Impact of Flavonoid-Enriched Antioxidant Nanoformulation Supplementation on In Vitro Maturation and Gene Expression of Buffalo Oocytes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization and Particle Size of EMD-300® and EMP3-H200®
2.2. 2,2-Diphenyl-1-Picrylhydrazyl Radical (DPPH) Scavenging Assay
2.3. Collection of the Ovaries and Cumulus–Oocyte Complexes (COCs)
2.4. Oocyte IVM
2.5. Evaluation of Cumulus Expansion and Nuclear Maturation
2.6. RNA Isolation and cDNA Synthesis
2.7. Quantitative Real Time PCR (qRT PCR)
2.8. TAC and MDA Level Estimation in Spent IVM Medium
2.9. Statistical Analysis
3. Results
3.1. Oocyte Recovery Rate and Grading
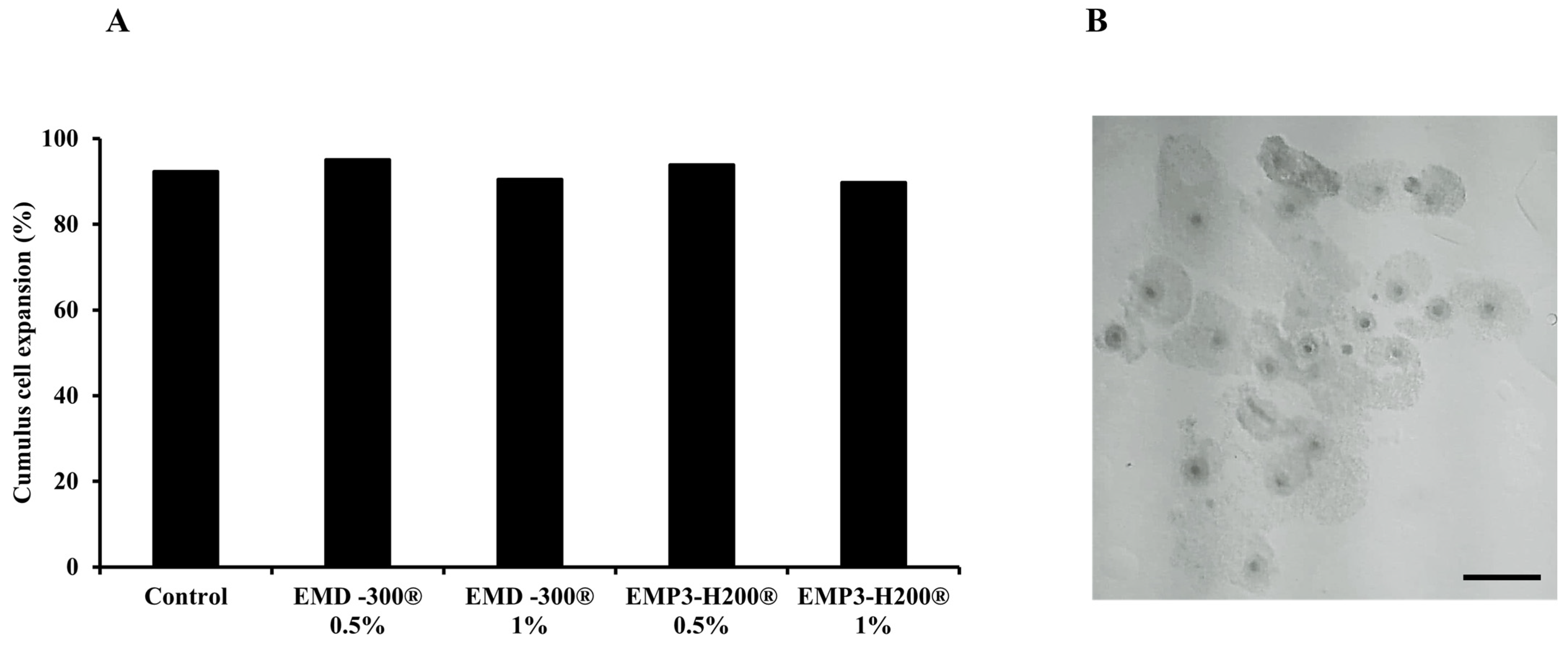
3.2. The Effect of EMD-300® and EMP3-H200® Supplementation During IVM of Buffalo COCs on Cumulus Cell Expansion
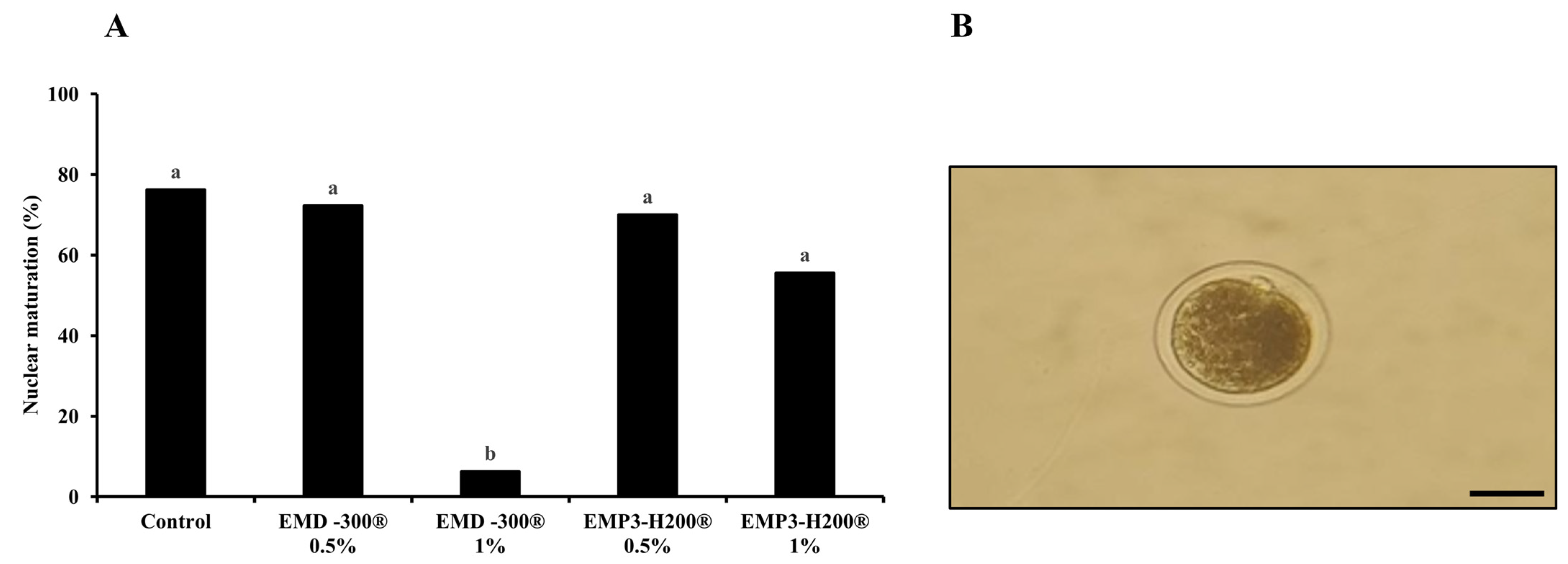
3.3. The Effect of EMD-300® and EMP3-H200® Supplementation During IVM of Buffalo Oocytes on Nuclear Maturation Rates
3.4. The Effect of EMD-300® and EMP3-H200® Supplementation During IVM of Buffalo COCs on the Expression Pattern of Oxidative Stress Response-Associated Genes
3.5. The Effect of EMD-300® and EMP3-H200® Supplementation During IVM of Buffalo COCs on the Expression Pattern of Apoptotic and Endoplasmic Reticulum Stress-Associated Genes
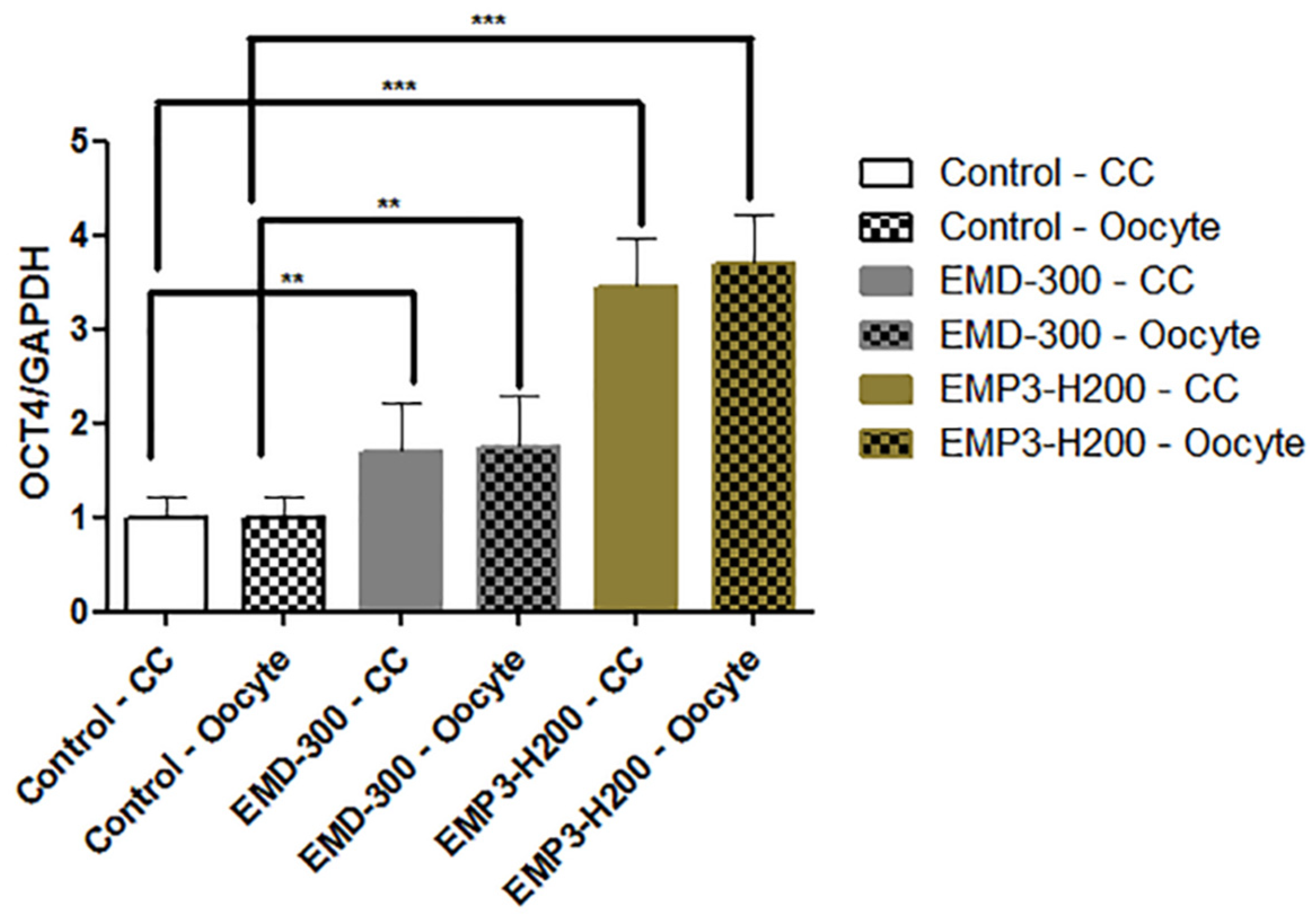
3.6. The Effect of EMD-300® and EMP3-H200® Supplementation During IVM of Buffalo COCs on the Expression Pattern of the OCT4 Gene
3.7. Levels of TAC and MDA in Spent IVM Medium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Presicce, G.A.; Gasparrini, B.; Salzano, A.; Neglia, G.; Campanile, G.; Zicarelli, L. Reproductive technologies in the buffalo (Bubalus bubalis). In Reproductive Technologies in Animals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 81–98. [Google Scholar] [CrossRef]
- Samad, M. A systematic review of research findings on buffalo health and production published during the last six decades in Bangladesh. J. Vet. Med. One Health Res. 2020, 2, 1–62. [Google Scholar] [CrossRef] [PubMed]
- Danell, B. Oestrus behaviour, ovarian morphology and cyclical variation. In Follicular System and Endocrine Pattern in Water Buffalo Heifers; Swedish University of Agricultural Sciences: Uppsala, Sweden, 1987; 124p. [Google Scholar]
- El-Wishy, A.B. The postpartum buffalo: I. Acyclicity and anestrus. Anim. Reprod. Sci. 2007, 97, 216–236. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chaves, M.S.; da Silva, A.F.B.; Vale, W.G.; Filho, S.T.R.; Ferreira-Silva, J.C. Factors affecting the in vitro embryo production in buffalo (Bubalus bubalis): A review. Vet. Med. 2023, 68, 45–56. [Google Scholar] [CrossRef]
- Arias-Álvarez, M.; García-García, R.M.; López-Tello, J.; Rebollar, P.; Gutiérrez-Adán, A.; Lorenzo, P.L. In vivo and in vitro maturation of rabbit oocytes differently affects the gene expression profile, mitochondrial distribution, apoptosis and early embryo development. Reprod. Fertil. Dev. 2017, 29, 1667–1679. [Google Scholar] [CrossRef]
