Diel Vertical Migration and Transport Pattern of Larvae and Juveniles of the Small Yellow Croaker (Larimichthys polyactis) in the Yangtze River Estuary
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Data Analysis
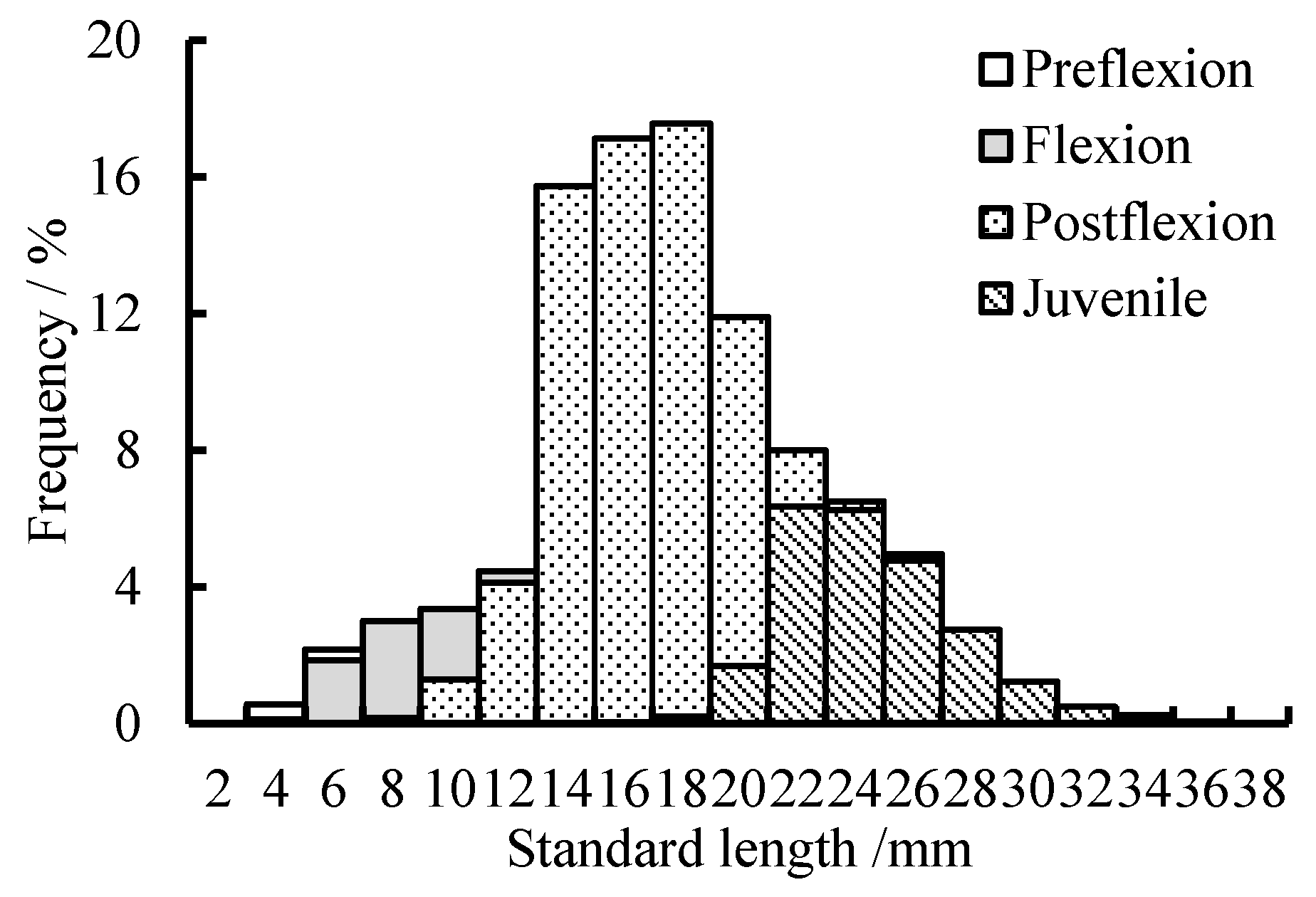
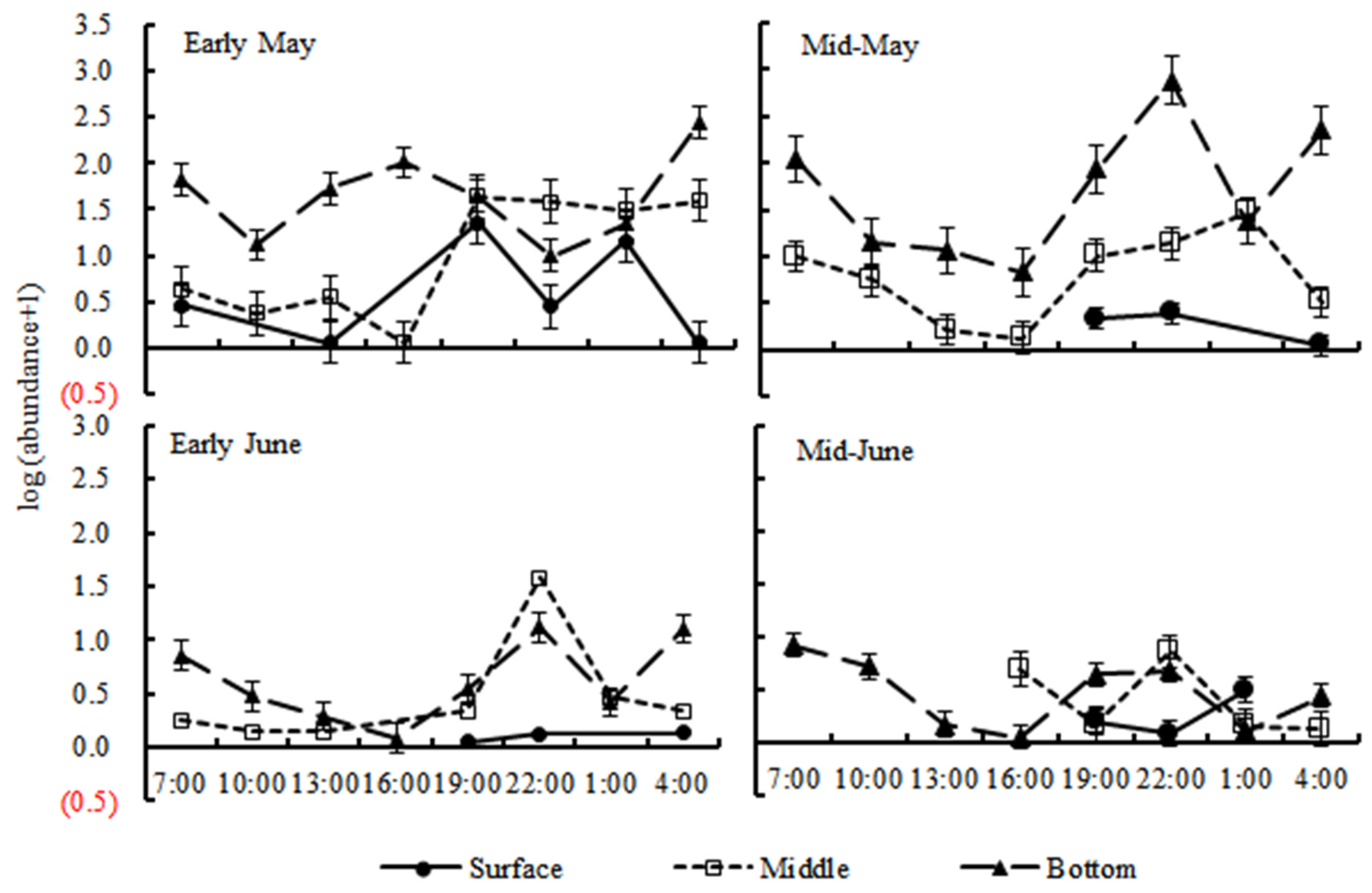
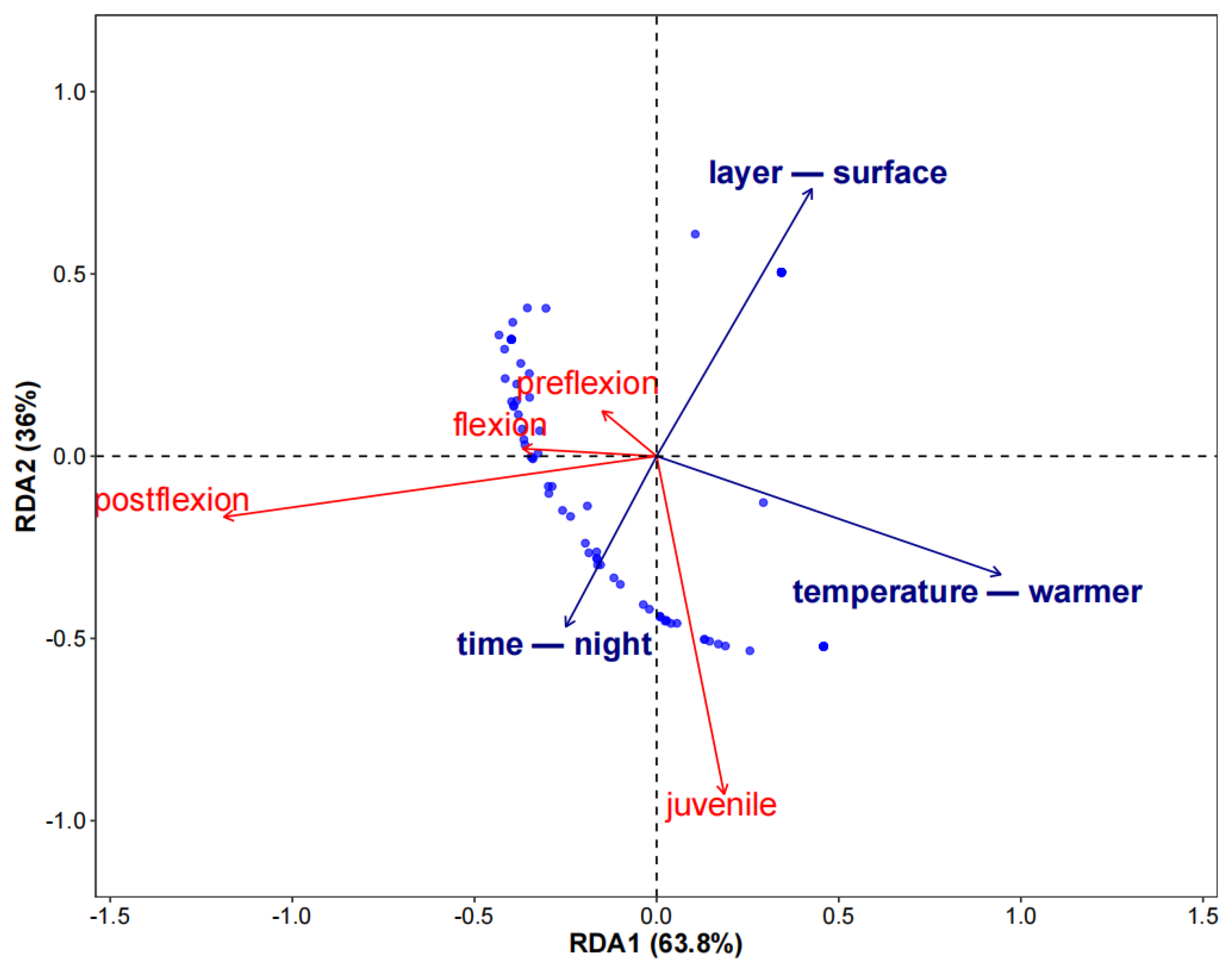
3. Results
3.1. Hydrographic Conditions
3.2. Abundance
3.3. Diel Vertical Distribution
3.4. Relationship Between Abundance and Hydrographic Conditions
4. Discussion
4.1. Diel Vertical Migration
4.2. Transport Pattern
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Islam, M.S.; Hibino, M.; Tanaka, M. Tidal and diurnal variations in larval fish abundance in an estuarine inlet in Ariake bay, Japan: Implication for selective tidal stream transport. Ecol. Res. 2007, 22, 165–171. [Google Scholar] [CrossRef]
- Pattrick, P.; Strydom, N. Recruitment of fish larvae and juveniles into two estuarine nursery areas with evidence of ebb tide use. Estuar. Coast. Shelf Sci. 2014, 149, 120–132. [Google Scholar] [CrossRef]
- Forward, R.B.J.; Tankersley, R.A.; Reinsel, K.A. Selective tidal stream transport of spot (Leistomus xanthurus Lacepede) and pinfish [Lagodon rhomboides (Linnaeus)] larvae: Contribution of circatidal rhythms in activity. J. Exp. Mar. Biol. Ecol. 1998, 226, 19–32. [Google Scholar] [CrossRef]
- Swearer, S.E.; Caselle, J.E.; Lea, D.W.; Warner, R.R. Larval retention and recruitment in an island population of a coral-reef fish. Nature 1999, 402, 799–802. [Google Scholar] [CrossRef]
