The Behavioral Responses of Koi Carp (Cyprinus carpio) to Different Temperatures: Which Is Better, Infrared or Quadrupole Technology?
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
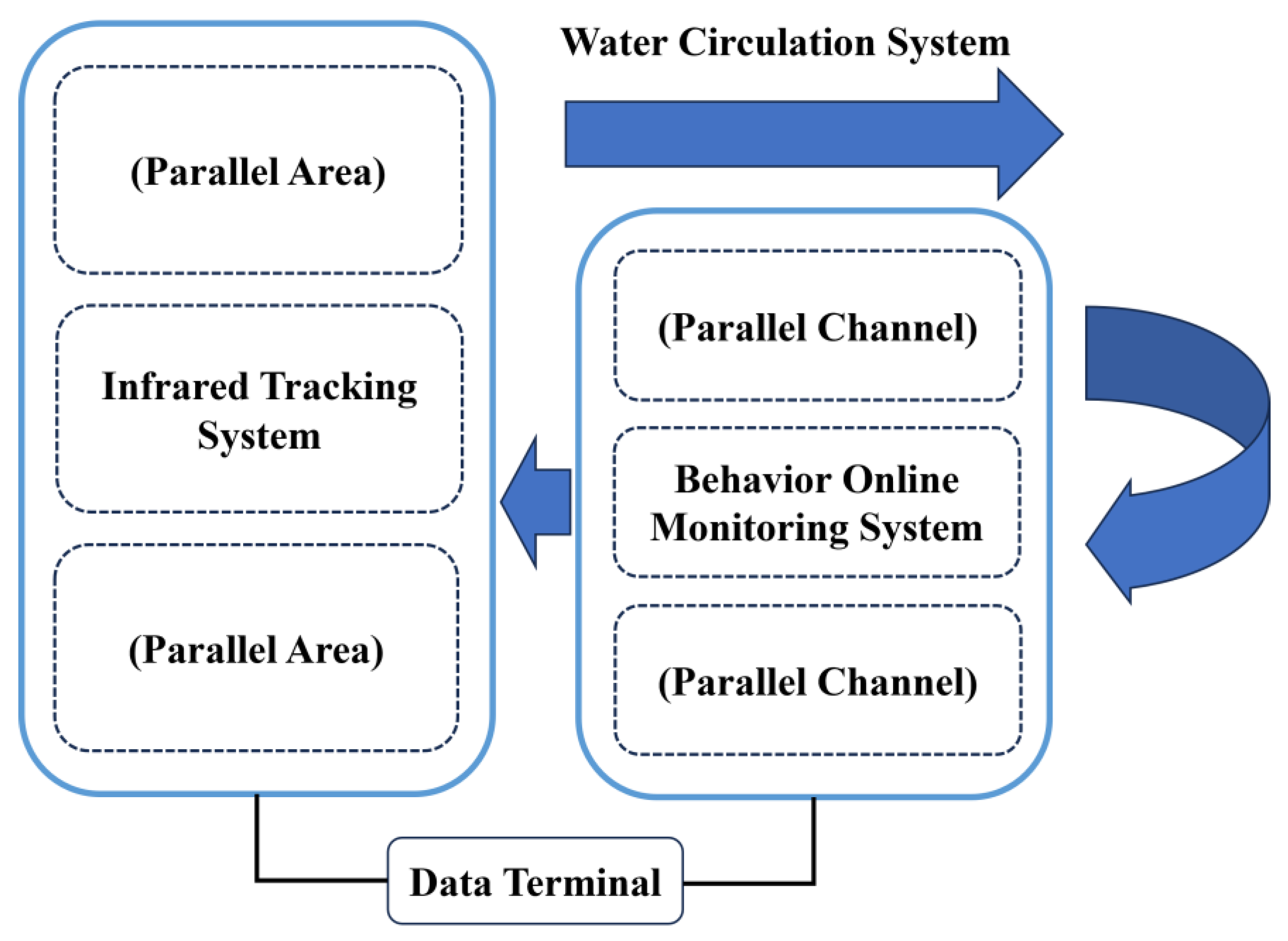
2.1. Experimental Apparatus
2.1.1. Infrared Tracking System
2.1.2. Behavior Online Monitoring System
2.1.3. Water Circulation System
2.1.4. Experimental Subject: Koi Carp
2.2. Experimental Design
2.3. Analytical Methods
3. Results
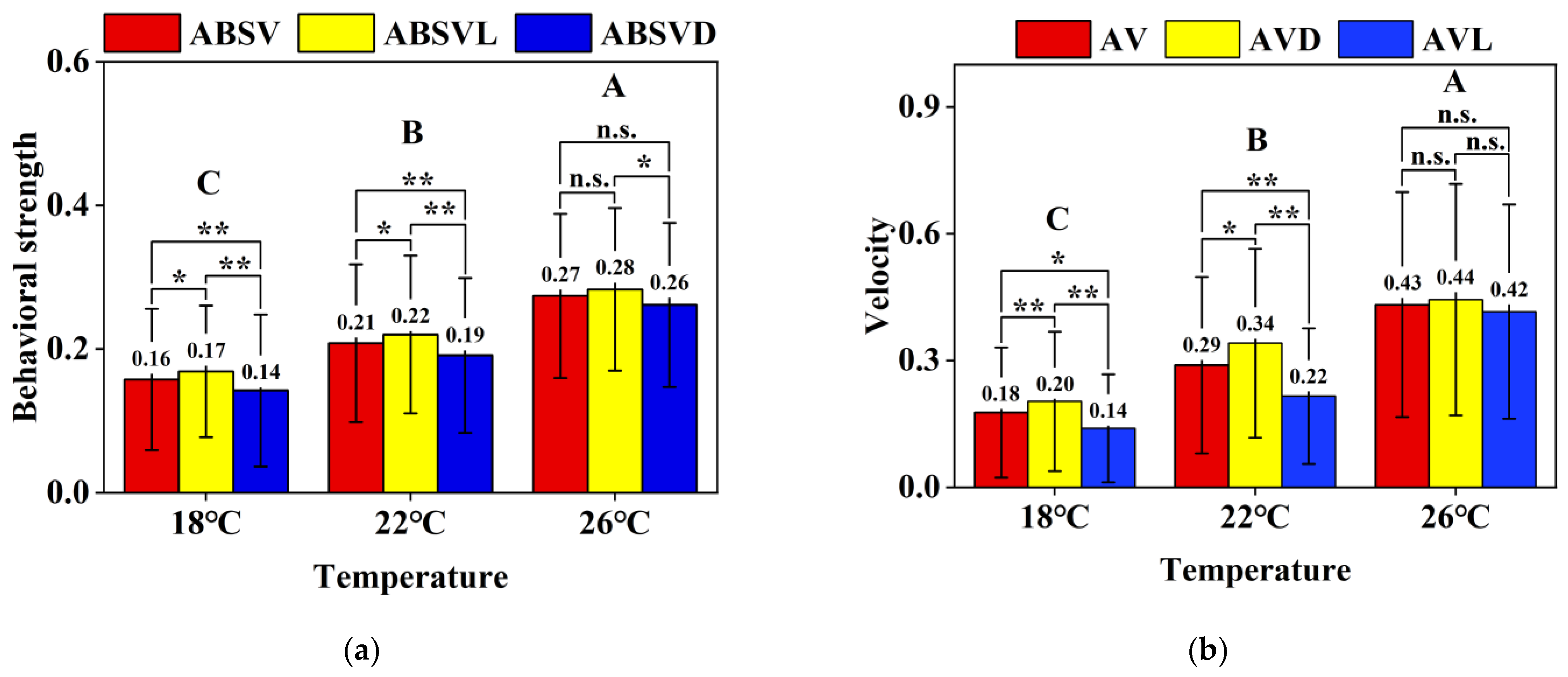
3.1. Behavioral Responses of Koi Carp
- (a)
- A significant main effect of temperature was observed (p < 0.001), with behavioral strength increasing progressively across temperature gradients: 0.16 ± 0.10 (18 °C) < 0.21 ± 0.11 (22 °C) < 0.27 ± 0.11 (26 °C). Photoperiod exerted a significant main effect at 18 °C (p < 0.001) and 22 °C (p < 0.001), following the hierarchy ABSVL > ABSV > ABSVD (21.4% and 15.8% differences, respectively). However, this photoperiod effect diminished at 26 °C (7.7% difference, p < 0.05), indicating a significant temperature × photoperiod interaction (p < 0.05).
- (b)
- Similar thermal dependence was evident in swimming velocity (p < 0.001): 0.18 ± 0.15 cm/s (18 °C) < 0.29 ± 0.21 cm/s (22 °C) < 0.43 ± 0.27 cm/s (26 °C). Photoperiod significantly influenced velocities at 18 °C (42.9% difference, p < 0.001) and 22 °C (54.5%, p < 0.001), adhering to the AVL > AV > AVD hierarchy. Notably, the temperature × photoperiod interaction was significant (p < 0.05), as photoperiod effects vanished at 26 °C (4.8% difference, p > 0.05), with no diurnal activity variation observed.
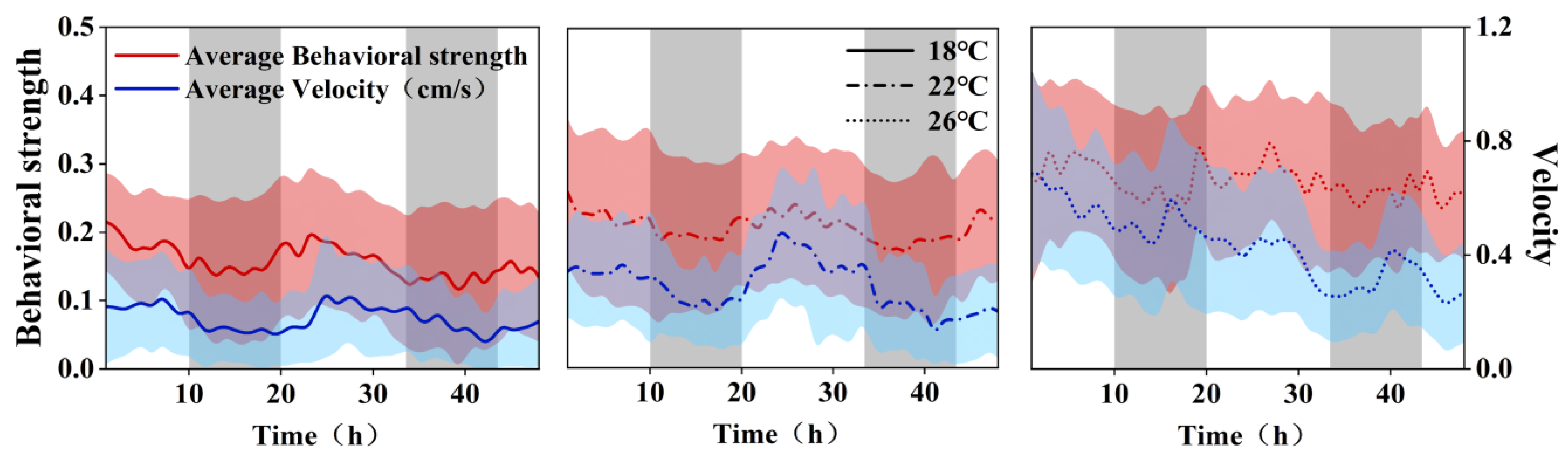
3.2. Autocorrelation Analysis
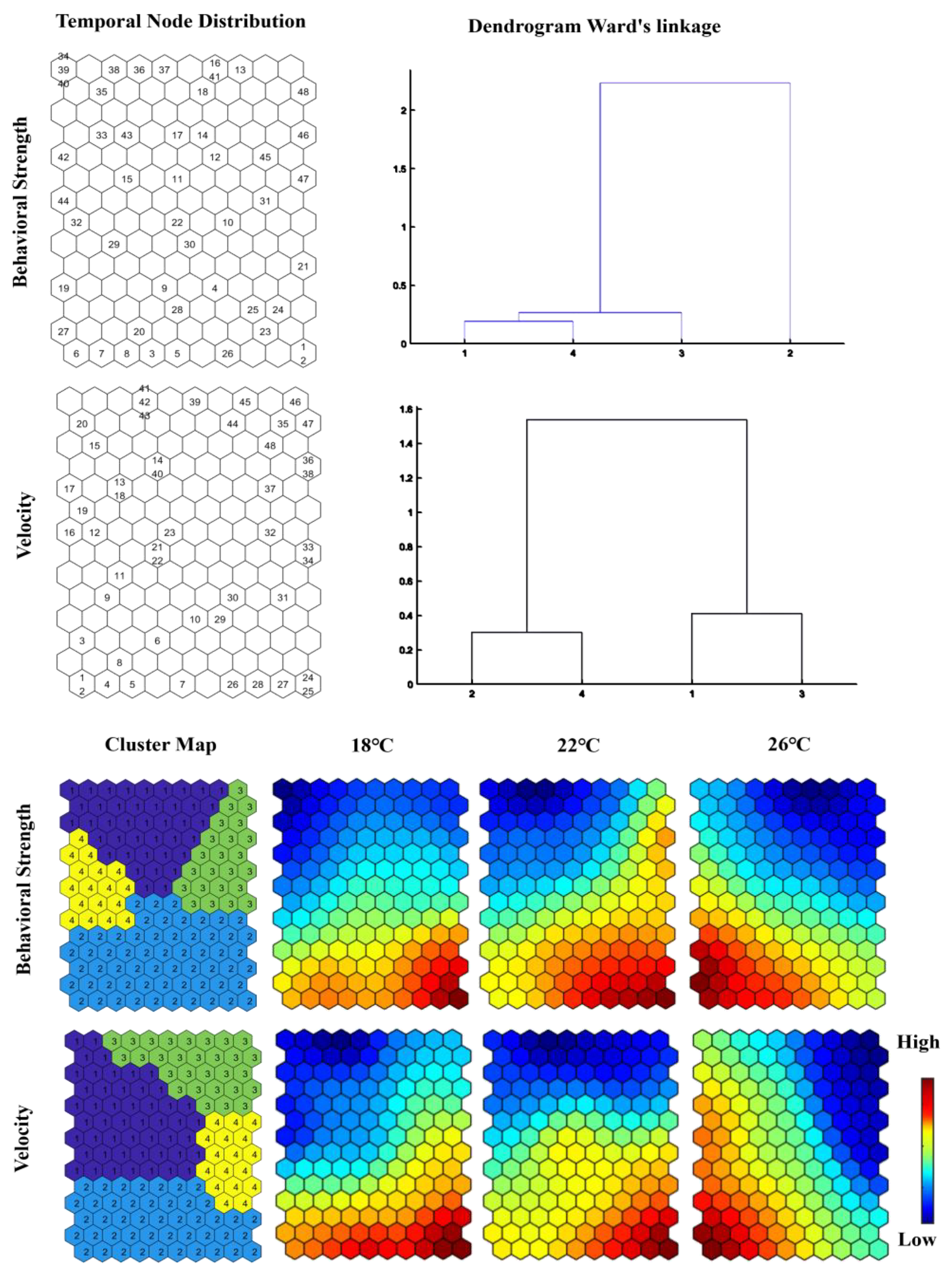
3.3. SOM Analysis
3.4. Comparative Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABSV | Average Behavioral Strength Values |
| ABSVL | Average Behavioral Strength Values in Light Periods |
| ABSVD | Average Behavioral Strength Values in Dark Periods |
| AV | Average Velocity |
| AVL | Average Velocity in Light Periods |
| AVD | Average Velocity in Dark Periods |
| D (ABSVL-ABSVD) | Differences between ABSVL and ABSVD |
| D (AVL-AVD) | Differences between AVL and AVD |
References
- Yuan, F.; Lu, L.; Zhang, Y.; Wang, S.; Cai, Y. Data mining of the cancer-related lncRNAs GO terms and KEGG pathways by using mRMR method. Math. Biosci. 2018, 304, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Feng, M.; Cheng, Z.; Zhao, M.; Mao, J.; Mirowski, L. Water quality monitoring using abnormal tail-beat frequency of crucian carp. Ecotoxicol. Environ. Saf. 2015, 111, 185–191. [Google Scholar] [CrossRef] [PubMed]
- de Campos Júnior, E.O.; de Campos, J.M.S.; Dias, R.J.P.; Barros, N.O. Novelties on tradescantia: Perspectives on water quality monitoring. Chemosphere 2024, 368, 122564. [Google Scholar] [CrossRef]
- Bownik, A.; Wlodkowic, D. Advances in real-time monitoring of water quality using automated analysis of animal behaviour. Sci. Total Environ. 2021, 789, 147796. [Google Scholar] [CrossRef]
