Methionine Antagonizes Liver and Kidney Antioxidant Function Damage in Heat-Stressed Rex Rabbits
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Organ Index and Histopathological Observation
2.3. Quantifying Methionine Concentrations Using the LC-MS External Standard Method
2.4. Serum Biochemical
2.5. Skin Oxidative Stress Indicator
2.6. Oxidant Stress Index of Liver and Kidney
2.7. RT-PCR
2.8. Statistical Analysis
3. Results
3.1. Effects on Liver and Kidney Physiological Indicators
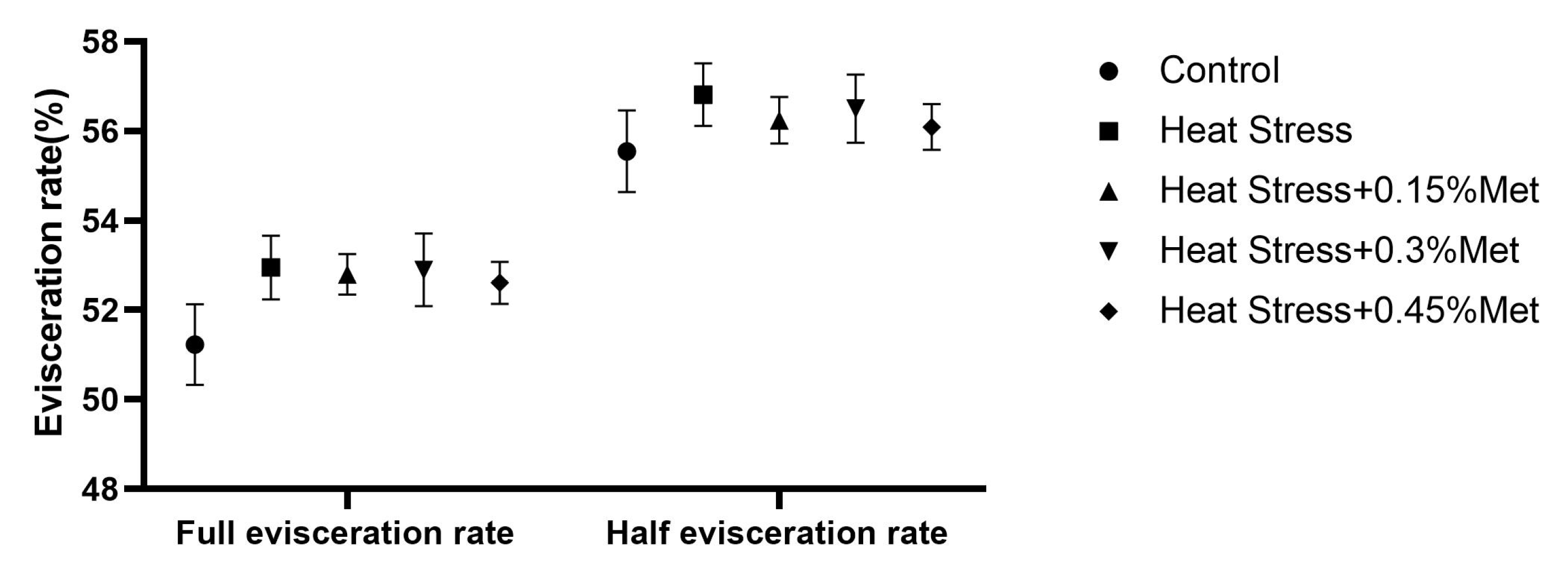
3.2. Slaughter Performance
3.3. Methionine Concentration and Apparent Digestibility
3.4. Other Amino Acid Concentrations in Serum
3.5. Skin Oxidative Stress Assessment
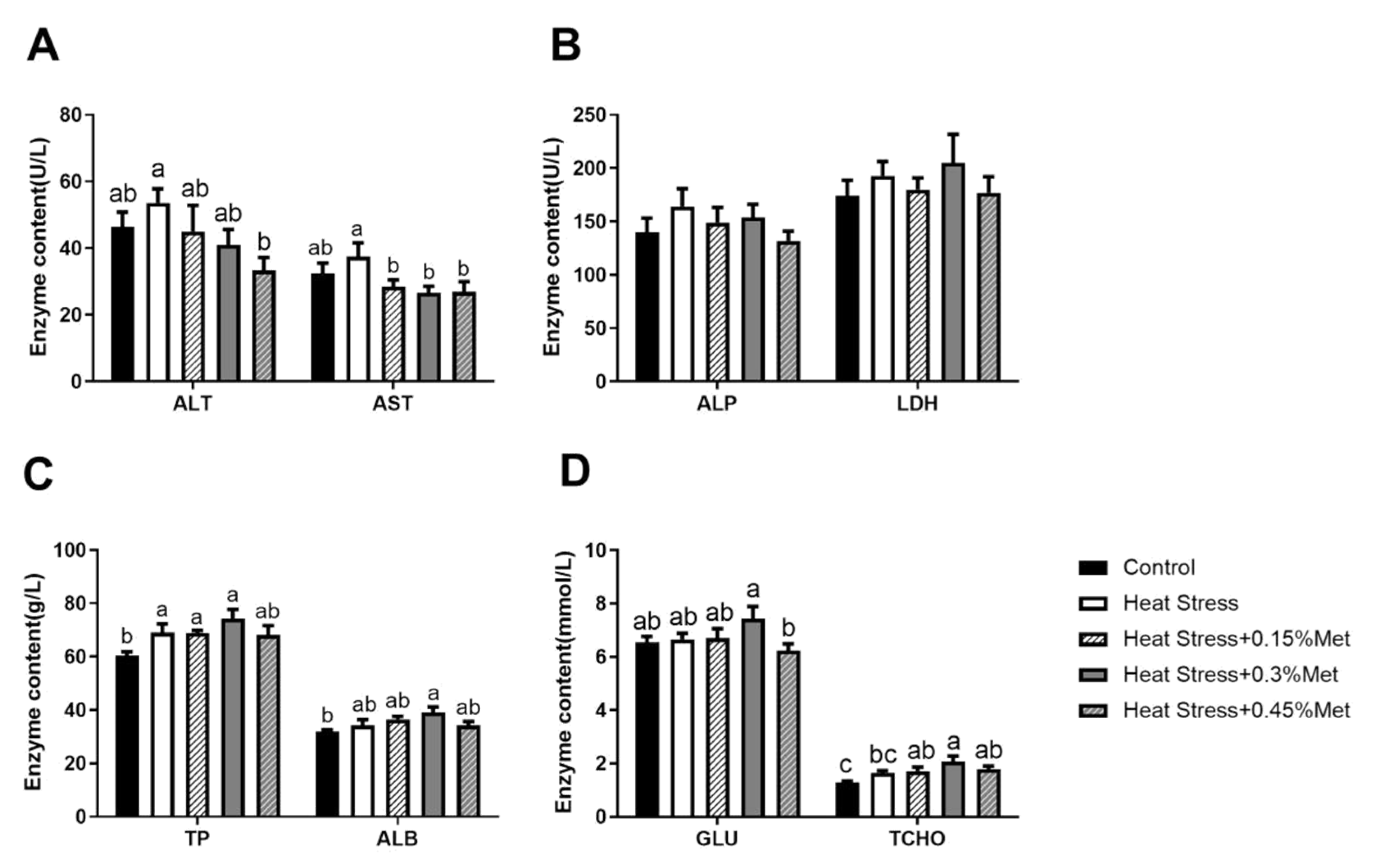
