Effects of Embryonic Thermal Manipulation on Body Performance and Cecum Microbiome in Broiler Chickens Following a Post-Hatch Lipopolysaccharide Challenge
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
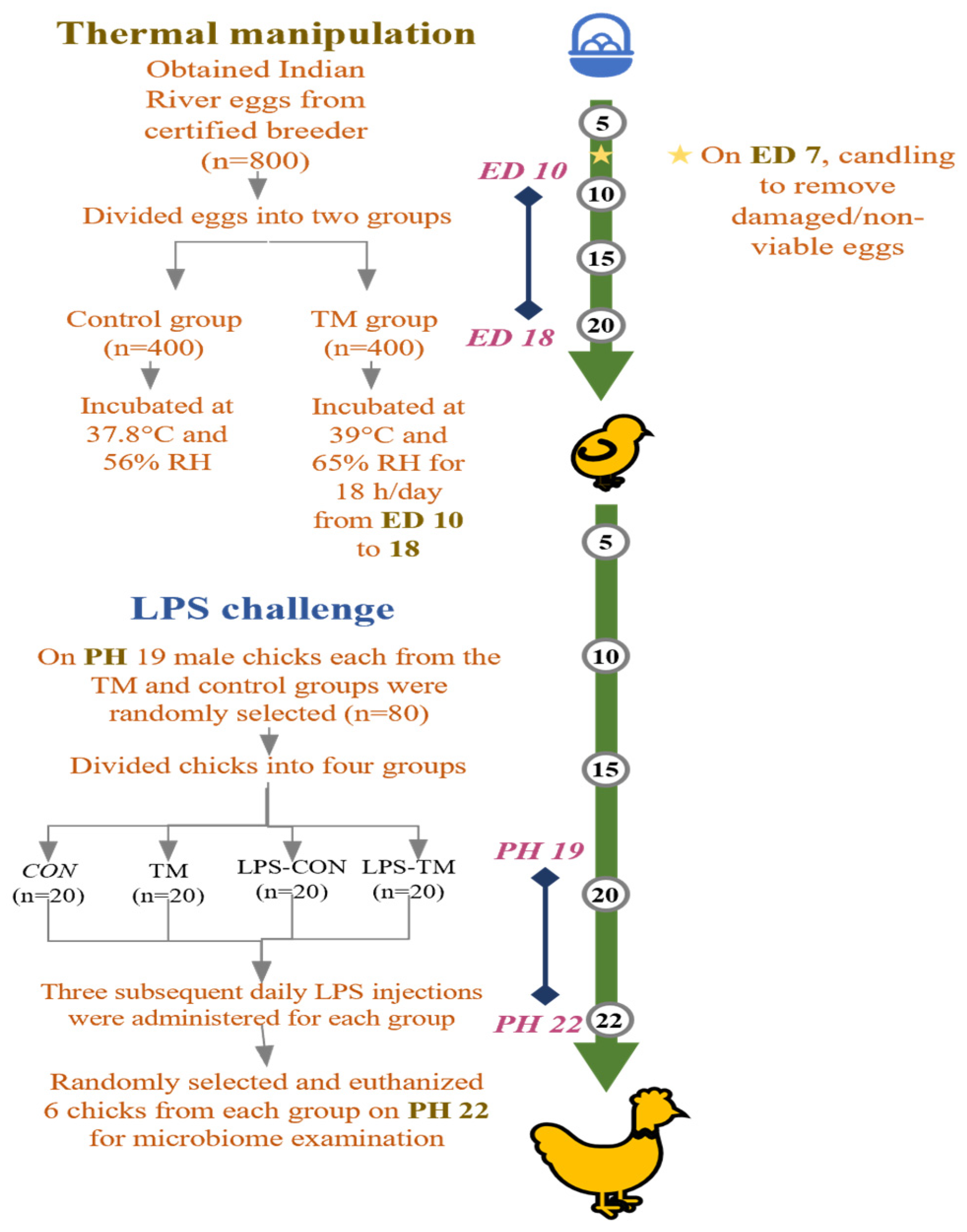
2.1. Study Population and Incubation
2.2. Hatching Management and Post-Hatching Rearing
2.3. LPS Challenge
2.4. Microbiological Analysis
2.4.1. DNA Isolation and Sequencing
2.4.2. Data Analysis by Bioinformatics Tools
2.5. Statistical Analyses
3. Results
3.1. Effects of TM and LPS on Alpha Diversity
3.2. Rarefaction Curve Analysis
3.3. Effects of TM and LPS on Beta Diversity
3.4. Effects of TM and LPS on Cecal Microbiota: Phylum-Level Composition
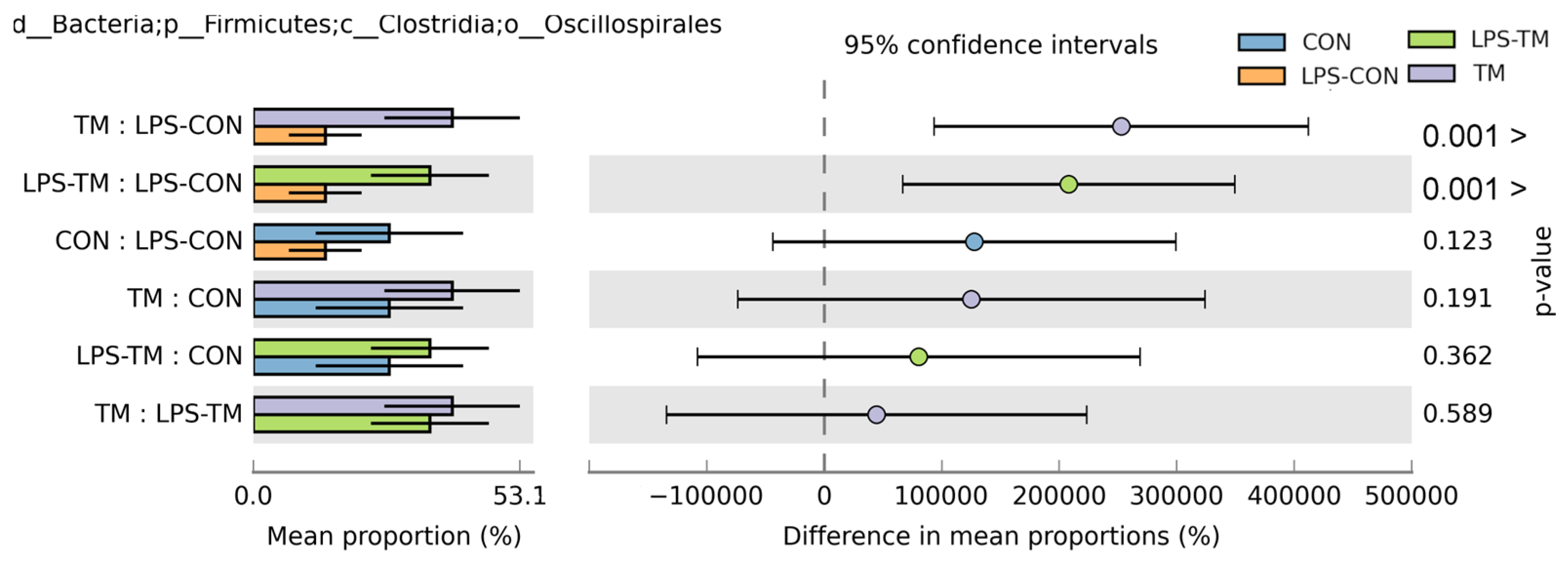
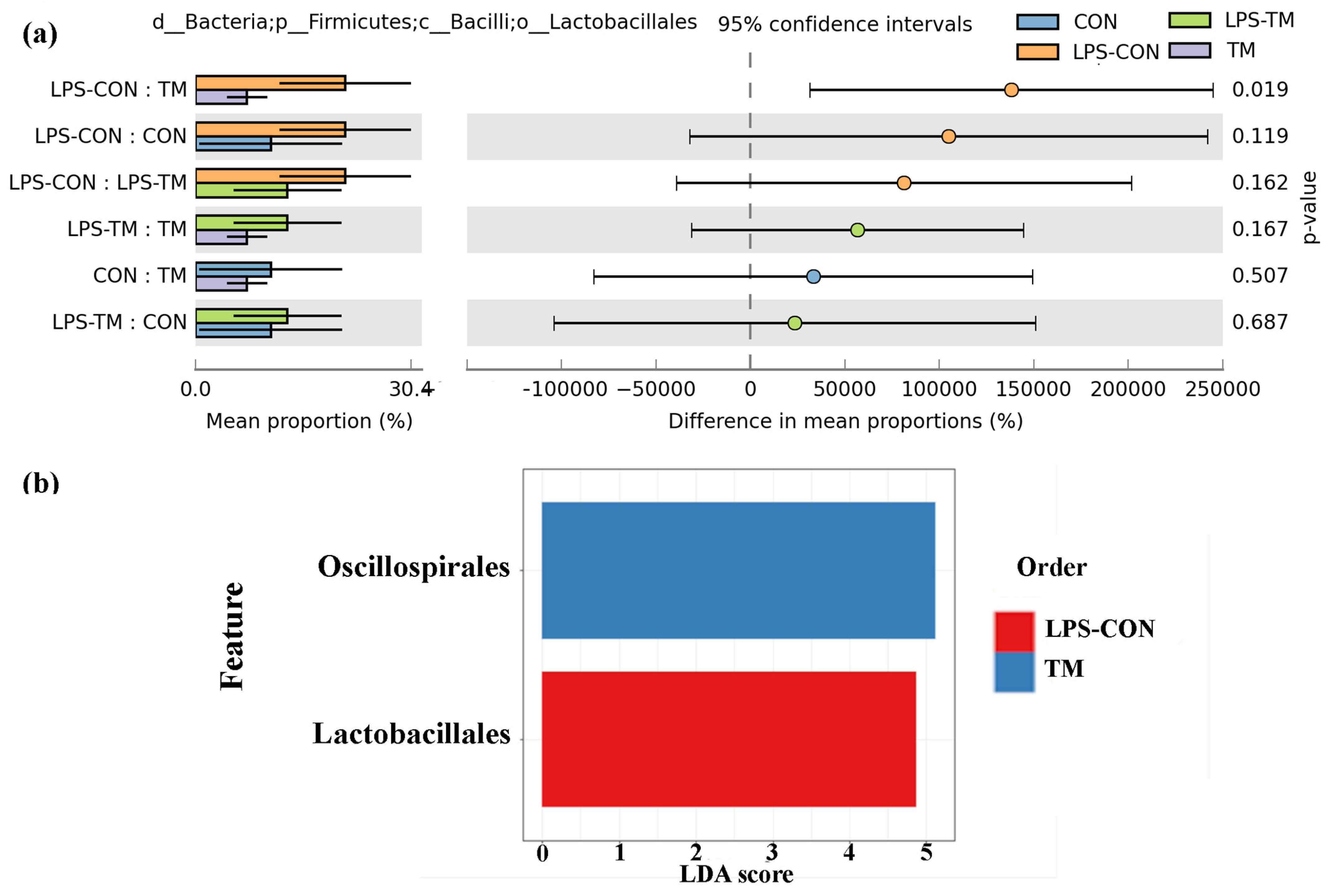
3.5. Effects of TM and LPS on Cecal Microbiota: Order-Level Composition
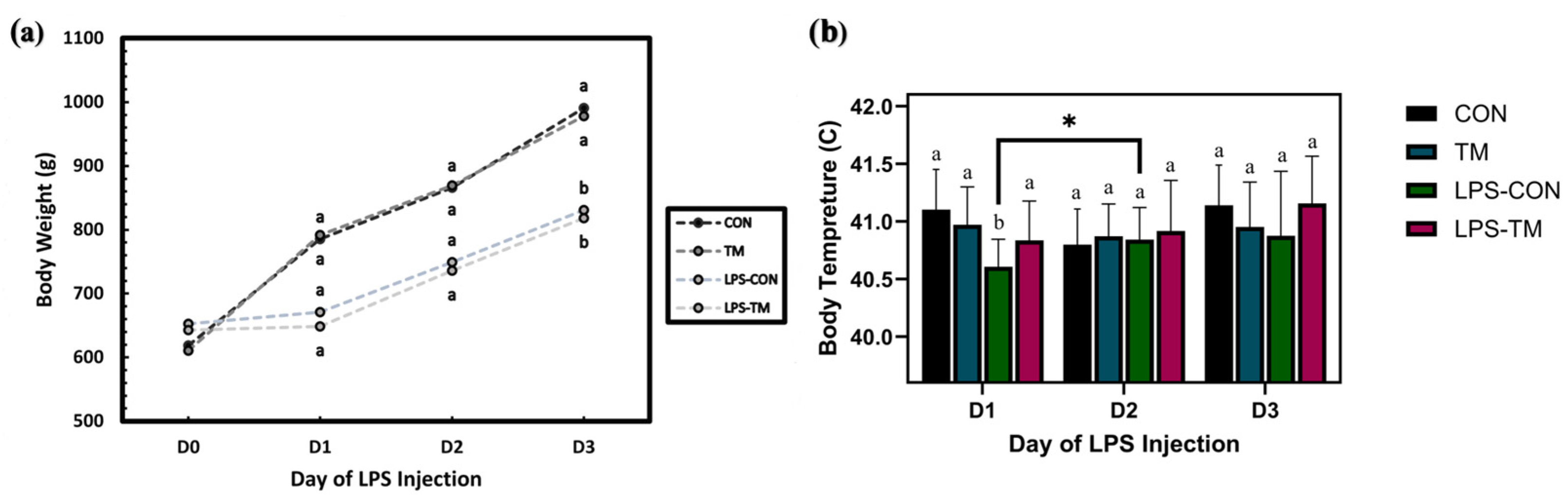
3.6. TM and LPS Challenge Effects on Body Temperature (BT)
3.7. TM and LPS Challenge Effects on Body Weight (BW)
4. Discussion
5. Conclusions
6. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TM | Thermal manipulation |
| CON | Control |
| LPS | Lipopolysaccharide |
| LPS-CON | Control with lipopolysaccharide challenge |
| LPS-TM | Thermal manipulation with lipopolysaccharide challenge |
| RH | Relative humidity |
| ED | Embryonic day |
| PH | Post-hatch |
| ASV | Amplicon sequencing variant |
| CCA | Canonical correspondence analysis |
| PERMANOVA | Permutational Multivariate Analysis of Variance |
| PERMDISP | Permutational analysis of multivariate dispersions |
References
- FAO. World Food and Agriculture—Statistical Yearbook 2023; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Zou, A.; Nadeau, K.; Wang, P.W.; Lee, J.Y.; Guttman, D.S.; Sharif, S.; Korver, D.R.; Brumell, J.H.; Parkinson, J. Accumulation of genetic variants associated with immunity in the selective breeding of broilers. BMC Genet. 2020, 21, 5. [Google Scholar] [CrossRef]
- Shterzer, N.; Sbehat, Y.; Poudel, B.; Rothschild, N.; Oloko, O.E.; Headrick, J.; Petersen, E.; Druyan, S.; Mills, E. Differences in gut bacterial community composition between modern and slower-growing broiler breeder lines: Implications of growth selection on microbiome composition. Front. Physiol. 2023, 14, 1151151. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, J.P.; Wilkie, D.C.; Van Kessel, A.G.; Drew, M.D. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim. Feed. Sci. Technol. 2006, 129, 60–88. [Google Scholar] [CrossRef]
- Nisha, A. Antibiotic residues—A global health hazard. Vet. World 2008, 1, 375. [Google Scholar] [CrossRef]
- O’Neill, J. Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations. Rev. Antimicrob. Resist. 2014. [Google Scholar]