- Wei, D.; Wang, L.; Poopal, R.-K.; Ren, Z. IR-based device to acquire real-time online heart ECG signals of fish (Cyprinus carpio) to evaluate the water quality. Environ. Pollut. 2023, 337, 112564. [Google Scholar] [CrossRef]
- Hassan, S.H.A.; Gurung, A.; Kang, W.-c.; Shin, B.-S.; Rahimnejad, M.; Jeon, B.-H.; Kim, J.R.; Oh, S.-E. Real-time monitoring of water quality of stream water using sulfur-oxidizing bacteria as bio-indicator. Chemosphere 2019, 223, 58–63. [Google Scholar] [CrossRef]
- Storey, M.V.; van der Gaag, B.; Burns, B.P. Advances in on-line drinking water quality monitoring and early warning systems. Water Res. 2011, 45, 741–747. [Google Scholar] [CrossRef]
- Sun, L.; Wang, B.; Yang, P.; Wang, X.; Li, D.; Wang, J. Water quality parameter analysis model based on fish behavior. Comput. Electron. Agric. 2022, 203, 107500. [Google Scholar] [CrossRef]
- Cui, Z.; Pimentel, M.S.; Faleiro, F.; Diniz, M.; Machado, J.; Pousão-Ferreira, P.; Peck, M.A.; Pörtner, H.O.; Rosa, R. Oxidative Stress and Digestive Enzyme Activity of Flatfish Larvae in a Changing Ocean. PLoS ONE 2015, 10, e0134082. [Google Scholar] [CrossRef]
- Sampaio, E.; Lopes, A.R.; Francisco, S.; Paula, J.R.; Pimentel, M.; Maulvault, A.L.; Repolho, T.; Grilo, T.F.; Pousão Ferreira, P.; Marques, A.; et al. Ocean acidification dampens physiological stress response to warming and contamination in a commercially-important fish (Argyrosomus regius). Sci. Total Environ. 2018, 618, 388–398. [Google Scholar] [CrossRef]
- Lesser, M.P. Oxidative stress in marine environments: Biochemistry and physiological ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Vaccarella, E.; Massimi, L.; Canepari, S. Assessment of oxidative stress induced by atmospheric particulate matter: From acellular and cellular assays to the use of model and experimental organisms. Sci. Total Environ. 2025, 965, 178651. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Dominguez, A.; Connell, S.D.; Leung, J.Y.S.; Nagelkerken, I. Adaptive responses of fishes to climate change: Feedback between physiology and behaviour. Sci. Total Environ. 2019, 692, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lee, S.-H.; Chon, T.-S. Analysis of behavioral changes of zebrafish (Danio rerio) in response to formaldehyde using Self-organizing map and a hidden Markov model. Ecol. Model. 2011, 222, 2191–2201. [Google Scholar] [CrossRef]
- Brunelle, L.D.; Huang, I.J.; Angeles, L.F.; Running, L.S.; Sirotkin, H.I.; McElroy, A.E.; Aga, D.S. Comprehensive assessment of chemical residues in surface and wastewater using passive sampling, chemical, biological, and fish behavioral assays. Sci. Total Environ. 2022, 828, 154176. [Google Scholar] [CrossRef]
- Green, A.J.; Planchart, A. The neurological toxicity of heavy metals: A fish perspective. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 208, 12–19. [Google Scholar] [CrossRef]
- Freitas, L.M.; Valadares, L.P.d.A.; Camozzi, M.G.M.; de Oliveira, P.G.; Ferreira Machado, M.R.; Lima, F.C. Animal models in the neurotoxicology of 2,4-D. Hum. Exp. Toxicol. 2019, 38, 1178–1182. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, J.; Zhang, X.; Li, H.; Liu, Y.; Gao, L. Effects of temperature and photoperiod on growth, physiological, and behavioral performance in steelhead trout (Oncorhynchus mykiss) under indoor aquaculture condition. Front. Mar. Sci. 2023, 10, 1114662. [Google Scholar] [CrossRef]