3.6. Serum Biochemistry
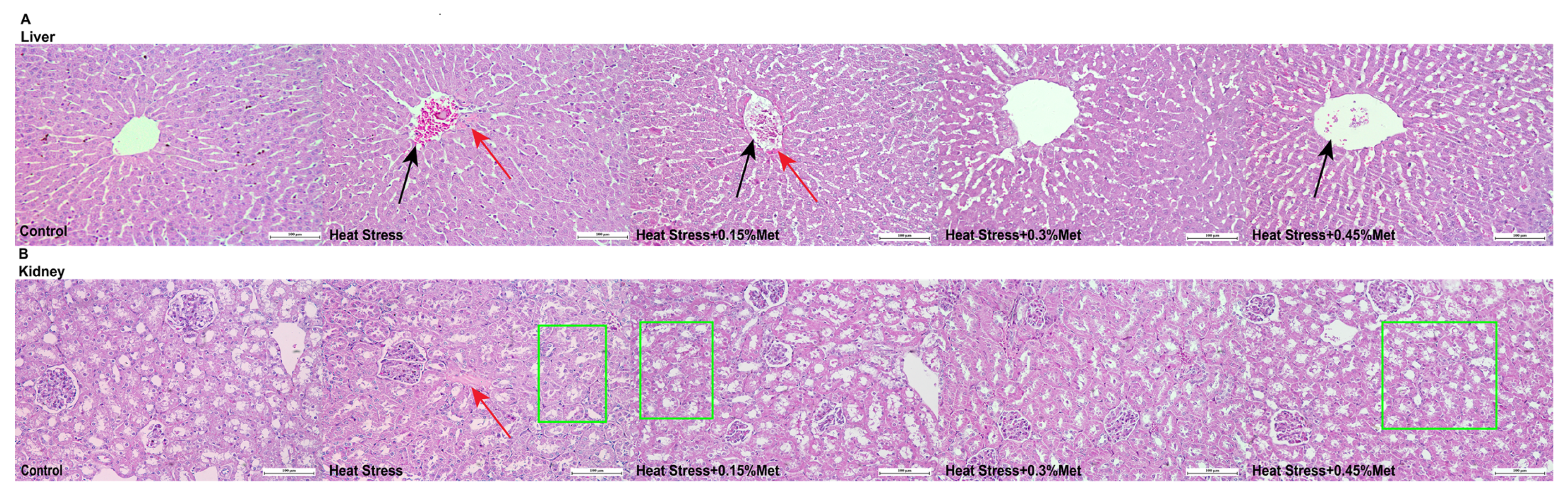
3.7. Liver and Kidney Pathological Structure Diagnosis
3.8. Liver and Kidney Antioxidant Defense
3.9. Expression of Antioxidant Genes in the Liver and Kidney
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| MDA | Malondialdehyde |
| MSRA | Methionine sulfoxide reductase A |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GPx | Glutathione peroxidase |
| HS | Heat stress |
| ALP | Alkaline phosphatase |
| TP | Total protein |
| ALB | Albumin |
| Glu | Glucose |
| TCHO | Total cholesterol |
| LDH | Lactate dehydrogenase |
| Met | Methionine |
Appendix A
| Ingredients | Content (%) | Nutrient Levels | Content |
|---|---|---|---|
| Corn | 6.00 | DM (%) | 87.45 |
| Wheat middling | 12.00 | DE (2) (MJ/kg) | 9.87 |
| Wheat bran | 15.00 | CP (%) | 17.04 |
| Corn germ meal | 24.00 | EE (%) | 3.8 |
| Alfalfa powder | 9.00 | CF (%) | 18.78 |
| Soybean meal | 8.00 | Ash (%) | 7.26 |
| Soybean oil | 1.00 | Ca (%) | 0.87 |
| Beanstalk powder | 21.00 | P (%) | 0.43 |
| Premix (1) | 4.00 | Lys (%) | 0.59 |
| Total | 100.00 | Met (%) | 0.19 |
References
- Kumar, N.; Latha Telagarapu, V.M.; Fornoni, A. Climate Change, Heat Stress, and Kidney Disease-Associated Mortality and Health Care Utilization. Kidney Int. Rep. 2024, 9, 2844–2847. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO) Database. Available online: https://www.fao.org/ (accessed on 19 December 2024).