- Macaulay, A.D.; Gilbert, I.; Scantland, S.; Fournier, E.; Ashkar, F.; Bastien, A.; Saadi, H.A.S.; Gagné, D.; Sirard, M.-A.; Khandjian, É.W. Cumulus cell transcripts transit to the bovine oocyte in preparation for maturation. Biol. Reprod. 2016, 94, 16. [Google Scholar] [CrossRef]
- Zhou, C.-J.; Wu, S.-N.; Shen, J.-P.; Wang, D.-H.; Kong, X.-W.; Lu, A.; Li, Y.-J.; Zhou, H.-X.; Zhao, Y.-F.; Liang, C.-G. The beneficial effects of cumulus cells and oocyte-cumulus cell gap junctions depends on oocyte maturation and fertilization methods in mice. PeerJ 2016, 4, e1761. [Google Scholar] [CrossRef]
- Auclair, S.; Uzbekov, R.; Elis, S.; Sanchez, L.; Kireev, I.; Lardic, L.; Dalbies-Tran, R.; Uzbekova, S. Absence of cumulus cells during in vitro maturation affects lipid metabolism in bovine oocytes. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E599–E613. [Google Scholar] [CrossRef]
- Kakkassery, M.P.; Vijayakumaran, V.; Sreekumaran, T. Effect of cumulus oocyte complex morphology on in vitro maturation of bovine oocytes. J. Vet. Anim. Sci. 2010, 41, 12–17. [Google Scholar]
- Landim-Alvarenga, F.; Maziero, R. Control of oocyte maturation. Anim. Reprod. 2018, 11, 150–158. [Google Scholar]
- Ferreira, E.; Vireque, A.; Adona, P.R.; Meirelles, F.V.; Ferriani, R.A.; Navarro, P.A.d.A.S. Cytoplasmic maturation of bovine oocytes: Structural and biochemical modifications and acquisition of developmental competence. Theriogenology 2009, 71, 836–848. [Google Scholar] [CrossRef]
- Agarwal, A.; Said, T.M.; Bedaiwy, M.A.; Banerjee, J.; Alvarez, J.G. Oxidative stress in an assisted reproductive techniques setting. Fertil. Steril. 2006, 86, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Noda, Y.; Goto, Y.; Mori, T. Effects of visible light and other environmental factors on the production of oxygen radicals by hamster embryos. Theriogenology 1994, 41, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Liebel, F.; Kaur, S.; Ruvolo, E.; Kollias, N.; Southall, M.D. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J. Investig. Dermatol. 2012, 132, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Khoudja, R.Y.; Xu, Y.; Li, T.; Zhou, C. Better ivf outcomes following improvements in laboratory air quality. J. Assist. Reprod. Genet. 2013, 30, 69–76. [Google Scholar] [CrossRef]
- Torres-Osorio, V.; Urrego, R.; Echeverri-Zuluaga, J.J.; López-Herrera, A. Estrés oxidativo y el uso de antioxidantes en la producción in vitro de embriones mamíferos. Revisión. Rev. Mex. Cienc. Pecu. 2019, 10, 433–459. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sikka, S. The role of free radicals and antioxidants in reproduction. Curr. Opin. Obstet. Gynecol. 2006, 18, 325–332. [Google Scholar] [CrossRef]