- Gerhardt, A.; Schmidt, S.; Höss, S. Measurement of movement patterns of Caenorhabditis elegans (Nematoda) with the Multispecies Freshwater Biomonitor (MFB)—A potential new method to study a behavioral toxicity parameter of nematodes in sediments. Environ. Pollut. 2002, 120, 513–516. [Google Scholar] [CrossRef]
- Borcherding, J. Ten Years of Practical Experience with the Dreissena-Monitor, a Biological Early Warning System for Continuous Water Quality Monitoring. Hydrobiologia 2006, 556, 417–426. [Google Scholar] [CrossRef]
- Xia, C.; Chon, T.-S.; Liu, Y.; Chi, J.; Lee, J. Posture tracking of multiple individual fish for behavioral monitoring with visual sensors. Ecol. Inform. 2016, 36, 190–198. [Google Scholar] [CrossRef]
- Zarfl, C.; Lumsdon, A.E.; Berlekamp, J.; Tydecks, L.; Tockner, K. A global boom in hydropower dam construction. Aquat. Sci. 2014, 77, 161–170. [Google Scholar] [CrossRef]
- Lai, X.; Jiang, J.; Yang, G.; Lu, X.X. Should the Three Gorges Dam be blamed for the extremely low water levels in the middle–lower Yangtze River? Hydrol. Process. 2013, 28, 150–160. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, G.; Liang, J.; Zhang, J.; Cai, Q.; Huang, L.; Li, X.; Zhu, H.; Hu, C.; Shen, S. Changes of soil microbial biomass and bacterial community structure in Dongting Lake: Impacts of 50,000 dams of Yangtze River. Ecol. Eng. 2013, 57, 72–78. [Google Scholar] [CrossRef]
- Chen, M.; Qin, X.; Zeng, G.; Li, J. Impacts of human activity modes and climate on heavy metal “spread” in groundwater are biased. Chemosphere 2016, 152, 439–445. [Google Scholar] [CrossRef]
- Suzuki, F.M.; Pompeu, P.S. Influence of abiotic factors on ichthyoplankton occurrence in stretches with and without dams in the upper Grande River basin, south-eastern Brazil. Fish. Manag. Ecol. 2016, 23, 99–108. [Google Scholar] [CrossRef]
- Silva, A.T.; Lucas, M.C.; Castro-Santos, T.; Katopodis, C.; Baumgartner, L.J.; Thiem, J.D.; Aarestrup, K.; Pompeu, P.S.; O’Brien, G.C.; Braun, D.C.; et al. The future of fish passage science, engineering, and practice. Fish Fish. 2017, 19, 340–362. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2018, 94, 849–873. [Google Scholar] [CrossRef]
- Gao, X.; Li, M.Z.; Lin, P.C.; Duan, Z.H.; Liu, H.Z. Environmental cues for natural reproduction of the Chinese sturgeon, Acipenser sinensis Gray, 1835, in the Yangtze River, China. J. Appl. Ichthyol. 2013, 29, 1389–1394. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Castello, L.; Murphy, B.R.; Xie, S. Potential effects of dam cascade on fish: Lessons from the Yangtze River. Rev. Fish Biol. Fish. 2015, 25, 569–585. [Google Scholar] [CrossRef]
- Baumgartner, L.J.; Boys, C.; Marsden, T.; McPherson, J.; Ning, N.; Phonekhampheng, O.; Robinson, W.; Singhanouvong, D.; Stuart, I.G.; Thorncraft, G. A Cone Fishway Facilitates Lateral Migrations of Tropical River-Floodplain Fish Communities. Water 2020, 12, 513. [Google Scholar] [CrossRef]