- Kisliouk, T.; Ziv, M.; Meiri, N. Epigenetic control of translation regulation: Alterations in histone H3 lysine 9 post-translation modifications are correlated with the expression of the translation initiation factor 2B (Eif2b5) during thermal control establishment. Dev. Neurobiol. 2010, 70, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Yahav, S.; Collin, A.; Shinder, D.; Picard, M. Thermal manipulations during broiler chick embryogenesis: Effects of timing and temperature. Poult. Sci. 2004, 83, 1959–1963. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; Jaradat, Z.W.; Ababneh, M.M.; Okour, M.Z.; Saleh, K.M.M.; Alkofahi, A.; Alboom, M.H. Effects of embryonic thermal manipulation on the immune response to post-hatch Escherichia coli challenge in broiler chicken. Vet. World. 2023, 16, 918–928. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; El-Bahr, S.M. Thermal manipulation of the broilers embryos: Expression of muscle markers genes and weights of body and internal organs during embryonic and post-hatch days. BMC. Vet. Res. 2019, 15, 166. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; Alliftawi, A.R.S.; Saleh, K.M.M.; Jaradat, Z.W. Expression of digestive enzyme and intestinal transporter genes during chronic heat stress in the thermally manipulated broiler chicken. Poult. Sci. 2019, 98, 4113–4122. [Google Scholar] [CrossRef]
- Loyau, T.; Bedrani, L.; Berri, C.; Metayer-Coustard, S.; Praud, C.; Coustham, V.; Mignon-Grasteau, S.; Duclos, M.J.; Tesseraud, S.; Rideau, N.; et al. Cyclic variations in incubation conditions induce adaptive responses to later heat exposure in chickens: A review. Animal 2015, 9, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Li, D.; Jiao, H.C.; Zhao, J.P.; Lin, H. Lipopolysaccharide inhibits hypothalamic Agouti-related protein gene expression via activating mechanistic target of rapamycin signaling in chicks. Gen. Comp. Endocrinol. 2021, 313, 113876. [Google Scholar] [CrossRef] [PubMed]
- Al-Zghoul, M.d.B.; Gerrard, D.; Dalloul, R. Dynamics of cytokines and heat shock proteins mRNA expression in thermally manipulated chicken challenged with intraperitoneally injection of Lipopolysaccharide (LPS). FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Wick, M.; Lilburn, M.S. Effect of embryonic thermal manipulation on heat shock protein 70 (HSP70) expression and subsequent immune response to post-hatch lipopolysaccharide challenge in Pekin ducklings. Poult. Sci. 2019, 98, 722–733. [Google Scholar] [CrossRef]
- Clavijo, V.; Florez, M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: A review. Poult. Sci. 2018, 97, 1006–1021. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, F.T.; Ding, J.; Zhou, H.; Xu, K.; He, C.; Han, C.; Zheng, Y.; Luo, H.; Yang, K.; Gu, C.; et al. Dynamic distribution of gut microbiota during embryonic development in chicken. Poult. Sci. 2020, 99, 5079–5090. [Google Scholar] [CrossRef]