- Schultz, E.T.; Cowen, R.K.; Lwiza, K.; Gospodarek, A.M. Explaining advection: Do larval bay anchovy (Anchoa mitchilli) show selective tidal-stream transport? Ices J. Mar. Sci. 2000, 57, 360–371. [Google Scholar] [CrossRef]
- Paris, C.B.; Cowen, R.K. Direct evidence of a biophysical retention mechanism for coral reef fish larvae. Limnol. Oceanogr. 2004, 49, 1964–1979. [Google Scholar] [CrossRef]
- Hurst, T.P.; Cooper, D.W.; Scheingross, J.S.; Seale, E.M.; Laurel, B.J.; Spencer, M.L. Effects of ontogeny, temperature, and light on vertical movements of larval pacific cod (Gadus macrocephalus). Fish. Oceanogr. 2009, 18, 301–311. [Google Scholar] [CrossRef]
- Heath, M.R. Field investigations of the early life stages of marine fish. Adv. Mar. Biol. 1992, 28, 1–174. [Google Scholar]
- Röpke, A. Do larvae of mesopelagic fishes in the Arabian Sea adjust their vertical distribution to physical and biological gradients? Mar. Ecol. Prog. Ser. 1993, 101, 223–235. [Google Scholar] [CrossRef]
- Gray, C.A. Do thermoclines explain the vertical distributions of larval fishes in the dynamic coastal waters of south-eastern Australia? Mar. Freshw. Res. 1996, 47, 183–190. [Google Scholar] [CrossRef]
- Auth, T.D.; Brodeur, R.D.; Fisher, K.M. Diel variation in vertical distribution of an offshore ichthyoplankton community off the Oregon coast. Fish. Bull. 2007, 105, 313–326. [Google Scholar]