- Oladimeji, A.M.; Johnson, T.G.; Metwally, K.; Farghly, M.; Mahrose, K.M. Environmental heat stress in rabbits: Implications and ameliorations. Int. J. Biometeorol. 2022, 66, 1–11. [Google Scholar] [CrossRef] [PubMed]
- El-Raghi, A.A.; Hassan, M.A.E.; Hashem, N.M.; Abdelnour, S.A. Struggling Thermal Stress Impacts on Growth Performance and Health Status of Newly Weaned Rabbits Using Nanoemulsion of Origanum majorana Considering the Economic Efficiency of Supplementation. Animals 2023, 13, 1772. [Google Scholar] [CrossRef]
- Lacetera, N. Impact of climate change on animal health and welfare. Anim. Front. 2019, 9, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Chen, J.; Xu, J.; Zhang, L.; Liu, L.; Li, F. Protective effect of chlorogenic acid on liver injury in heat-stressed meat rabbits. J. Anim. Physiol. Anim. Nutr. 2024, 108, 1203–1213. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef]
- Ismail, R.; Hassan, M.A.E.; Moustafa, M.; Al-Shehri, M.; Alazragi, R.S.; Khojah, H.; El-Raghi, A.A.; Abdelnour, S.A.; Gad, A.M.A. The Influence of a Nanoemulsion of Cardamom Essential Oil on the Growth Performance, Feed Utilization, Carcass Characteristics, and Health Status of Growing Rabbits under a High Ambient Temperature. Animals 2023, 13, 2990. [Google Scholar] [CrossRef]
- Davis, B.C.; Tillman, H.; Chung, R.T.; Stravitz, R.T.; Reddy, R.; Fontana, R.J.; McGuire, B.; Davern, T.; Lee, W.M. Heat stroke leading to acute liver injury & failure: A case series from the Acute Liver Failure Study Group. Liver Int. 2017, 37, 509–513. [Google Scholar] [CrossRef]
- Delli Bovi, A.P.; Marciano, F.; Mandato, C.; Siano, M.A.; Savoia, M.; Vajro, P. Oxidative Stress in Non-alcoholic Fatty Liver Disease. An Updated Mini Review. Front. Med. 2021, 8, 595371. [Google Scholar] [CrossRef]
- Devarajan, P. Update on mechanisms of ischemic acute kidney injury. J. Am. Soc. Nephrol. 2006, 17, 1503–1520. [Google Scholar] [CrossRef]
- El-Ratel, I.T.; Mekawy, A.; Hassab, S.H.M.; Abdelnour, S. Enhancing growing rabbit heat stress resilience through dietary supplementation with natural antioxidants. BMC Vet. Res. 2025, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, X.; Liu, G.; Li, F. Methionine Modulates the Growth and Development of Heat-Stressed Dermal Papilla Cells via the Wnt/β-Catenin Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 1495. [Google Scholar] [CrossRef] [PubMed]
- Veredas, F.J.; Cantón, F.R.; Aledo, J.C. Methionine residues around phosphorylation sites are preferentially oxidized in vivo under stress conditions. Sci. Rep. 2017, 7, 40403. [Google Scholar] [CrossRef]
- Elfarra, A.A.; Krause, R.J. Potential roles of flavin-containing monooxygenases in sulfoxidation reactions of l-methionine, N-acetyl-l-methionine and peptides containing l-methionine. Biochim. Biophys. Acta 2005, 1703, 183–189. [Google Scholar] [CrossRef]
- Cabreiro, F.; Picot, C.R.; Perichon, M.; Friguet, B.; Petropoulos, I. Overexpression of methionine sulfoxide reductases A and B2 protects MOLT-4 cells against zinc-induced oxidative stress. Antioxid. Redox Signal. 2009, 11, 215–225. [Google Scholar] [CrossRef]