- Uni, Z.; Gal-Garber, O.; Geyra, A.; Sklan, D.; Yahav, S. Changes in Growth and Function of Chick Small Intestine Epithelium Due to Early Thermal Conditioning. Poult. Sci. 2001, 80, 438–445. [Google Scholar] [CrossRef]
- Amaz, S.A.; Chaudhary, A.; Mahato, P.L.; Jha, R.; Mishra, B. Pre-hatch thermal manipulation of embryos and post-hatch baicalein supplementation mitigated heat stress in broiler chickens. J. Anim. Sci. Biotechnol. 2024, 15, 8. [Google Scholar] [CrossRef]
- Wu, D.; Liang, S.; Du, X.; Xiao, J.; Feng, H.; Ren, Z.; Yang, X.; Yang, X. Effects of fecal microbiota transplantation and fecal virome transplantation on LPS-induced intestinal injury in broilers. Poult. Sci. 2024, 103, 103316. [Google Scholar] [CrossRef]
- Chen, J.Y.; Yu, Y.H. Bacillus subtilis-fermented products ameliorate the growth performance and alter cecal microbiota community in broilers under lipopolysaccharide challenge. Poult. Sci. 2021, 100, 875–886. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Lucke, A.; Doupovec, B.; Zebeli, Q.; Bohm, J. A multicomponent mycotoxin deactivator modifies the response of the jejunal mucosal and cecal bacterial community to deoxynivalenol contaminated feed and oral lipopolysaccharide challenge in chickens1. J. Anim. Sci. 2020, 98, skz377. [Google Scholar] [CrossRef] [PubMed]
- Al-Zghoul, M.B.; Mohammad Saleh, K.M. Effects of thermal manipulation of eggs on the response of jejunal mucosae to posthatch chronic heat stress in broiler chickens. Poult. Sci. 2020, 99, 2727–2735. [Google Scholar] [CrossRef] [PubMed]
- Al-Zghoul, M.B.; Sukker, H.; Ababneh, M.M. Effect of thermal manipulation of broilers embryos on the response to heat-induced oxidative stress. Poult. Sci. 2019, 98, 991–1001. [Google Scholar] [CrossRef]
- Shakouri, M.D.; Malekzadeh, M. Responses of broiler chickens to the nutrient recommendations of NRC (1994) and the Ross broiler management manual. Rev. Colom. Cienc. Pecua. 2016, 29, 91–98. [Google Scholar]
- Aviagen. Indian River Broiler Management Handbook. Available online: https://www.scribd.com/document/638577219/Untitled (accessed on 25 March 2024).
- Wu, Y.; Li, Q.; Liu, J.; Liu, Y.; Xu, Y.; Zhang, R.; Yu, Y.; Wang, Y.; Yang, C. Integrating Serum Metabolome and Gut Microbiome to Evaluate the Benefits of Lauric Acid on Lipopolysaccharide- Challenged Broilers. Front. Immunol. 2021, 12, 759323. [Google Scholar] [CrossRef]
- Zheng, Y.W.; Zhang, J.Y.; Zhou, H.B.; Guo, Y.P.; Ma, Q.G.; Ji, C.; Zhao, L.H. Effects of dietary pyrroloquinoline quinone disodium supplementation on inflammatory responses, oxidative stress, and intestinal morphology in broiler chickens challenged with lipopolysaccharide. Poult. Sci. 2020, 99, 5389–5398. [Google Scholar] [CrossRef]
- Pichler, M.; Coskun, O.K.; Ortega-Arbulu, A.S.; Conci, N.; Worheide, G.; Vargas, S.; Orsi, W.D. A 16S rRNA gene sequencing and analysis protocol for the Illumina MiniSeq platform. Microbiologyopen 2018, 7, e00611. [Google Scholar] [CrossRef]