- Sakuma, K.M.; Ralston, S.; Roberts, D.A. Diel vertical distribution of postflexion larval Citharichthys spp. and Sebastes spp. off central California. Fish. Oceanogr. 1999, 8, 68–76. [Google Scholar] [CrossRef]
- Huebert, K.B. Barokinesis and depth regulation by pelagic coral reef fish larvae. Mar. Ecol. Prog. Ser. 2008, 367, 261–269. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Cabrero, A.; Gago, J.; Guevara-Fletcher, C.; Herrero, M.; de Rojas, A.H.; Garcia, A.; Laiz-Carrion, R.; Vergara, A.R.; Alvarez, P.; et al. Vertical distribution and migration of fish larvae in the NW Iberian upwelling system during the winter mixing period: Implications for cross-shelf distribution. Fish. Oceanogr. 2015, 24, 274–290. [Google Scholar] [CrossRef]
- Neilson, J.D.; Perry, R.I. Diel vertical migrations of marine fishes: An obligate or facultative process? Adv. Mar. Biol. 1990, 26, 115–168. [Google Scholar]
- Song, X.J.; Hu, F.; Xu, M.; Zhang, Y.; Jin, Y.; Gao, X.D.; Liu, Z.L.; Ling, J.Z.; Li, S.F.; Cheng, J.H. Spatiotemporal Distribution and Dispersal Pattern of Early Life Stages of the Small Yellow Croaker (Larimichthys Polyactis) in the Southern Yellow Sea. Diversity 2024, 16, 521. [Google Scholar] [CrossRef]
- Lin, N.; Chen, Y.G.; Jin, Y.; Yuan, X.W.; Ling, J.Z.; Jiang, Y.Z. Distribution of the early life stages of small yellow croaker in the Yangtze River estuary and adjacent waters. Fish. Sci. 2018, 84, 357–363. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Lu, H.F.; Zhao, C.Y.; Chen, L.F.; Zang, Z.J.; Jiang, Y.W. Fish Eggs and Larvae in the Offshore Waters of China; Shanghai Science and Technology Press: Shanghai, China, 1985. (In Chinese) [Google Scholar]