- Yalçinkaya-Demirsöz, S.; Depboylu, B.; Dogru-Abbasoglu, S.; Unlüçerçi, Y.; Uysal, M. Effects of high methionine diet on oxidative stress in serum, apo-B containing lipoproteins, heart, and aorta in rabbits. Ann. Clin. Lab. Sci. 2009, 39, 386–391. [Google Scholar]
- Kong, D.; Xu, J.; Zhang, Q.; Luo, D.; Lv, Q.; Li, S.; Chen, X.; Wei, L.; Zhu, X.; Liu, Y.; et al. Selenomethionine Attenuates Aflatoxin B(1)-induced Liver Injury by Modulating the Gut Microbiota and Metabolites in Rabbits. J. Agric. Food Chem. 2025, 73, 3080–3094. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.S.; Rashamol, V.P.; Bagath, M.; Sejian, V.; Dunshea, F.R. Impacts of heat stress on immune responses and oxidative stress in farm animals and nutritional strategies for amelioration. Int. J. Biometeorol. 2021, 65, 1231–1244. [Google Scholar] [CrossRef]
- Liang, M.; Wang, Z.; Li, H.; Cai, L.; Pan, J.; He, H.; Wu, Q.; Tang, Y.; Ma, J.; Yang, L. l-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem. Toxicol. 2018, 115, 315–328. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Li, Y.; Ji, F.; Ge, G.; Xu, H. Midazolam Ameliorates Acute Liver Injury Induced by Carbon Tetrachloride via Enhancing Nrf2 Signaling Pathway. Front. Pharmacol. 2022, 13, 940137. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Z.; Liu, H.; Liu, G.; Li, F. Methionine promotes the development of hair follicles via the Wnt/β-catenin signalling pathway in Rex rabbits. J. Anim. Physiol. Anim. Nutr. 2020, 104, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Marai, I.F.; Ayyat, M.S.; Abd el-Monem, U.M. Growth performance and reproductive traits at first parity of New Zealand white female rabbits as affected by heat stress and its alleviation under Egyptian conditions. Trop. Anim. Health Prod. 2001, 33, 451–462. [Google Scholar] [CrossRef]
- Yue, Z.; Liu, M.; Zhang, B.; Li, F.; Li, C.; Chen, X.; Li, F.; Liu, L. Vitamin A regulates dermal papilla cell proliferation and apoptosis under heat stress via IGF1 and Wnt10b signaling. Ecotoxicol. Environ. Saf. 2023, 262, 115328. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Liu, L.; Li, F. Methionine can subside hair follicle development prejudice of heat-stressed rex rabbits. FASEB J. 2022, 36, e22464. [Google Scholar] [CrossRef]
- Wu, Z.L.; Yang, X.; Chen, S.Y.; Deng, F.L.; Jia, X.B.; Hu, S.Q.; Wang, J.; Lai, S.J. Liver Transcriptome Changes of Hyla Rabbit in Response to Chronic Heat Stress. Animals 2019, 9, 1141. [Google Scholar] [CrossRef] [PubMed]
- Tyritzis, S.I.; Kyroudi, A.; Liatsikos, E.; Manousakas, T.; Karayannacos, P.; Kostomitsopoulos, N.; Zervas, A.; Pavlakis, K.; Stolzenburg, J.U.; Constantinides, C. Comparison of prolonged warm and cold ischemia on the solitary kidney during partial nephrectomy in a rabbit model. World J. Urol. 2007, 25, 635–640. [Google Scholar] [CrossRef]
- Wilson, T.E. Renal sympathetic nerve, blood flow, and epithelial transport responses to thermal stress. Auton. Neurosci. 2017, 204, 25–34. [Google Scholar] [CrossRef]