- QIAGEN. DNeasy® PowerSoil® Pro Kit Handbook; QIAGEN: Hilden, Germany, 2023; Available online: https://www.qiagen.com/jp/resources/download.aspx?id=9bb59b74-e493-4aeb-b6c1-f660852e8d97&lang=en (accessed on 25 March 2024).
- Weinroth, M.D.; Oakley, B.; Ramirez, G.A.; Reyes, A.; Harris, C.E.; Buhr, R.J. 16S rRNA gene-based assessment of common broiler chicken sampling methods: Evaluating intra-flock sample size, cecal pair similarity, and cloacal swab similarity to other alimentary tract locations. Front. Physiol. 2022, 13, 996654. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J. Alpha Diversity. In Bioinformatic and Statistical Analysis of Microbiome Data; Xia, Y., Sun, J., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 289–333. [Google Scholar]
- Willis, A.D. Rarefaction, Alpha Diversity, and Statistics. Front. Microbiol. 2019, 10, 2407. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Kers, J.G.; Saccenti, E. The power of microbiome studies: Some considerations on which alpha and beta metrics to use and how to report results. Front. Microbiol. 2022, 12, 796025. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Cui, L.Y.; Hu, Z.F.; Du, X.P.; Abid, H.M.; Wang, H.B. Environmental and host factors shaping the gut microbiota diversity of brown frog Rana dybowskii. Sci. Total Environ. 2020, 741, 140142. [Google Scholar] [CrossRef] [PubMed]
- de Jong, I.C.; Schokker, D.; Gunnink, H.; van Wijhe, M.; Rebel, J.M.J. Early life environment affects behavior, welfare, gut microbiome composition, and diversity in broiler chickens. Front. Vet. Sci. 2022, 9, 977359. [Google Scholar] [CrossRef]
- David, S.A.; Vitorino Carvalho, A.; Gimonnet, C.; Brionne, A.; Hennequet-Antier, C.; Piegu, B.; Crochet, S.; Courousse, N.; Bordeau, T.; Bigot, Y.; et al. Thermal Manipulation During Embryogenesis Impacts H3K4me3 and H3K27me3 Histone Marks in Chicken Hypothalamus. Front. Genet. 2019, 10, 1207. [Google Scholar] [CrossRef]
- Dunislawska, A.; Pietrzak, E.; Wishna Kadawarage, R.; Beldowska, A.; Siwek, M. Pre-hatching and post-hatching environmental factors related to epigenetic mechanisms in poultry. J. Anim. Sci. 2021, 100, skab370. [Google Scholar] [CrossRef]
- Sikorska, M.; Siwek, M.; Slawinska, A.; Dunislawska, A. miRNA Profiling in the Chicken Liver under the Influence of Early Microbiota Stimulation with Probiotic, Prebiotic, and Synbiotic. Genes 2021, 12, 685. [Google Scholar] [CrossRef]
- Dunislawska, A.; Slawinska, A.; Stadnicka, K.; Bednarczyk, M.; Gulewicz, P.; Jozefiak, D.; Siwek, M. Synbiotics for broiler chickens—In Vitro design and evaluation of the influence on host and selected microbiota populations following in ovo delivery. PLoS ONE 2017, 12, e0168587. [Google Scholar] [CrossRef]