- Silva, E.; Greene, A.F.; Strauss, K.; Herrick, J.R.; Schoolcraft, W.B.; Krisher, R.L. Antioxidant supplementation during in vitro culture improves mitochondrial function and development of embryos from aged female mice. Reprod. Fertil. Dev. 2015, 27, 975–983. [Google Scholar] [CrossRef]
- Gasparrini, B.; Sayoud, H.; Neglia, G.; de Matos, D.G.; Donnay, I.; Zicarelli, L. Glutathione synthesis during in vitro maturation of buffalo (bubalus bubalis) oocytes: Effects of cysteamine on embryo development. Theriogenology 2003, 60, 943–952. [Google Scholar] [CrossRef]
- Patel, P.A.; Chaudhary, S.S.; Puri, G.; Singh, V.K.; Odedara, A.B. Effects of β-mercaptoethanol on in vitro maturation and glutathione level of buffalo oocytes. Vet. World 2015, 8, 213. [Google Scholar] [CrossRef]
- de Oliveira Santos, M.V.; Borges, A.A.; de Queiroz Neta, L.B.; Bertini, L.M.; Pereira, A.F. Use of natural antioxidants in in vitro mammalian embryo production. Semin. Cienc. Agrar. 2018, 39, 431–443. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils–a review. Food. Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Bahgat, H.S.; Abdel-Ghafar, A.; Badr, M.R.; Shafik, B.; Azab, R.E.-s.; Ramadan, S.; Mohamed, M.E.-R. Ameliorating effects of kaempferol on buffalo oocytes developmental competence (part-i). Benha Vet. Med. J. 2023, 45, 60–63. [Google Scholar] [CrossRef]
- Sovernigo, T.; Adona, P.; Monzani, P.; Guemra, S.; Barros, F.; Lopes, F.; Leal, C. Effects of supplementation of medium with different antioxidants during in vitro maturation of bovine oocytes on subsequent embryo production. Reprod. Domest. Anim. 2017, 52, 561–569. [Google Scholar] [CrossRef]
- Cajas, Y.N.; Cañón-Beltrán, K.; Ladrón de Guevara, M.; Millán de La Blanca, M.G.; Ramos-Ibeas, P.; Gutiérrez-Adán, A.; Rizos, D.; González, E.M. Antioxidant nobiletin enhances oocyte maturation and subsequent embryo development and quality. Int. J. Mol. Sci. 2020, 21, 5340. [Google Scholar] [CrossRef]
- Karimian, M.; Zandi, M.; Sanjabi, M.R.; Masoumian, M.; Ofoghi, H. Effects of grape seed extract, quercetin and vitamin c on ovine oocyte maturation and subsequent embryonic development. Cell. Mol. Biol. 2018, 64, 98–102. [Google Scholar] [CrossRef]
- Hashem, N.M.; Gonzalez-Bulnes, A. Nanotechnology and reproductive management of farm animals: Challenges and advances. Animals 2021, 11, 1932. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Silva, J.R.V.; Barroso, P.A.A.; Nascimento, D.R.; Figueira, C.S.; Azevedo, V.A.N.; Silva, B.R.; Santos, R.P.d. Benefits and challenges of nanomaterials in assisted reproductive technologies. Mol. Reprod. Dev. 2021, 88, 707–717. [Google Scholar] [CrossRef]