- Tan, J.; Gao, Z.; Dai, H.; Yang, Z.; Shi, X. Effects of turbulence and velocity on the movement behaviour of bighead carp (Hypophthalmichthys nobilis) in an experimental vertical slot fishway. Ecol. Eng. 2019, 127, 363–374. [Google Scholar] [CrossRef]
- Conallin, J.; Tun, N.N.; Swe, A.M.; Baumgartner, L.J.; Lunn, Z.; Mallen-Cooper, M.; Marsden, T.; Ning, N.; Robinson, W.; Senevirathna, L.; et al. Using fish swimming ability to refine criteria for fishway construction in Myanmar. Fish. Res. 2023, 262. [Google Scholar] [CrossRef]
- Ziqi, G.; Guangning, L.; Zhihong, Q.; Shuangke, S.; Haitao, L.; Tiegang, Z.; Cen, W. Study on effect of water temperature on migration of schizothorax prenanti in vertical slot fishway. J. China Inst. Water Resour. Hydropower Res. 2021, 19, 255–261. [Google Scholar] [CrossRef]
- Zutshi, B.; Singh, A.; Dasgupta, P. Impact of transient temperature disturbance on the oxidative stress indices and glucose levels of juvenile Koi carps (Cyprinus carpio var koi). J. Basic Appl. Zool. 2020, 81, 1–8. [Google Scholar] [CrossRef]
- Freitas, R.; De Marchi, L.; Bastos, M.; Moreira, A.; Velez, C.; Chiesa, S.; Wrona, F.J.; Figueira, E.; Soares, A.M.V.M. Effects of seawater acidification and salinity alterations on metabolic, osmoregulation and oxidative stress markers in Mytilus galloprovincialis. Ecol. Indic. 2017, 79, 54–62. [Google Scholar] [CrossRef]
- Pereira, E.; Cardoso, G.R.; Quintella, B.R.; Mateus, C.S.; Alexandre, C.M.; Oliveira, R.L.; Belo, A.F.; Telhado, A.; Quadrado, M.F.; Batista, C.M.; et al. Proposals for optimizing sea lamprey passage through a vertical-slot fishway. Ecohydrology 2019, 12, e2087. [Google Scholar] [CrossRef]
- Campos, D.F.; Val, A.L.; Almeida-Val, V.M.F. The influence of lifestyle and swimming behavior on metabolic rate and thermal tolerance of twelve Amazon forest stream fish species. J. Therm. Biol. 2018, 72, 148–154. [Google Scholar] [CrossRef]
- Madeira, D.; Vinagre, C.; Diniz, M.S. Are fish in hot water? Effects of warming on oxidative stress metabolism in the commercial species Sparus aurata. Ecol. Indic. 2016, 63, 324–331. [Google Scholar] [CrossRef]
- Allan, B.J.M.; Domenici, P.; Munday, P.L.; McCormick, M.I. Feeling the heat: The effect of acute temperature changes on predator–prey interactions in coral reef fish. Conserv. Physiol. 2015, 3, cov011. [Google Scholar] [CrossRef]
- Dong, Y.; Dong, S.; Ji, T. Effect of different thermal regimes on growth and physiological performance of the sea cucumber Apostichopus japonicus Selenka. Aquaculture 2008, 275, 329–334. [Google Scholar] [CrossRef]
- Reidy, S.P.; Kerr, S.R.; Nelson, J.A. Aerobic and anaerobic swimming performance of individual Atlantic cod. J. Exp. Biol. 2000, 203, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Mathes, M.T.; Hinch, S.G.; Cooke, S.J.; Crossin, G.T.; Patterson, D.A.; Lotto, A.G.; Farrell, A.P. Effect of water temperature, timing, physiological condition, and lake thermal refugia on migrating adult Weaver Creek sockeye salmon (Oncorhynchus nerka). Can. J. Fish. Aquat. Sci. 2010, 67, 70–84. [Google Scholar] [CrossRef]