- Trevisi, P.; Luise, D.; Correa, F.; Bosi, P. Timely Control of Gastrointestinal Eubiosis: A Strategic Pillar of Pig Health. Microorganisms 2021, 9, 313. [Google Scholar] [CrossRef]
- Diaz Carrasco, J.M.; Casanova, N.A.; Fernández Miyakawa, M.E. Microbiota, Gut Health and Chicken Productivity: What Is the Connection? Microorganisms 2019, 7, 374. [Google Scholar] [CrossRef]
- Timmerman, H.M.; Koning, C.J.; Mulder, L.; Rombouts, F.M.; Beynen, A.C. Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. Int. J. Food Microbiol. 2004, 96, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Luo, L.; Wang, X.; Wen, Q.; Zhou, L.; Wu, K. Characterization of the cecal microbiome composition of Wenchang chickens before and after fattening. PLoS ONE 2019, 14, e0225692. [Google Scholar] [CrossRef]
- Mancabelli, L.; Ferrario, C.; Milani, C.; Mangifesta, M.; Turroni, F.; Duranti, S.; Lugli, G.A.; Viappiani, A.; Ossiprandi, M.C.; van Sinderen, D.; et al. Insights into the biodiversity of the gut microbiota of broiler chickens. Environ. Microbiol. 2016, 18, 4727–4738. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Ge, Y.; Xu, Z.; Zhang, D.; Li, D. The digestive and reproductive tract microbiotas and their association with body weight in laying hens. Poult. Sci. 2021, 100, 101422. [Google Scholar] [CrossRef]
- Polansky, O.; Sekelova, Z.; Faldynova, M.; Sebkova, A.; Sisak, F.; Rychlik, I. Important metabolic pathways and biological processes expressed by chicken cecal microbiota. Appl. Environ. Microbiol. 2016, 82, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ma, S.; Farooq, U.; Liu, B.; Wang, Z.; Sun, H.; Cui, Y.; Li, D.; Shi, Y. Chronological dynamics of the gut microbiome in response to the pasture grazing system in geese. Microbiol. Spectr. 2024, 12, e0418823. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wen, Z.; Liu, W.; Meng, L.; Huang, H. Oscillospira—A candidate for the next-generation probiotics. Gut Microbes 2021, 13, 1987783. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.P.; Zhang, Y.N.; Li, Y.; Gu, L.T.; Sun, H.H.; Liu, M.D.; Zhou, H.L.; Wang, Y.S.; Xu, Z.X. Short-chain fatty acids in diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef]
- Wlazło, Ł.; Kowalska, D.; Bielański, P.; Ossowski, M.; Czech, A.; Łukaszewicz, M.; Nowakowicz-Dębek, B. Study of the Possibility of Modulating the Composition of the Gastrointestinal Microbiome of Rabbits Fed Fermented Rapeseed Meal. Ann. Anim. Sci. 2025, 25, 271–279. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Umar, M.; Hassan, F.-u.; Alagawany, M.; Arif, M.; Taha, A.E.; Elnesr, S.S.; El-Tarabily, K.A.; Abd El-Hack, M.E. Applications of butyric acid in poultry production: The dynamics of gut health, performance, nutrient utilization, egg quality, and osteoporosis. Anim. Health Res. Rev. 2022, 23, 136–146. [Google Scholar] [CrossRef]