- Coates, T.; Kirkland, G.S.; Dymock, R.B.; Murphy, B.F.; Brealey, J.K.; Mathew, T.H.; Disney, A.P. Cutaneous necrosis from calcific uremic arteriolopathy. Am. J. Kidney Dis. 1998, 32, 384–391. [Google Scholar] [CrossRef]
- Attia, Y.A.; Bovera, F.; El-Tahawy, W.S.; El-Hanoun, A.M.; Al-Harthi, M.A.; Habiba, H.I. Productive and reproductive performance of rabbits does as affected by bee pollen and/or propolis, inulin and/or mannan-oligosaccharides. World Rabbit. Sci. 2015, 23, 273. [Google Scholar] [CrossRef]
- Zhang, B.; Boyuan, N.; Chen, X.; Li, C.; Liu, M.; Yue, Z.; Liu, L.; Li, F. Effects of the SLC38A2–mTOR Pathway Involved in Regulating the Different Compositions of Dietary Essential Amino Acids–Lysine and Methionine on Growth and Muscle Quality in Rabbits. Animals 2022, 12, 3406. [Google Scholar] [CrossRef] [PubMed]
- Romanovsky, A.A. Skin temperature: Its role in thermoregulation. Acta Physiol. 2014, 210, 498–507. [Google Scholar] [CrossRef]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Dong, X.F.; Tong, J.M.; Zhang, Q. Alfalfa polysaccharides improve the growth performance and antioxidant status of heat-stressed rabbits. Livest. Sci. 2010, 131, 88–93. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, L.; Li, J.; Xing, T.; Jiang, Y.; Gao, F. Heat stress alters muscle protein and amino acid metabolism and accelerates liver gluconeogenesis for energy supply in broilers. Poult. Sci. 2021, 100, 215–223. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Emara, A.M.; Gan, X.; Li, H. Effects of malondialdehyde as a byproduct of lipid oxidation on protein oxidation in rabbit meat. Food Chem. 2019, 288, 405–412. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Jacobs, P.J.; Oosthuizen, M.K.; Mitchell, C.; Blount, J.D.; Bennett, N.C. Heat and dehydration induced oxidative damage and antioxidant defenses following incubator heat stress and a simulated heat wave in wild caught four-striped field mice Rhabdomys dilectus. PLoS ONE 2020, 15, e0242279. [Google Scholar] [CrossRef]
- Tang, J.-Y.; Wang, L.-Q.; Jia, G.; Liu, G.-M.; Chen, X.-L.; Tian, G.; Cai, J.-Y.; Shang, H.-Y.; Zhao, H. The hydroxy-analogue of selenomethionine alleviated lipopolysaccharide-induced inflammatory responses is associated with recover expression of several selenoprotein encoding genes in the spleens of Kunming mice. RSC Adv. 2019, 9, 40462–40470. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, N.; Jiang, H.; Meng, X.; Ge, H.; Yang, X.; Xu, X.; Qian, K.; Park, Y.; Zheng, Y.; et al. 20-hydroxyecdysone regulates expression of methioninesulfoxide reductases through transcription factor FOXO in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2021, 131, 103546. [Google Scholar] [CrossRef]
- Moskovitz, J.; Bar-Noy, S.; Williams, W.M.; Requena, J.; Berlett, B.S.; Stadtman, E.R. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc. Natl. Acad. Sci. USA 2001, 98, 12920–12925. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Tang, X.D.; Chen, M.L.; Joiner, M.L.; Sun, G.; Brot, N.; Weissbach, H.; Heinemann, S.H.; Iverson, L.; Wu, C.F.; et al. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc. Natl. Acad. Sci. USA 2002, 99, 2748–2753. [Google Scholar] [CrossRef] [PubMed]