- McCann, E.L.; Johnson, N.S.; Pangle, K.L. Corresponding long-term shifts in stream temperature and invasive fish migration. Can. J. Fish. Aquat. Sci. 2018, 75, 772–778. [Google Scholar] [CrossRef]
- Binder, T.R.; McDonald, D.G. The role of temperature in controlling diel activity in upstream migrant sea lampreys (Petromyzon marinus). Can. J. Fish. Aquat. Sci. 2008, 65, 1113–1121. [Google Scholar] [CrossRef]
- Vatine, G.; Vallone, D.; Gothilf, Y.; Foulkes, N.S. It’s time to swim! Zebrafish and the circadian clock. FEBS Lett. 2011, 585, 1485–1494. [Google Scholar] [CrossRef]
- Rensing, L.; Ruoff, P. Temperature Effect on Entrainment, Phase Shifting, and Amplitude of Circadian Clocks and Its Molecular Bases. Chronobiol. Int. 2009, 19, 807–864. [Google Scholar] [CrossRef]
- Vera, L.M.; de Alba, G.; Santos, S.; Szewczyk, T.M.; Mackenzie, S.A.; Sánchez-Vázquez, F.J.; Rey Planellas, S. Circadian rhythm of preferred temperature in fish: Behavioural thermoregulation linked to daily photocycles in zebrafish and Nile tilapia. J. Therm. Biol. 2023, 113, 103544. [Google Scholar] [CrossRef]
- Panda, S.; Hogenesch, J.B.; Kay, S.A. Circadian rhythms from flies to human. Nature 2002, 417, 329–335. [Google Scholar] [CrossRef]
- Mistlberger, R.E. Food-anticipatory circadian rhythms: Concepts and methods. Eur. J. Neurosci. 2009, 30, 1718–1729. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Zhang, R.; Li, H.; Zhu, H. Targeted metabolomics revealed the seasonal plasticity of skin color and pigment metabolites in ornamental koi carp. Ecotoxicol. Environ. Saf. 2024, 281. [Google Scholar] [CrossRef]
- Chen, B.-J.; Fu, S.-J.; Cao, Z.-D.; Wang, Y.-X. Effect of temperature on critical oxygen tension (Pcrit) and gill morphology in six cyprinids in the Yangtze River, China. Aquaculture 2019, 508, 137–146. [Google Scholar] [CrossRef]
- Betsy, C.J.; Sangavi, S.; Ajith, J.; Saravanan, M.; Sampath Kumar, J.S. Influence of antioxidants on the growth performance, gonadosomatic index and biochemical properties of gonad and fertilization success in koi carp (Cyprinus carpio L.). Aquac. Res. 2021, 52, 5719–5729. [Google Scholar] [CrossRef]
- Weber, M.J.; Brown, M.L. Density-Dependence and Environmental Conditions Regulate Recruitment and First-Year Growth of Common Carp in Shallow Lakes. Trans. Am. Fish. Soc. 2013, 142, 471–482. [Google Scholar] [CrossRef]
- Shapiro Goldberg, D.; van Rijn, I.; Kiflawi, M.; Belmaker, J.; Heino, M. Decreases in length at maturation of Mediterranean fishes associated with higher sea temperatures. ICES J. Mar. Sci. 2019, 76, 946–959. [Google Scholar] [CrossRef]
- Wu, J.; Cuddington, K. Effect of Temperature on Black Carp (Mylopharyngodon piceus) Age at Sexual Maturity. Ecol. Freshw. Fish 2024, 34, e12823. [Google Scholar] [CrossRef]
- Ndong, D.; Chen, Y.-Y.; Lin, Y.-H.; Vaseeharan, B.; Chen, J.-C. The immune response of tilapia Oreochromis mossambicus and its susceptibility to Streptococcus iniae under stress in low and high temperatures. Fish Shellfish Immunol. 2007, 22, 686–694. [Google Scholar] [CrossRef]