- El-Naby, A.-s.A.-H.H.; Ibrahim, S.; Hozyen, H.F.; Sosa, A.; Mahmoud, K.G.M.; Farghali, A.A. Impact of nano-selenium on nuclear maturation and genes expression profile of buffalo oocytes matured in vitro. Mol. Biol. Rep. 2020, 47, 8593–8603. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, H.N.; Kandil, O.M.; Ahmad, I.M.; Mansour, M.; El-Debaky, H.A.; Ali, K.A.; Ismail, E.A.; Abedelaziz, S.A. Effect of melatonin-loaded chitosan nanoparticles (cmn) on gene expression of in-vitro matured buffalo oocyte. J. Adv. Vet. Res. 2023, 13, 656–663. [Google Scholar]
- Remião, M.H.; Lucas, C.G.; Domingues, W.B.; Silveira, T.; Barther, N.N.; Komninou, E.R.; Basso, A.C.; Jornada, D.S.; Beck, R.C.R.; Pohlmann, A.R. Melatonin delivery by nanocapsules during in vitro bovine oocyte maturation decreased the reactive oxygen species of oocytes and embryos. Reprod. Toxicol. 2016, 63, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, B.; Helmy, N.A. Effect of nano-selenium and nano-zinc particles during in vitro maturation on the developmental competence of bovine oocytes. Anim. Prod. Sci. 2017, 58, 2021–2028. [Google Scholar] [CrossRef]
- Najjaa, H.; Zerria, K.; Fattouch, S.; Ammar, E.; Neffati, M. Antioxidant and antimicrobial activities of allium roseum l.“Lazoul,” a wild edible endemic species in north africa. Int. J. Food Prop. 2011, 14, 371–380. [Google Scholar] [CrossRef]
- El-Naby, A.H.; Mahmoud, K.; Ahmed, Y.F.; Abouel-Roos, M.E.; Abdel-Ghaffar, A.E. Effect of season of the year and ovarian structures on oocytes recovery rate, quality and meiotic competence in egyptian buffaloes. Glob. Vet. 2013, 10, 408–412. [Google Scholar] [CrossRef]
- El-Shalofy, A.S. Some Trials for Cryobanking of Egyptian Buffalo Oocytes. Master’s Thesis, Faculty of Veterinary Medicine, Cairo University, Cairo, Egypt, 2016. [Google Scholar]
- Moawad, A.R.; Zhu, J.; Choi, I.; Amarnath, D.; Chen, W.; Campbell, K.H. Production of good-quality blastocyst embryos following ivf of ovine oocytes vitrified at the germinal vesicle stage using a cryoloop. Reprod. Fertil. Dev. 2013, 25, 1204–1215. [Google Scholar] [CrossRef]
- Kandil, O.M.; Rahman, S.M.A.E.; Ali, R.S.; Ismail, E.A.; Ibrahim, N.M. Effect of melatonin on developmental competence, mitochondrial distribution, and intensity of fresh and vitrified/thawed in vitro matured buffalo oocytes. Reprod. Biol. Endocrinol. 2024, 22, 39. [Google Scholar] [CrossRef]
- Saber, Y.H.; Ibrahim, S.; Mahmoud, K.G.M.; Ahmed, W.M.; Ragab, R.S.; Seida, A.A. Expression profile of viability and stress response genes as a result of resveratrol supplementation in vitrified and in vitro produced cattle embryos. Mol. Biol. Rep. 2024, 51, 692. [Google Scholar] [CrossRef]
- Rocha-Frigoni, N.A.; Leão, B.C.; Dall’Acqua, P.C.; Mingoti, G.Z. Improving the cytoplasmic maturation of bovine oocytes matured in vitro with intracellular and/or extracellular antioxidants is not associated with increased rates of embryo development. Theriogenology 2016, 86, 1897–1905. [Google Scholar] [CrossRef]
- Menezo, Y.J.; Silvestris, E.; Dale, B.; Elder, K. Oxidative stress and alterations in DNA methylation: Two sides of the same coin in reproduction. Reprod. Biomed. Online 2016, 33, 668–683. [Google Scholar] [CrossRef] [PubMed]