- Ayari, D.; Boukazoula, F.; Soumati, B.; Souiki, L. Evaluation of oxidative stress biomarkers of rabbits’ liver exposed to thermooxidized virgin olive oil obtained from blanquette olive cultivars. Biomarkers 2019, 24, 407–413. [Google Scholar] [CrossRef]
- Alzeer, A.H.; el-Hazmi, M.A.; Warsy, A.S.; Ansari, Z.A.; Yrkendi, M.S. Serum enzymes in heat stroke: Prognostic implication. Clin. Chem. 1997, 43, 1182–1187. [Google Scholar] [CrossRef]
- Hu, B.; Wei, H.; Song, Y.; Chen, M.; Fan, Z.; Qiu, R.; Zhu, W.; Xu, W.; Wang, F. NF-κB and Keap1 Interaction Represses Nrf2-Mediated Antioxidant Response in Rabbit Hemorrhagic Disease Virus Infection. J. Virol. 2020, 94, 1128. [Google Scholar] [CrossRef] [PubMed]
- Osama, A.; Zhang, J.; Yao, J.; Yao, X.; Fang, J. Nrf2: A dark horse in Alzheimer’s disease treatment. Ageing Res. Rev. 2020, 64, 101206. [Google Scholar] [CrossRef]
- Shu, G.; Yusuf, A.; Dai, C.; Sun, H.; Deng, X. Piperine inhibits AML-12 hepatocyte EMT and LX-2 HSC activation and alleviates mouse liver fibrosis provoked by CCl4: Roles in the activation of the Nrf2 cascade and subsequent suppression of the TGF-β1/Smad axis. Food Funct. 2021, 12, 11686–11703. [Google Scholar] [CrossRef]
- Wang, Y.R.; Chen, K.L.; Li, C.M.; Li, L.; Wang, G.L. Heme oxygenase 1 regulates apoptosis induced by heat stress in bovine ovarian granulosa cells via the ERK1/2 pathway. J. Cell. Physiol. 2019, 234, 3961–3972. [Google Scholar] [CrossRef]
- Preethi, S.; Arthiga, K.; Patil, A.B.; Spandana, A.; Jain, V. Review on NAD(P)H dehydrogenase quinone 1 (NQO1) pathway. Mol. Biol. Rep. 2022, 49, 8907–8924. [Google Scholar] [CrossRef]


| Gene | GenBank | Primer Sequences (5′→3′) | Product Size (bp) |
|---|---|---|---|
| SOD | XM_051854201.1 | F: TTTCTGGACAAACCTGAGCCCTAAC R: CCGTCAGCCTCTCCTTGAACTTG | 110 |
| CAT | XM_002721425.3 | F: CCAGTCTATTAGGTTCCATGTTCC R: CGATTATTGGCGTTTTGGTC | 117 |
| GPx | NM_001085444.1 | F: CAGGAGAACGCCAAGAATGAGGAG R: GTTCACCTCGCACTTCTGGAAGAG | 105 |
| NQO1 | XM_002711667.4 | F: AGCGGCTCCATGTACTCTCTCC R: GAGTGTGCCCGATGCTGTATGTG | 136 |
| HO-1 | XM_051846030.1 | F: CCACCAAGTTCAAGCAGCTCTACC R: TTAGCCTCTTCCACCACCCTCTG | 88 |
| Nrf2 | XM_051849401.1 | F: AAGCAACTCAGCACCTTGTATCTGG R: GAATACATTGCCGTCCCTCGTCTG | 114 |
| GAPDH | NM_001082253.1 | F: GGCTGCTTTTAACTCTGGCAAA R: CGTGGGTGGAATCATACTGGAA | 101 |
| Keap1 | XM_008251549.3 | F: CCTCAACCGCCTGCTCTATGC R: ATCCGCCACTCGTTCCTCTCC | 96 |
| Items | Control | Heat Stress | Heat Stress +0.15% Met | Heat Stress +0.3% Met | Heat Stress +0.45% Met | R-MSE | p-Value |
|---|---|---|---|---|---|---|---|
| Initial weigh (kg) | 1.97 ± 0.05 | 1.97 ± 0.04 | 1.96 ± 0.03 | 1.95 ± 0.04 | 1.93 ± 0.04 | 0.2220 | 0.9388 |
| Final Weight (kg) | 2.46 ± 0.05 a | 2.35 ± 0.04 ab | 2.37 ± 0.03 ab | 2.36 ± 0.04 ab | 2.28 ± 0.04 b | 0.2200 | 0.0546 |
| Liver (g) | 55 ± 1.1 a | 46 ± 1.3 c | 50 ± 1.2 bc | 51 ± 1.5 ab | 48 ± 1.8 bc | 3.929 | 0.0017 |