- Sano, M.; Ito, T.; Matsuyama, T.; Nakayasu, C.; Kurita, J. Effect of water temperature shifting on mortality of Japanese flounder Paralichthys olivaceus experimentally infected with viral hemorrhagic septicemia virus. Aquaculture 2009, 286, 254–258. [Google Scholar] [CrossRef]
- Perelberg, A.; Ilouze, M.; Kotler, M.; Steinitz, M. Antibody response and resistance of Cyprinus carpio immunized with cyprinid herpes virus 3 (CyHV-3). Vaccine 2008, 26, 3750–3756. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Zhang, R.; Li, H.; Zhu, H. Correlation of skin color and plasma carotenoid-related metabolites of ornamental koi carp under temperature fluctuations. Ecotoxicol. Environ. Saf. 2024, 273, 116165. [Google Scholar] [CrossRef]
- Slominski, A.T.; Jiang, Y.; Zhang, S.; Xu, J.; Feng, J.; Mahboob, S.; Al-Ghanim, K.A.; Sun, X.; Xu, P. Comparative Transcriptome Analysis Reveals the Genetic Basis of Skin Color Variation in Common Carp. PLoS ONE 2014, 9, e108200. [Google Scholar] [CrossRef]
- Chen, X. Construction of Online Water Quality Monitoring Instrument Technology Based on Fish Physiological Ecology. Master’s Thesis, Shandong Normal University, Jinan, Chian, 2023. [Google Scholar]
- Lin, J.; Zhang, Z.; Wang, X.; Yin, J.; Wu, Y.; Zhang, X.; Zhang, D.; Jin, Q. A Novel Life Sign Interpretation System Based on Electrical Impedance Spectroscopy for Swimming Aquatic Animals. IEEE Trans. Instrum. Meas. 2024, 73, 1–13. [Google Scholar] [CrossRef]
- Liu, Q.; Ren, Z. Study on behavioral response of zebrafish (Danio rerio) under cadmium stress. Oceanol. Limnol. Sin. 2024, 55, 566–576. (In Chinese) [Google Scholar] [CrossRef]
- He, Y.; Ren, Z. Behavior response of zebrafish (Danio rerio) under ammonia nitrogen stress based on online biomonitoring system. Acta Hydrobiol. Sin. 2022, 46, 903–913. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, R.; Peng, X.; Jia, R.; Ren, Z. A review on the development and application of the online biomonitoring technologies for water pollution incidents. Asian J. Ecotoxicol. 2019, 14, 42–52. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, L.; Chen, J.; Ren, Z.; Wang, Z.; Chon, T.-S. Evidence for the Stepwise Behavioral Response Model (SBRM): The effects of Carbamate Pesticides on medaka (Oryzias latipes) in an online monitoring system. Chemosphere 2012, 87, 734–741. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, L.; Fu, R.; Miao, M. The Stepwise Behavioral Responses: Behavioral Adjustment of the Chinese Rare Minnow (Gobiocypris rarus) in the Exposure of Carbamate Pesticides. BioMed Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Han, F. Study on oxygen consumption rate and asphyxiation point of Cyprinus carpio haematopterus. Jiangsu Agric. Sci. 2019, 47, 174–176. (In Chinese) [Google Scholar] [CrossRef]
- Chen, S.; Fan, Z.; Chen, W. The relationship of respiratory rate and oxygen consumption rate in common carp (Cyprinus (C.) carpio haematopterus Temminck et Schlegel) under different temperature. J. Northeast Agric. Univ. 2006, 37, 352–356. [Google Scholar] [CrossRef]