- Boni, R.; Santella, L.; Dale, B.; Roviello, S.; Di Palo, R.; Barbieri, V. An ultrastructural study of maturation in buffalo oocytes. Acta. Med. Vet. 1992, 38, 153-l. [Google Scholar]
- Bhardwaj, R.L.; Roy, K.S. Follicular development in the ovary of Indian buffalo (Bubalus bubalis). Indian J. Anim. Sci. 2006, 76, 506–509. [Google Scholar]
- Yousaf, M.R.; Chohan, K.R. Nuclear morphology, diameter and meiotic competence of buffalo oocytes relative to follicle. Reprod. Fertil. Dev. 2003, 15, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Shahid, B.; Jalali, S.; Khan, M.I.; Shami, S.A. Different methods of oocytes recovery for in vitro maturation in nili ravi buffalo’s oocytes. APCBEE Procedia 2014, 8, 359–363. [Google Scholar] [CrossRef]
- Roy, P.K.; Qamar, A.Y.; Fang, X.; Kim, G.; Bang, S.; De Zoysa, M.; Shin, S.T.; Cho, J. Chitosan nanoparticles enhance developmental competence of in vitro-matured porcine oocytes. Reprod. Domest. Anim. 2021, 56, 342–350. [Google Scholar] [CrossRef]
- Adwas, A.A.; Elsayed, A.; Azab, A.E.; Quwaydir, F.A. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar] [CrossRef]
- Wang, F.; Tian, X.; Zhang, L.; He, C.; Ji, P.; Li, Y.; Tan, D.; Liu, G. Beneficial effects of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization. Fertil. Steril. 2014, 101, 577.e1–586.e1. [Google Scholar] [CrossRef]
- Kang, J.-T.; Moon, J.H.; Choi, J.-Y.; Park, S.J.; Kim, S.J.; Saadeldin, I.M.; Lee, B.C. Effect of Antioxidant Flavonoids (Quercetin and Taxifolin) on In vitro Maturation of Porcine Oocytes. Asian-Australas. J. Anim. Sci. 2016, 29, 352–358. [Google Scholar] [CrossRef]
- Waiz, S.A.; Raies-ul-Haq, M.; Dhanda, S.; Kumar, A.; Goud, T.S.; Chauhan, M.; Upadhyay, R. Heat stress and antioxidant enzyme activity in bubaline (bubalus bubalis) oocytes during in vitro maturation. Int. J. Biometeorol. 2016, 60, 1357–1366. [Google Scholar] [CrossRef]
- Corrêa, G.A.; Rumpf, R.; Mundim, T.C.D.; Franco, M.M.; Dode, M.A.N. Oxygen tension during in vitro culture of bovine embryos: Effect in production and expression of genes related to oxidative stress. Anim. Reprod. Sci. 2008, 104, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Tripathi, S.K.; Singh, P.K.; Gupta, P.S.; Mondal, S. Global DNA methylation, DNA methyltransferase and stress-related gene expression in ovine oocytes and embryos after exposure to metabolic stressors. Reprod. Domest. Anim. 2023, 58, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Arias-Álvarez, M.; García-García, R.M.; López-Tello, J.; Rebollar, P.; Gutiérrez-Adán, A.; Lorenzo, P.L. A-tocopherol modifies the expression of genes related to oxidative stress and apoptosis during in vitro maturation and enhances the developmental competence of rabbit oocytes. Reprod. Fertil. Dev. 2018, 30, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Khalil, W.A.; Marei, W.F.; Khalid, M. Protective effects of antioxidants on linoleic acid–treated bovine oocytes during maturation and subsequent embryo development. Theriogenology 2013, 80, 161–168. [Google Scholar] [CrossRef]
