questions. Remote Sens. Ecol. Conserv. 2024, 10, 142–157. [Google Scholar] [CrossRef]
- Marra, P.P.; Cohen, E.B.; Loss, S.R.; Rutter, J.E.; Tonra, C.M. A call for full annual cycle research in animal ecology. Biol. Lett. 2015, 11, 20150552. [Google Scholar] [CrossRef]
- Canney, A.C.; McGough, L.M.; Bickford, N.A.; Wallen, K.E. Systematic map of human–raptor interaction and coexistence research. Animals 2021, 12, 45. [Google Scholar] [CrossRef]
- Bühler, R.; Schalcher, K.; Séchaud, R.; Michler, S.; Apolloni, N.; Roulin, A.; Almasi, B. Influence of prey availability on habitat selection during the non-breeding period in a resident bird of prey. Mov. Ecol. 2023, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J. Response of small rodents to manipulations of vegetation height in agro-ecosystems. Integr. Zool. 2008, 3, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Butet, A.; Rantier, Y.; Bergerot, B. Land use changes and raptor population trends: A twelve-year monitoring of two common species in agricultural landscapes of western France. Glob. Ecol. Conserv. 2022, 34, e02027. [Google Scholar] [CrossRef]
- Edwards, P.D.; Palme, R.; Boonstra, R. Is chronic stress a causal mechanism for small mammal population cycles? Reconciling the evidence. Oecologia 2023, 201, 609–623. [Google Scholar] [CrossRef]
- Tkadlec, E.; Zejda, J. Small rodent population fluctuations: The effects of age structure and seasonality. Evol. Ecol. 1998, 12, 191–210. [Google Scholar] [CrossRef]
- Pinot, A.; Barraquand, F.; Tedesco, E.; Lecoustre, V.; Bretagnolle, V.; Gauffre, B. Density-dependent reproduction causes winter crashes in a common vole population. Popul. Ecol. 2016, 58, 395–405. [Google Scholar] [CrossRef]
- Bieringer, G.; Laber, J. Erste Ergebnisse von Greifvogel-Winterzählungen im pannonischen Raum Niederösterreichs. Egretta 1999, 42, 30–39. [Google Scholar]
- Probst, R. Greifvogelüberwinterung 1998 bis 2002 im Bleistätter Moos, Kärnten. Carinthia II 2004, 194./114., 509–516. [Google Scholar]
- Dvorak, M.; Wendelin, B. Greifvogel-Bestände auf der Parndorfer Platte und im Heideboden (Nordburgenland) in den Wintern 2001/2002–2006/2007. Vogel Kdl. Nacht. Ostösterr. 2008, 19, 1–7. [Google Scholar]
- Harrison, X.A.; Blount, J.D.; Inger, R.; Norris, D.R.; Bearhop, S. Carry-over effects as drivers of fitness differences in animals: Carry-over effects in animal populations. J. Anim. Ecol. 2011, 80, 4–18. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Norris, D.R.; Crossin, G.T.; Cooke, S.J. Biological carryover effects: Linking common concepts and mechanisms in ecology and evolution. Ecosphere 2014, 5, art28. [Google Scholar] [CrossRef]
- Mueller, T.; Fagan, W.F. Search and navigation in dynamic environments—From individual behaviors to population distributions. Oikos 2008, 117, 654–664. [Google Scholar] [CrossRef]
- Amar, A.; Picozzi, N.; Meek, E.R.; Redpath, S.M.; Lambin, X. Decline of the Orkney hen harrier Circus cyaneus population: Do changes to demographic parameters and mating system fit a declining food hypothesis? Bird Study 2005, 52, 18–24. [Google Scholar] [CrossRef]
- Morollón, S.; Lee, S.; Urios, V. Movements of juvenile hen harriers (Circus cyaneus) tracked by satellite telemetry in Spain. Birds 2024, 5, 832–844. [Google Scholar] [CrossRef]
- Cornulier, T.; Yoccoz, N.G.; Bretagnolle, V.; Brommer, J.E.; Butet, A.; Ecke, F.; Elston, D.A.; Framstad, E.; Henttonen, H.; Hörnfeldt, B.; et al. Europe-wide dampening of population cycles in keystone herbivores. Science 2013, 340, 63–66. [Google Scholar] [CrossRef]
- Ewing, S.R.; Thomas, C.E.; Butcher, N.; Denman, B.; Douglas, D.J.T.; Anderson, D.I.K.; Anderson, G.Q.A.; Bray, J.; Downing, S.; Dugan, R.; et al. Illegal killing associated with gamebird management accounts for up to three-quarters of annual mortality in hen harriers (Circus cyaneus). Biol. Conserv. 2023, 283, 110072. [Google Scholar] [CrossRef]
- Murgatroyd, M.; Redpath, S.M.; Murphy, S.G.; Douglas, D.J.T.; Saunders, R.; Amar, A. Patterns of satellite tagged hen harrier disappearances suggest widespread illegal killing on British grouse moors. Nat. Commun. 2019, 10, 1094. [Google Scholar] [CrossRef]
- O’Donoghue, B.; O’Donoghue, T.A.; King, F. Opinion article: The hen harrier in Ireland: Conservation issues for the 21st century. Biol. Environ. Proc. R. Ir. Acad. 2011, 111, 83–93. [Google Scholar] [CrossRef]