- Zhang, W.H.; Jiang, Y.; Zhu, Q.; Gao, F.; Dai, S.; Chen, J.; Zhou, G. Sodium butyrate maintains growth performance by regulating the immune response in broiler chickens. Br. Poult. Sci. 2011, 52, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Kruh, J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol. Cell. Biochem. 1981, 42, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, K.E.; Al-Zghoul, M.B.; Saleh, K.M.M. Molecular and morphometric changes in the small intestine during hot and cold exposure in thermally manipulated broiler chickens. Vet. World 2021, 14, 1511. [Google Scholar] [CrossRef] [PubMed]
- Gałęcka, I.; Rychlik, A.; Całka, J. Influence of selected dosages of plastic microparticles on the porcine fecal microbiome. Sci. Rep. 2025, 15, 1269. [Google Scholar] [CrossRef]
- De Boever, S.; Croubels, S.; Meyer, E.; Sys, S.; Beyaert, R.; Ducatelle, R.; De Backer, P. Characterization of an intravenous lipopolysaccharide inflammation model in broiler chickens. Avian Pathol. 2009, 38, 403–411. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, J.; Pan, L.; Zhang, Y. Roles and applications of probiotic Lactobacillus strains. Appl. Microbiol. Biotechnol. 2018, 102, 8135–8143. [Google Scholar] [CrossRef]
- Bartley, A.; Yang, T.; Arocha, R.; Malphurs, W.L.; Larkin, R.; Magee, K.L.; Vickroy, T.W.; Zubcevic, J. Increased Abundance of Lactobacillales in the Colon of Beta-Adrenergic Receptor Knock Out Mouse Is Associated With Increased Gut Bacterial Production of Short Chain Fatty Acids and Reduced IL17 Expression in Circulating CD4+ Immune Cells. Front. Physiol. 2018, 9, 1593. [Google Scholar] [CrossRef]
- Pérez-Santiago, J.; Gianella, S.; Massanella, M.; Spina, C.A.; Karris, M.Y.; Var, S.R.; Patel, D.; Jordan, P.S.; Young, J.A.; Little, S.J.; et al. Gut Lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS 2013, 27, 1921–1931. [Google Scholar] [CrossRef]
- Ludwig, W.; Schleifer, K.-H.; Whitman, W.B. Lactobacillales ord. nov. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons: Hoboken, NJ, USA, 2015; p. 1. [Google Scholar]
- Alakomi, H.-L.; Skytta, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef]
- Alaqil, A. The Effect of Lactobacillus Acidophilus on Alleviating Stress Response and Production Impairment Induced by Escherichia Coli Lipopolysaccharide in Laying Hens. Adv. Anim. Vet. Sci. 2023, 11, 1183–1192. [Google Scholar] [CrossRef]
- Dai, X.; Hackmann, T.J.; Lobo, R.R.; Faciola, A.P. Lipopolysaccharide Stimulates the Growth of Bacteria That Contribute to Ruminal Acidosis. Appl. Environ. Microbiol. 2020, 86, e02193-19. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Iraqi, E.; Hady, A.A.; Elsayed, N.; Khalil, H.; El-Saadany, A.; El-Sabrout, K. Effect of thermal manipulation on embryonic development, hatching process, and chick quality under heat-stress conditions. Poult. Sci. 2024, 103, 103257. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203. [Google Scholar] [PubMed]