- Mao, X.L.; Yu, J.C.; Qin, Y.X. The Small Yellow Croaker. In The Fishery Resources Survey and Division in the East China Sea Region; East China Normal University Press: Shanghai, China, 1987. (In Chinese) [Google Scholar]
- Wang, J.H.; Sun, Y.W.; Liu, C.C.; Qin, Y.T.; Cheng, X.S.; Xu, R. The ichthyoplankton resource investigation in Changjiang Estuary. J. Mar. Sci. 2007, 25, 40–50. (In Chinese) [Google Scholar]
- Liu, S.D.; Xian, W.W.; Liu, D. Characteristics of ichthyoplankton assemblages in Yangtze Estuary and adjacent waters in spring. Chin. J. Appl. Ecol. 2008, 19, 2284–2292. (In Chinese) [Google Scholar]
- Liu, S.D.; Xian, W.W. Ichthyoplankton community structure characteristics during spring in Yangtze River Estuary before and after impoundment of Three Gorges Reservoir. J. Yangtze River Sci. Res. Inst. 2010, 27, 82–87. (In Chinese) [Google Scholar]
- Liu, S.H.; Wang, J.H.; Liu, C.C.; Qin, Y.T.; Liu, Z.G.; Deng, B.P. Inter-annual variation in pelagic fish egg, larval, and juvenile assemblages during summer in the Yangtze River Estuary, China. Acta Ecol. Sin. 2015, 35, 7190–7197. (In Chinese) [Google Scholar]
- Xu, M.; Wang, Y.H.; Liu, Z.L.; Liu, Y.; Zhang, Y.; Yang, L.L.; Wang, F.; Wu, H.; Cheng, J.H. Seasonal distribution of the early life stages of the small yellow croaker (Larimichthys polyactis) and its dynamic controls adjacent to the Changjiang River Estuary. Fish. Oceanogr. 2023, 32, 390–404. [Google Scholar] [CrossRef]