- Arroyo, B.; Leckie, F.; Amar, A.; McCluskie, A.; Redpath, S. Ranging behaviour of hen harriers breeding in Special Protection Areas in Scotland. Bird Study 2014, 61, 48–55. [Google Scholar] [CrossRef]
- Maeso, S.; Morollón, S.; García-Macía, J.; Lee, S.; Urios, V. Habitat use of the hen harrier (Circus cyaneus) during the breeding season in Spain. Birds 2024, 5, 558–570. [Google Scholar] [CrossRef]
- Morollón, S.; García-Macía, J.; Onrubia, A.; Lee, S.; Urios, V. Migration patterns of breeding hen harriers (Circus cyaneus) in Spain. Bird Study 2024, 71, 40–47. [Google Scholar] [CrossRef]
- Bontzorlos, V.; Cain, S.; Leshem, Y.; Spiegel, O.; Motro, Y.; Bloch, I.; Cherkaoui, S.I.; Aviel, S.; Apostolidou, M.; Christou, A.; et al. Barn owls as a nature-based solution for pest control: A multinational initiative around the Mediterranean and other regions. Conservation 2024, 4, 627–656. [Google Scholar] [CrossRef]
- Hochleitner, L.; Korpimäki, E.; Chakarov, N.; Isaksson, C.; Nebel, C.; Renner, S.C.; Vasko, V.; Voigt, C.C.; Terraube, J.; Sumasgutner, P. Diet diversity, individual heterozygosity and habitat heterogeneity influence health parameters in Eurasian kestrels (Falco tinnunculus). Ibis 2025, 167, 145–160. [Google Scholar] [CrossRef]
- Roberge, J.; Angelstam, P. Usefulness of the umbrella species concept as a conservation tool. Conserv. Biol. 2004, 18, 76–85. [Google Scholar] [CrossRef]
- Natsukawa, H.; Sergio, F. Top predators as biodiversity indicators: A meta-analysis. Ecol. Lett. 2022, 25, 2062–2075. [Google Scholar] [CrossRef]
- Dvorak, M.; Landmann, A.; Teufelbauer, N.; Wichmann, G.; Berg, H.-M.; Probst, R. The conservation status of the breeding birds of Austria: Red List (5th version) and Birds of Conservation Concern (1st version). Egretta 2017, 55, 6–42. [Google Scholar]
- BirdLife International. European Red List of Birds; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]

| Behavior | Multiplier | Female | Male |
|---|---|---|---|
| Sitting Daytime Sitting Nighttime | 1.7 | 3.939 | 3.009 |
| 1.7 | 3.152 | 2.417 | |
| Soaring | 7 | 16.219 | 12.390 |
| Quartering | 8 | 18.536 | 14.160 |
| Flying | 11.5 | 26.646 | 20.355 |
| High-Speed Flying | 12.5 | 28.963 | 22.125 |
| Sitting | Soaring | Quartering | Flying | High-Speed Flying | Total (Min) | |
| Males | 2871.5 (62.5%) | 42.0 (0.9%) | 1157.0 (25.2%) | 231.5 (5.0%) | 289.0 (6.3%) | 4591.0 |
| Non-Territorial Females | 2696.5 (57.5%) | 43.0 (0.9%) | 1469.0 (31.3%) | 230.0 (4.9%) | 249.5 (5.3%) | 4688.0 |
| Territorial Females | 3016.5 (74.5%) | 7.0 (0.2%) | 808.0 (20.0%) | 87.5 (2.2%) | 131.0 (3.2%) | 4050.0 |
| Sitting | Soaring | Quartering | Flying | High-Speed Flying | Subtotal | Night | Total (kcal) | |
| Males | 19.673 | 0.709 | 36.899 | 7.396 | 9.634 | 74.312 | 33.838 | 108.150 |
| Non-Territorial Females | 22.657 | 1.488 | 58.083 | 13.073 | 15.414 | 110.715 | 44.128 | 154.843 |
| Territorial Females | 29.338 | 0.280 | 36.980 | 5.757 | 9.368 | 81.724 | 44.128 | 125.852 |
| Requirement (kcal) | Voles (22.1 kcal) | Voles (32.5 kcal) | Requirement * (kcal) | Voles * (22.1 kcal) | Voles * (32.5 kcal) | |
| Males | 108.150 | 4.9 | 3.3 | 117.448 | 5.3 | 3.6 |
| Non-Territorial Females | 154.843 | 7.0 | 4.8 | 169.282 | 7.7 | 5.2 |
| Territorial Females | 125.852 | 5.7 | 3.9 | 135.212 | 6.1 | 4.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Probst, R.; Probst, R. Winter Ecology of the Hen Harrier, Circus cyaneus: Bridging Behavioral Insights and Conservation Requirements. Animals 2025, 15, 1057. https://doi.org/10.3390/ani15071057
Probst R, Probst R. Winter Ecology of the Hen Harrier, Circus cyaneus: Bridging Behavioral Insights and Conservation Requirements. Animals. 2025; 15(7):1057. https://doi.org/10.3390/ani15071057
Chicago/Turabian StyleProbst, Remo, and Renate Probst. 2025. "Winter Ecology of the Hen Harrier, Circus cyaneus: Bridging Behavioral Insights and Conservation Requirements" Animals 15, no. 7: 1057. https://doi.org/10.3390/ani15071057
APA StyleProbst, R., & Probst, R. (2025). Winter Ecology of the Hen Harrier, Circus cyaneus: Bridging Behavioral Insights and Conservation Requirements. Animals, 15(7), 1057. https://doi.org/10.3390/ani15071057