- Tetel, M.J.; de Vries, G.J.; Melcangi, R.C.; Panzica, G.; O’Mahony, S.M. Steroids, stress and the gut microbiome-brain axis. J. Neuroendocrinol. 2018, 30, e12548. [Google Scholar] [CrossRef] [PubMed]
- Black, B.L. Influence of hormones on glycogen and glucose metabolism in embryonic chick intestine. Am. J. Physiol. 1988, 254, G65–G73. [Google Scholar] [CrossRef]
- Chotinsky, D.; Mihaylov, R. Effect of probiotics and avotan on the level of thyroid hormones in the blood plasma of broiler chickens. Bulgarian J. Agric. Sci. 2013, 19, 817–821. [Google Scholar]
- Li, N.; Ansari, A.R.; Sun, Z.; Huang, H.; Cui, L.; Hu, Y.; Zhao, X.; Zhong, J.; Abdel-Kafy, E.M.; Liu, H. Toll like receptor 4 signaling pathway participated in Salmonella lipopolysaccharide-induced spleen injury in young chicks. Microb. Pathog. 2017, 112, 288–294. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; Al-Natour, M.; Dalab, A.; Alturki, O.; Althnaian, T.; Al-Ramadan, S.; Hannon, K.; El-Bahr, S. Thermal manipulation mid-term broiler chicken embryogenesis: Effect on muscle growth factors and muscle marker genes. Braz. J. Poult. Sci. 2016, 18, 607–618. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Wang, K.; Liu, L.; Chen, K.; Shan, W.; Liu, L.; Kahiel, M.; Li, C. Embryo thermal manipulation enhances mitochondrial function in the skeletal muscle of heat-stressed broilers by regulating transient receptor potential V2 expression. Poult. Sci. 2024, 103, 104034. [Google Scholar] [CrossRef] [PubMed]
- Dalab, A.S.; Ali, A.M.; Althnaian, T.A.; Alkhodair, K.M.; Al-Ramadan, S.Y. Molecular and ultrastructural investigations of the effect of thermal manipulation during embryogenesis on pectoral and thigh muscles growth factors in broilers. J. Appl. Poult. Res. 2022, 31, 100188. [Google Scholar] [CrossRef]
- Piestun, Y.; Harel, M.; Barak, M.; Yahav, S.; Halevy, O. Thermal manipulations in late-term chick embryos have immediate and longer term effects on myoblast proliferation and skeletal muscle hypertrophy. J. Appl. Physiol. 2009, 106, 233–240. [Google Scholar] [CrossRef]
- Piestun, Y.; Yahav, S.; Halevy, O. Thermal manipulation during embryogenesis affects myoblast proliferation and skeletal muscle growth in meat-type chickens. Poult. Sci. 2015, 94, 2528–2536. [Google Scholar] [CrossRef] [PubMed]
- Tzschentke, B.; Halle, I. Influence of temperature stimulation during the last 4 days of incubation on secondary sex ratio and later performance in male and female broiler chicks. Br. Poult. Sci. 2009, 50, 634–640. [Google Scholar] [CrossRef]