- Hinckley, S.; Hermann, A.J.; Megrey, B.A. Development of a spatial explicit, individual-based model of marine fish early life history. Mar. Ecol. Prog. Ser. 1996, 139, 47–68. [Google Scholar] [CrossRef]
- Cowen, R.K.; Sponaugle, S. Larval dispersal and marine population connectivity. Annu. Rev. Mar. Sci. 2009, 1, 443–466. [Google Scholar] [CrossRef]
- Aceves-Medina, G.; Saldierna-Martínez, R.; Hinojosa-Medina, A.; Jiménez-Rosenberg, S.P.A.; Hernández-Rivas, M.E.; Morales-Avila, R. Vertical structure of larval fish assemblages during diel cycles in summer and winter in the southern part of Bahía de La Paz, México. Estuar. Coast. Shelf Sci. 2008, 76, 889–901. [Google Scholar] [CrossRef]
- Kendall, A.W.J.; Ahlstrom, E.H.; Moser, H.G. Early Life History Stages of Fishes and Their Characters. In Ontogeny and Systematics of Fishes; American Society of Ichthyologists and Herpetologists: New York, NY, USA, 1984. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Oxford, UK, 2012. [Google Scholar]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Blanchet, F.G.; Legendre, P.; Borcard, D. Forward selection of explanatory variables. Ecology 2008, 89, 2623–2632. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. Version 2.6-10. 2025. Available online: https://CRAN.R-project.org/package=vegan (accessed on 25 February 2025).