- Huang, Z.; Pang, Y.; Hao, H.; Du, W.; Zhao, X.; Zhu, H. Effects of epigallocatechin-3-gallate on bovine oocytes matured in vitro. Asian-Australas. J. Anim. Sci. 2018, 31, 1420. [Google Scholar] [CrossRef]
- Marques, T.C.; da Silva Santos, E.C.; Diesel, T.O.; Leme, L.O.; Martins, C.F.; Dode, M.A.N.; Alves, B.G.; Costa, F.P.H.; De Oliveira, E.B.; Gambarini, M.L. Melatonin reduces apoptotic cells, sod 2 and hspb 1 and improves the in vitro production and quality of bovine blastocysts. Reprod. Domest. Anim. 2018, 53, 226–236. [Google Scholar] [CrossRef]
- Cetica, P.; Pintos, L.; Dalvit, G.; Beconi, M. Antioxidant enzyme activity and oxidative stress in bovine oocyte in vitro maturation. IUBMB Life 2001, 51, 57–64. [Google Scholar] [CrossRef]
- Fathi, M.; Salama, A.; El-Shahat, K.; El-Sherbiny, H.; Abdelnaby, E.A. Effect of melatonin supplementation during ivm of dromedary camel oocytes (camelus dromedarius) on their maturation, fertilization, and developmental rates in vitro. Theriogenology 2021, 172, 187–192. [Google Scholar] [CrossRef]
- Khalil, W.A.; Yang, C.-Y.; El-Moghazy, M.M.; El-Rais, M.S.; Shang, J.-H.; El-Sayed, A. Effect of zinc chloride and sodium selenite supplementation on in vitro maturation, oxidative biomarkers, and gene expression in buffalo (bubalus bubalis) oocytes. Zygote 2021, 29, 393–400. [Google Scholar] [CrossRef]
- Boumela, I.; Assou, S.; Aouacheria, A.; Haouzi, D.; Dechaud, H.; De Vos, J.; Handyside, A.; Hamamah, S. Involvement of bcl2 family members in the regulation of human oocyte and early embryo survival and death: Gene expression and beyond. Reproduction 2011, 141, 549. [Google Scholar] [CrossRef]
- Asadi, M.; Taghizadeh, S.; Kaviani, E.; Vakili, O.; Taheri-Anganeh, M.; Tahamtan, M.; Savardashtaki, A. Caspase-3: Structure, function, and biotechnological aspects. Biotechnol. Appl. Biochem. 2022, 69, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Saini, S.; Thakur, S.; Sharma, S.; Punetha, M.; Kumar, P.; Sango, C.; Sharma, R.; Datta, T.; Yadav, P. Enhancing the quality of inferior oocytes of buffalo for in vitro embryo production: The impact of melatonin on maturation, scnt, and epigenetic modifications. Tissue Cell 2024, 89, 102480. [Google Scholar] [CrossRef]
- Xiang, D.-C.; Jia, B.-Y.; Fu, X.-W.; Guo, J.-X.; Hong, Q.-H.; Quan, G.-B.; Wu, G.-Q. Role of astaxanthin as an efficient antioxidant on the in vitro maturation and vitrification of porcine oocytes. Theriogenology 2021, 167, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Ghemrawi, R.; Battaglia-Hsu, S.-F.; Arnold, C. Endoplasmic reticulum stress in metabolic disorders. Cells 2018, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, H.; Nishitoh, H. Signaling pathways from the endoplasmic reticulum and their roles in disease. Genes 2013, 4, 306–333. [Google Scholar] [CrossRef]