- Stockman, J.; Weber, E.S.P.; Kass, P.H.; Pascoe, P.J.; Paul-Murphy, J. Physiologic and biochemical measurements and response to noxious stimulation at various concentrations of MS-222 in Koi (Cyprinus carpio). Vet. Anaesth. Analg. 2013, 40, 35–47. [Google Scholar] [CrossRef]
- Stehlik, L.L. Effects of seasonal change on activity rhythms and swimming behavior of age-0 bluefish (Pomatomus saltatrix) and a description of gliding behavior. Fish. Bull. 2009, 107, 1–12. [Google Scholar]
- Lokkeborg, S.; Fernö, A.; Jorgensen, T. Effect of position-fixing interval on estimated swimming speed and movement pattern of fish tracked with a stationary positioning system. Hydrobiologia 2002, 483, 259–264. [Google Scholar] [CrossRef]
- Kim, J.; Kato, S.; Takeuchi, K.; Tatsuma, T.; Kang, I.J. Evaluation on potential for assessing indoor formaldehyde using biosensor system based on swimming behavior of Japanese medaka (oryzias latipes). Build. Environ. 2011, 46, 849–854. [Google Scholar] [CrossRef]
- Das, S.K.; Noor, N.M.; Kai, K.S.; Juan, Q.Z.; Mohd Iskandar, N.S.; De, M. Effects of temperature on the growth, gastric emptying time, and oxygen consumption rate of mahseer (Tor tambroides) under laboratory conditions. Aquac. Rep. 2018, 12, 20–24. [Google Scholar] [CrossRef]
- Andrian, K.N.; Wihadmadyatami, H.; Wijayanti, N.; Karnati, S.; Haryanto, A. A comprehensive review of current practices, challenges, and future perspectives in Koi fish (Cyprinus carpio var. koi) cultivation. Vet. World 2024, 17, 1846–1854. [Google Scholar] [CrossRef]
- Yanuhar, U.; Musa, M.; Evanuarini, H.; Wuragil, D.K.; Permata, F.S. Water Quality in Koi Fish (Cyprinus carpio) Concrete Ponds with Filtration in Nglegok District, Blitar Regency. Univers. J. Agric. Res. 2022, 10, 814–820. [Google Scholar] [CrossRef]
- Staaks, G.; Kirschbaum, F.; Williot, P. Experimental studies on thermal behaviour and diurnal activity rhythms of juvenile European sturgeon (Acipenser sturio). J. Appl. Ichthyol. 1999, 15, 243–247. [Google Scholar] [CrossRef]
- Bartolini, T.; Butail, S.; Porfiri, M. Temperature influences sociality and activity of freshwater fish. Environ. Biol. Fishes 2014, 98, 825–832. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Zhang, L.; Wu, Z.; Zhu, S.; Li, J.; Li, X. Site Fidelity, Habitat Use, and Movement Patterns of the Common Carp during Its Breeding Season in the Pearl River as Determined by Acoustic Telemetry. Water 2020, 12, 2233. [Google Scholar] [CrossRef]
- Shiau, J.; Watson, J.R.; Cramp, R.L.; Gordos, M.A.; Franklin, C.E. Interactions between water depth, velocity and body size on fish swimming performance: Implications for culvert hydrodynamics. Ecol. Eng. 2020, 156, 105987. [Google Scholar] [CrossRef]
- Gerringer, M.E.; Drazen, J.C.; Yancey, P.H. Metabolic enzyme activities of abyssal and hadal fishes: Pressure effects and a re-evaluation of depth-related changes. Deep Sea Res. Part I Oceanogr. Res. Pap. 2017, 125, 135–146. [Google Scholar] [CrossRef]
- Ju, Z.; Dunham, R.; Liu, Z. Differential gene expression in the brain of channel catfish (Ictalurus punctatus) in response to cold acclimation. Mol. Genet. Genom. 2002, 268, 87–95. [Google Scholar] [CrossRef]