- Ginestet, C. ggplot2: Elegant Graphics for Data Analysis. J. R. Stat. Soc. A Stat. 2011, 174, 245–246. [Google Scholar] [CrossRef]
- Hohenwarter, M.; Preiner, J. Dynamic Mathematics with GeoGebra. J. Online Math. Its Appl. 2007, 7, 2–12. [Google Scholar]
- Voss, R.; Schmidt, J.O.; Schnack, D. Vertical distribution of Baltic sprat larvae: Changes in patterns of diel migration? Ices J. Mar. Sci. 2007, 64, 956–962. [Google Scholar] [CrossRef]
- Hibino, M.; Ohta, T.; Isoda, T.; Nakayama, K.; Tanaka, M. Diel and tidal changes in the distribution and feeding habits of Japanese temperate bass Lateolabrax japonicus juveniles in the surf zone of Ariake Bay. Ichthyol. Res. 2006, 53, 129–136. [Google Scholar] [CrossRef]
- Landaeta, M.F.; Castro, L.R. Spring spawning and early nursery zone of the mesopelagic fish Maurolicus parvipinnis at the coastal upwelling zone off Talcahuano, central Chile. Mar. Ecol. Prog. Ser. 2002, 226, 179–191. [Google Scholar] [CrossRef][Green Version]
- Shoji, J.; Maehara, T.; Tanaka, M. Diel Vertical Movement and Feeding Rhythm of Japanese Spanish Mackerel Larvae in the Central Seto Inland Sea. Fish. Sci. 1999, 65, 726–730. [Google Scholar] [CrossRef][Green Version]
- Somarakis, S.; Nikolioudakis, N. Oceanographic habitat, growth and mortality of larval anchovy (Engraulis encrasicolus) in the northern Aegean Sea (eastern Mediterranean). Mar. Biol. 2007, 152, 1143–1158. [Google Scholar] [CrossRef]
- Miller, B.S.; Kendall, A.W.J. Early Life History of Marine Fishes; University of California Press: London, UK, 2009. [Google Scholar]
- Olivar, M.P.; Sabatés, A. Vertical distribution of fish larvae in the north-west Mediterranean Sea in spring. Mar. Biol. 1997, 129, 289–300. [Google Scholar] [CrossRef]
- Yamashita, Y.; Kitagawa, D.; Aoyama, T. Diel vertical migration and feeding rhythm of the larvae of the Japanese sand-eel Ammodytes personatus. Bull. Jpn. Soc. Sci. Fish. 1985, 51, 1–5. [Google Scholar] [CrossRef]
- Munk, P.; Kiørboe, T.; Christensen, V. Vertical migrations of herring, Clupea harengus, larvae in relation to light and prey distribution. Environ. Biol. Fish. 1989, 26, 87–96. [Google Scholar] [CrossRef]