- Pagliassotti, M.J.; Kim, P.Y.; Estrada, A.L.; Stewart, C.M.; Gentile, C.L. Endoplasmic reticulum stress in obesity and obesity-related disorders: An expanded view. Metabolism 2016, 65, 1238–1246. [Google Scholar] [CrossRef]
- Song, B.-S.; Yoon, S.-B.; Sim, B.-W.; Kim, Y.-H.; Cha, J.-J.; Choi, S.-A.; Jeong, K.-J.; Kim, J.-S.; Huh, J.-W.; Lee, S.-R. Valproic acid enhances early development of bovine somatic cell nuclear transfer embryos by alleviating endoplasmic reticulum stress. Reprod. Fertil. Dev. 2014, 26, 432–440. [Google Scholar] [CrossRef]
- Sharma, A.; Agrawal, H.; Mullani, N.; Sandhu, A.; Singh, M.K.; Chauhan, M.S.; Singla, S.K.; Palta, P.; Manik, R.S. Supplementation of tauroursodeoxycholic acid during ivc did not enhance in vitro development and quality of buffalo ivf embryos but combated endoplasmic reticulum stress. Theriogenology 2015, 84, 200–207. [Google Scholar] [CrossRef]
- Wu, G.; Schöler, H.R. Role of oct4 in the early embryo development. Cell Regen. 2014, 3, 7. [Google Scholar] [CrossRef]
- Guo, Y.; Mantel, C.; Hromas, R.A.; Broxmeyer, H.E. Oct-4 is critical for survival/antiapoptosis of murine embryonic stem cells subjected to stress: Effects associated with stat3/survivin. Stem Cells 2008, 26, 30–34. [Google Scholar] [CrossRef]
- Zuccotti, M.; Merico, V.; Sacchi, L.; Bellone, M.; Brink, T.C.; Bellazzi, R.; Stefanelli, M.; Redi, C.A.; Garagna, S.; Adjaye, J. Maternal oct-4 is a potential key regulator of the developmental competence of mouse oocytes. BMC Dev. Biol. 2008, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, M.; Merico, V.; Sacchi, L.; Bellone, M.; Brink, T.C.; Stefanelli, M.; Redi, C.A.; Bellazzi, R.; Adjaye, J.; Garagna, S. Oct-4 regulates the expression of stella and foxj2 at the nanog locus: Implications for the developmental competence of mouse oocytes. Hum. Reprod. 2009, 24, 2225–2237. [Google Scholar] [CrossRef] [PubMed]
- Grossman, D.; Kalo, D.; Gendelman, M.; Roth, Z. Effect of di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate on in vitro developmental competence of bovine oocytes. Cell Biol. Toxicol. 2012, 28, 383–396. [Google Scholar] [CrossRef]
- Ritika, R.; Saini, S.; Shavi, S.; Ramesh, P.; Selokar, N.L.; Ludri, A.; Singh, M.K. Curcumin enhances developmental competence and ameliorates heat stress in in vitro buffalo (bubalus bubalis) embryos. Vet. World 2024, 17, 2433. [Google Scholar] [CrossRef]
- Abdulhasan, M.; Li, Q.; Dai, J.; Abu-Soud, H.; Puscheck, E.; Rappolee, D. Coq10 increases mitochondrial mass and polarization, atp and oct4 potency levels, and bovine oocyte mii during ivm while decreasing ampk activity and oocyte death. J. Assist. Reprod. Genet. 2017, 34, 1595–1607. [Google Scholar] [CrossRef]