| Liver index (g/kg) | 23 ± 0.7 a | 21 ± 0.8 b | 22 ± 0.5 ab | 23 ± 0.5 a | 22 ± 0.9 ab | 1.929 | 0.0604 |
| Kidney (g) | 13 ± 0.6 | 11 ± 0.4 | 10 ± 0.5 | 11 ± 0.7 | 10 ± 0.4 | 1.497 | 0.0072 |
| Renal index (g/kg) | 5.4 ± 0.28 a | 4.9 ± 0.17 ab | 4.5 ± 0.22 b | 5.0 ± 0.29 ab | 4.6 ± 0.18 b | 0.6586 | 0.0696 |
| Items | Control | Heat Stress | Heat Stress +0.15% Met | Heat Stress +0.3% Met | Heat Stress +0.45% Met | R-MSE | p-Value |
|---|---|---|---|---|---|---|---|
| Feed (mg/g) | 1.95 ± 0.06 d | 1.90 ± 0.02 d | 8.15 ± 0.09 c | 14.5 ± 0.15 b | 20.8 ± 0.25 a | 0.1922 | <0.0001 |
| Feces (μg/g) | 12.5 ± 0.87 b | 16.9 ± 4.7 b | 13.1 ± 2.8 b | 21.9 ± 3.8 b | 475 ± 216 a | 167.2 | 0.024 |
| Apparent digestibility (%) | 99.2 ± 0.05 b | 98.7 ± 0.08 c | 99.8 ± 0.01 a | 99.9 ± 0.01 a | 96.7 ± 0.18 d | 0.2587 | <0.0001 |
| Items | Control | Heat Stress | Heat Stress +0.15% Met | Heat Stress +0.3% Met | Heat Stress +0.45% Met | R-MSE | p-Value |
|---|---|---|---|---|---|---|---|
| ASP (μmol/L) | 0.20 ± 0.01 b | 0.29 ± 0.01 a | 0.28 ± 0.01 a | 0.25 ± 0.02 ab | 0.29 ± 0.01 ab | 0.030 | 0.029 |
| GLU (μmol/L) | 0.69 ± 0.04 b | 0.88 ± 0.04 a | 0.76 ± 0.01 ab | 0.70 ± 0.06 b | 0.73 ± 0.05 ab | 0.077 | 0.081 |
| PHE (μmol/L) | 0.62 ± 0.04 b | 0.75 ± 0.04 ab | 0.78 ± 0.02 a | 0.74 ± 0.03 ab | 0.75 ± 0.04 ab | 0.067 | 0.102 |
| MET (μmol/L) | 0.41 ± 0.03 b | 0.36 ± 0.01 c | 0.48 ± 0.01 a | 0.50 ± 0.01 a | 0.52 ± 0.01 a | 0.026 | <0.0001 |
| TRP (μmol/L) | 0.46 ± 0.02 ab | 0.39 ± 0.02 b | 0.50 ± 0.02 a | 0.49 ± 0.05 a | 0.44 ± 0.01 ab | 0.043 | 0.065 |
| Items | Control | Heat Stress | Heat Stress +0.15% Met | Heat Stress +0.3% Met | Heat Stress +0.45% Met | R-MSE | p-Value |
|---|---|---|---|---|---|---|---|
| MDA (nmol/mgprot) | 5.95 ± 0.97 | 7.36 ± 0.49 | 7.61 ± 4.4 | 3.82 ± 0.92 | 7.24 ± 0.61 | 5.11 | 0.6851 |
| ALT (U/gprot) | 7.28 ± 1.49 | 8.42 ± 1.99 | 7.94 ± 2.48 | 7.98 ± 2.49 | 6.11 ± 1.83 | 4.913 | 0.9219 |
| T-SOD (U/mgprot) | 12.2 ± 0.26 a | 10.2 ± 0.66 b | 11.1 ± 0.09 ab | 11.4 ± 0.34 ab | 10.5 ± 0.44 b | 0.994 | 0.0192 |
| MSRA (IU/L) | 6.64 ± 0.20 a | 4.08 ± 0.13 c | 3.41 ± 0.50 c | 5.34 ± 0.21 b | 6.80 ± 0.63 a | 0.863 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wang, X.; Liu, G.; Liu, L.; Li, F. Methionine Antagonizes Liver and Kidney Antioxidant Function Damage in Heat-Stressed Rex Rabbits. Animals 2025, 15, 1148. https://doi.org/10.3390/ani15081148
Li S, Wang X, Liu G, Liu L, Li F. Methionine Antagonizes Liver and Kidney Antioxidant Function Damage in Heat-Stressed Rex Rabbits. Animals. 2025; 15(8):1148. https://doi.org/10.3390/ani15081148
Chicago/Turabian StyleLi, Shu, Xiaosong Wang, Gongyan Liu, Lei Liu, and Fuchang Li. 2025. "Methionine Antagonizes Liver and Kidney Antioxidant Function Damage in Heat-Stressed Rex Rabbits" Animals 15, no. 8: 1148. https://doi.org/10.3390/ani15081148
APA StyleLi, S., Wang, X., Liu, G., Liu, L., & Li, F. (2025). Methionine Antagonizes Liver and Kidney Antioxidant Function Damage in Heat-Stressed Rex Rabbits. Animals, 15(8), 1148. https://doi.org/10.3390/ani15081148