- Hare, J.A.; Govoni, J.J. Comparison of average larval fish vertical distributions among species exhibiting different transport pathways on the southeast United States continental shelf. Fish. Bull.-Natl. Ocean. Atmos. Adm. 2005, 103, 728–736. [Google Scholar]
- Heath, M.R.; Henderson, E.W.; Baird, D.L. Vertical distribution of herring larvae in relation to physical mixing and illumination. Mar. Ecol. Prog. Ser. 1988, 47, 211–228. [Google Scholar] [CrossRef]
- Olla, B.L.; Davis, M.W. Effects of physical factors on the vertical distribution of larval walleye pollock Theragra chalcogramma under controlled laboratory conditions. Mar. Ecol. Prog. Ser. 1990, 63, 105–112. [Google Scholar] [CrossRef]
- Li, J.S.; Ling, J.Z.; Hu, F. Temporal and spatial distribution and growth characteristics of Larimichthys polyactis larvae and juveniles in the coastal waters of the Yangtze River Estuary. Mar. Fish. 2018, 40, 404–412. (In Chinese) [Google Scholar]
- Lin, L.S.; Cheng, J.H.; Jiang, Y.Z.; Yuan, X.W.; Li, J.S.; Gao, T.X. Spatial distribution and environmental characteristics of the spawning grounds of small yellow croaker in the southern Yellow Sea and the East China Sea. Acta Ecol. Sin. 2008, 28, 3485–3494. (In Chinese) [Google Scholar]
- Li, Y.X.; Tang, J.H.; Xu, X.M.; Xu, J.; Liu, Z.Y.; Xu, H.; Cheng, J.H. Comparision of otolith microstructures in small yellow croaker larvae and juveniles from Sanmen Bay and Lvsi. Mar. Fish. 2013, 35, 423–431. (In Chinese) [Google Scholar]
- Boehlert, G.W.; Mundy, B.C. Roles of behavioral and physical factors in larval and juvenile fish recruitment to estuarine nursery areas. Am. Fish. Soc. Symp. 1988, 3, 61–67. [Google Scholar]
- Churchill, J.H.; Forward, R.B.; Luettich, R.A.; Hench, J.L.; Hettler, W.F.; Crowder, L.B.; Blanton, J.O. Circulation and larval transport within a tidally dominated estuary. Fish. Oceanogr. 1999, 8, 173–189. [Google Scholar] [CrossRef]
- Shenker, J.M.; Hepner, D.J.; Frere, P.E.; Currence, L.E.; Wakefield, W.W. Upriver Migration and Abundance of Naked Goby (Gobiosoma bosci) Larvae in the Patuxent River Estuary, Maryland. Estuaries 1983, 6, 36–42. [Google Scholar] [CrossRef]
- GB/T 12763.6-2007; Specifications for Oceanographic Survey-Part 6: Marine Biological Survey. General Administration of Quality Supervision Inspection and Quarantine of the People’s Republic of China and China Standardization Administration: Beijing, China, 2008.