- Cordova, A.; Miranda, M.; King, W.; Mastromonaco, G. Effects of egf and melatonin on gene expression of cumulus cells and further in vitro embryo development in bovines. Zygote 2022, 30, 600–610. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, J.-J.; Yin, Y.-J.; Jiang, H.; Zhang, J.-B.; Liang, S.; Yuan, B. Ferulic acid enhances oocyte maturation and the subsequent development of bovine oocytes. Int. J. Mol. Sci. 2023, 24, 14804. [Google Scholar] [CrossRef]


| Gene | Sequence 5′–3′ | Accession No. | Product Size (bps) | Annealing °C |
|---|---|---|---|---|
| GPX4 | F: GCTCATTGAGAACGTAGCAT R: GTACTTCAGGCAATTCAGGAT | NM_174076.3 | 175 | 55 |
| SOD | F: GATACAGTCGTGGTAACTGGAT R: TCTCCTGAGAGTGAAATCAGA | NM_174615.2 | 248 | 54 |
| CAT | F: TCTCCACTGTTGCTGGAGAAT R: TGCGTTTGAGGGTTTCTCTT | NM_001035386.2 | 187 | 54 |
| ATF6 | F: AAGACAAGCCCATCATTGGT R: TGATTGTTTTTGCTGGAACG | XM_024989877.1 | 162 | 51 |
| CASP3 | F: GACTGTGGTATTGAGACAGACA R: CGTACTTTTTCAGCATCTCAC | XM_006075118.1 | 175 | 50 |
| OCT4 | F: CAGAAGAGGATCACACTAGGAT R: GTCTCTGCCTTGCATATCTC | NM_174580.3 | 212 | 53 |
| GAPDH | F: CTACATGGTCTACATGTTCCAG R: CCTTCTCCATGGTAGTGAAGA | XM_006065800.1 | 200 | 50 |
| Ovaries (n) | Oocytes (n) | Recovery Rate (Mean ± SEM) | Culturable Oocytes n (Mean% ± SEM) | Non-Culturable Oocytes n (Mean% ± SEM) |
|---|---|---|---|---|
| 346 | 634 | 2.02 ± 0.23 | 426 (65.92 ± 2.61) a | 208 (34.08 ± 2.61) b |
| Treatment | TAC (mmol/L) | MDA (nmol/mL) |
|---|---|---|
| Control | 0.29 ± 0.17 | 17.07 ± 3.91 |
| EMD-300® 0.5% | 0.41 ± 0.19 | 8.46 ± 2.14 |
| EMP3-H200® 0.5% | 0.35 ± 0.15 | 11.91 ± 4.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Saka, E.M.; El-Wishy, A.B.A.; Moawad, A.R.; Ibrahim, S.; Ibrahim, S.; Shahat, A.M. Impact of Flavonoid-Enriched Antioxidant Nanoformulation Supplementation on In Vitro Maturation and Gene Expression of Buffalo Oocytes. Animals 2025, 15, 1147. https://doi.org/10.3390/ani15081147
El-Saka EM, El-Wishy ABA, Moawad AR, Ibrahim S, Ibrahim S, Shahat AM. Impact of Flavonoid-Enriched Antioxidant Nanoformulation Supplementation on In Vitro Maturation and Gene Expression of Buffalo Oocytes. Animals. 2025; 15(8):1147. https://doi.org/10.3390/ani15081147
Chicago/Turabian StyleEl-Saka, Eman M., Abou Bakr A. El-Wishy, Adel R. Moawad, Sally Ibrahim, Saber Ibrahim, and Abdallah M. Shahat. 2025. "Impact of Flavonoid-Enriched Antioxidant Nanoformulation Supplementation on In Vitro Maturation and Gene Expression of Buffalo Oocytes" Animals 15, no. 8: 1147. https://doi.org/10.3390/ani15081147
APA StyleEl-Saka, E. M., El-Wishy, A. B. A., Moawad, A. R., Ibrahim, S., Ibrahim, S., & Shahat, A. M. (2025). Impact of Flavonoid-Enriched Antioxidant Nanoformulation Supplementation on In Vitro Maturation and Gene Expression of Buffalo Oocytes. Animals, 15(8), 1147. https://doi.org/10.3390/ani15081147