- Zhou, T.; Gui, L.; Liu, M.; Li, W.; Hu, P.; Duarte, D.F.C.; Niu, H.; Chen, L. Transcriptomic responses to low temperature stress in the Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2019, 84, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Penghan, L.Y.; Cao, Z.D.; Fu, S.J. Effect of temperature and dissolved oxygen on swimming performance in crucian carp. Aquat. Biol. 2014, 21, 57–65. [Google Scholar] [CrossRef]
- Baumann, P.; Stevanella, G. Fish passage principles to be considered for medium and large dams: The case study of a fish passage concept for a hydroelectric power project on the Mekong mainstem in Laos. Ecol. Eng. 2012, 48, 79–85. [Google Scholar] [CrossRef]
- Comte, L.; Olden, J.D. Climatic vulnerability of the world’s freshwater and marine fishes. Nat. Clim. Change 2017, 7, 718–722. [Google Scholar] [CrossRef]
- Knouft, J.H.; Ficklin, D.L. The Potential Impacts of Climate Change on Biodiversity in Flowing Freshwater Systems. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 111–133. [Google Scholar] [CrossRef]
- Raj, R.J.R.; Sasipraba, T.; Vasudev, M.; Gupta, S.; Rizwan, M.; Srivastava, P. Predicting the Impact of Climate Change on Tidal Zone Fishes Using SVM Approach. Procedia Comput. Sci. 2016, 92, 237–243. [Google Scholar] [CrossRef]
- van Weelden, C.; Towers, J.R.; Bosker, T. Impacts of climate change on cetacean distribution, habitat and migration. Clim. Change Ecol. 2021, 1, 100009. [Google Scholar] [CrossRef]
- Pomeranz, J.P.F.; Junker, J.R.; Wesner, J.S. Individual size distributions across North American streams vary with local temperature. Glob. Change Biol. 2021, 28, 848–858. [Google Scholar] [CrossRef]
- Baudron, A.R.; Needle, C.L.; Rijnsdorp, A.D.; Tara Marshall, C. Warming temperatures and smaller body sizes: Synchronous changes in growth of North Sea fishes. Glob. Change Biol. 2014, 20, 1023–1031. [Google Scholar] [CrossRef]
- de Visser, S.; Scherer, L.; Huijbregts, M.; Barbarossa, V. Characterization factors for the impact of climate change on freshwater fish species. Ecol. Indic. 2023, 150, 110238. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, G.; Ren, Z. The Behavioral Responses of Koi Carp (Cyprinus carpio) to Different Temperatures: Which Is Better, Infrared or Quadrupole Technology? Animals 2025, 15, 943. https://doi.org/10.3390/ani15070943
Zhong G, Ren Z. The Behavioral Responses of Koi Carp (Cyprinus carpio) to Different Temperatures: Which Is Better, Infrared or Quadrupole Technology? Animals. 2025; 15(7):943. https://doi.org/10.3390/ani15070943
Chicago/Turabian StyleZhong, Guoqing, and Zongming Ren. 2025. "The Behavioral Responses of Koi Carp (Cyprinus carpio) to Different Temperatures: Which Is Better, Infrared or Quadrupole Technology?" Animals 15, no. 7: 943. https://doi.org/10.3390/ani15070943
APA StyleZhong, G., & Ren, Z. (2025). The Behavioral Responses of Koi Carp (Cyprinus carpio) to Different Temperatures: Which Is Better, Infrared or Quadrupole Technology? Animals, 15(7), 943. https://doi.org/10.3390/ani15070943