| Source | Sum of Squares | df | Mean Square | F | Sig. | Partial Eta Squared |
|---|---|---|---|---|---|---|
| Corrected Model | 34.72 | 23.00 | 1.51 | 10.76 | 0.00 | 0.77 |
| Intercept | 38.34 | 1.00 | 38.34 | 273.33 | 0.00 | 0.79 |
| month | 10.64 | 3.00 | 3.55 | 25.30 | 0.00 | 0.51 |
| layer | 13.85 | 2.00 | 6.92 | 49.37 | 0.00 | 0.58 |
| day-night | 3.60 | 1.00 | 3.60 | 25.66 | 0.00 | 0.26 |
| month * layer | 4.11 | 6.00 | 0.68 | 4.88 | 0.00 | 0.29 |
| month * day-night | 0.48 | 3.00 | 0.16 | 1.15 | 0.34 | 0.05 |
| layer * day-night | 0.64 | 2.00 | 0.32 | 2.27 | 0.11 | 0.06 |
| Error | 1.40 | 6.00 | 0.23 | 1.66 | 0.14 | 0.12 |
| Total | 10.10 | 72.00 | 0.14 | |||
| Corrected Total | 83.15 | 96.00 |
| (I) Month | (J) Month | Mean Difference (I − J) | Std. Error | Sig. | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Early May | Mid-May | 0.20 | 0.11 | 0.27 | −0.09 | 0.48 |
| Early June | 0.70 | 0.11 | 0.00 | 0.42 | 0.99 | |
| Mid-June | 0.79 | 0.11 | 0.00 | 0.51 | 1.07 | |
| Mid-May | Early May | −0.20 | 0.11 | 0.27 | −0.48 | 0.09 |
| Early June | 0.51 | 0.11 | 0.00 | 0.22 | 0.79 | |
| Mid-June | 0.59 | 0.11 | 0.00 | 0.31 | 0.88 | |
| Early June | Early May | −0.70 | 0.11 | 0.00 | −0.99 | −0.42 |
| Mid-May | −0.51 | 0.11 | 0.00 | −0.79 | −0.22 | |
| Mid-June | 0.09 | 0.11 | 0.85 | −0.20 | 0.37 | |
| Mid-June | Early May | −0.79 | 0.11 | 0.00 | −1.07 | −0.51 |
| Mid-May | −0.59 | 0.11 | 0.00 | −0.88 | −0.31 | |
| Early June | −0.09 | 0.11 | 0.85 | −0.37 | 0.20 | |
| (I) layer | (J) layer | |||||
| Surface | Middle | −0.46 | 0.09 | 0.00 | −0.69 | −0.24 |
| Bottom | −0.93 | 0.09 | 0.00 | −1.15 | −0.71 | |
| Middle | Surface | 0.46 | 0.09 | 0.00 | 0.24 | 0.69 |
| Bottom | −0.47 | 0.09 | 0.00 | −0.69 | −0.25 | |
| Bottom | Surface | 0.93 | 0.09 | 0.00 | 0.71 | 1.15 |
| Middle | 0.47 | 0.09 | 0.00 | 0.25 | 0.69 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Hu, F.; Ling, J.; Yuan, X.; Liu, Z.; Jin, Y.; Li, S.; Jiang, Y. Diel Vertical Migration and Transport Pattern of Larvae and Juveniles of the Small Yellow Croaker (Larimichthys polyactis) in the Yangtze River Estuary. Animals 2025, 15, 1128. https://doi.org/10.3390/ani15081128
Song X, Hu F, Ling J, Yuan X, Liu Z, Jin Y, Li S, Jiang Y. Diel Vertical Migration and Transport Pattern of Larvae and Juveniles of the Small Yellow Croaker (Larimichthys polyactis) in the Yangtze River Estuary. Animals. 2025; 15(8):1128. https://doi.org/10.3390/ani15081128
Chicago/Turabian StyleSong, Xiaojing, Fen Hu, Jianzhong Ling, Xingwei Yuan, Zunlei Liu, Yan Jin, Shengfa Li, and Yazhou Jiang. 2025. "Diel Vertical Migration and Transport Pattern of Larvae and Juveniles of the Small Yellow Croaker (Larimichthys polyactis) in the Yangtze River Estuary" Animals 15, no. 8: 1128. https://doi.org/10.3390/ani15081128
APA StyleSong, X., Hu, F., Ling, J., Yuan, X., Liu, Z., Jin, Y., Li, S., & Jiang, Y. (2025). Diel Vertical Migration and Transport Pattern of Larvae and Juveniles of the Small Yellow Croaker (Larimichthys polyactis) in the Yangtze River Estuary. Animals, 15(8), 1128. https://doi.org/10.3390/ani15081128