- Collin, A.; Berri, C.; Tesseraud, S.; Rodon, F.E.; Skiba-Cassy, S.; Crochet, S.; Duclos, M.J.; Rideau, N.; Tona, K.; Buyse, J.; et al. Effects of thermal manipulation during early and late embryogenesis on thermotolerance and breast muscle characteristics in broiler chickens. Poult. Sci. 2007, 86, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Walstra, I.; Ten Napel, J.; Kemp, B.; van den Brand, H. Temperature manipulation during layer chick embryogenesis. Poult. Sci. 2010, 89, 1502–1508. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2–Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Waste not, want not: Why rarefying microbiome data is inadmissible. PLoS Comput. Biol. 2014, 10, e1003531. [Google Scholar] [CrossRef]
- Schloss, P.D. Waste not, want not: Revisiting the analysis that called into question the practice of rarefaction. Msphere 2024, 9, e00355-23. [Google Scholar] [CrossRef]
- Team RS. RStudio: Integrated Development Environment for R; RStudio Inc.: Boston, MA, USA, 2021. [Google Scholar]
- R Core Team. RA Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Andersen, K.S.; Kirkegaard, R.H.; Karst, S.M.; Albertsen, M. ampvis2: An R package to analyse and visualise 16S rRNA amplicon data. bioRxiv 2018. [CrossRef]
- ter Braak, C.J.F. Canonical Correspondence Analysis: A New Eigenvector Technique for Multivariate Direct Gradient Analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef]
- ter Braak, C.J.F. Ordination; Cambridge University Press: Cambridge, UK, 1995; Volume 5. [Google Scholar]
- Gauch, H.G., Jr.; Whittaker, R.H.; Singer, S.B. A comparative study of nonmetric ordinations. J. Ecol. 1981, 69, 135–152. [Google Scholar] [CrossRef]
- Gauch, H.G., Jr. Noise reduction by eigenvector ordinations. Ecology 1982, 63, 1643–1649. [Google Scholar] [CrossRef]
- Wilmes, P.; Bond, P.L. The application of two-dimensional polyacrylamide gel electrophoresis and downstream analyses to a mixed community of prokaryotic microorganisms. Environ. Microbiol. 2004, 6, 911–920. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Anderson, M.J.; Walsh, D.C. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol. Monogr. 2013, 83, 557–574. [Google Scholar] [CrossRef]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [PubMed]




| Ingredient (% of Diet) | Starter | Grower |
|---|---|---|
| Corn | 56.80 | 64.76 |
| Soybean Meal (CP 44%) | 35.40 | 27.85 |
| Fish Meal | 1.00 | 1.00 |
| Soybean Oil | 2.31 | 1.39 |
| Oyster Shell | 1.34 | 1.84 |
| Dicalcium Phosphate | 1.53 | 1.66 |
| Common Salt | 0.396 | 0.326 |
| Vitamin Premix a | 0.50 | 0.50 |
| Mineral Premix b | 0.50 | 0.50 |
| DL-Methionine | 0.151 | 0.055 |
| L-Lysine HCl | 0.073 | 0.119 |
| Total | 100 | 100 |
| Nutrient Composition | ||
| Nutrient | Starter | Grower |
| AMEn (Kcal/Kg) | 2950 | 2960 |
| Crude Protein (%) | 21.203 | 18.499 |
| Arg (%) | 1.365 | 1.157 |
| Lys (%) | 1.208 | 1.052 |
| Met (%) | 0.490 | 0.360 |
| Met + Cys (%) | 0.832 | 0.666 |
| Ca (%) | 0.997 | 1.200 |
| Available p (%) | 0.453 | 0.467 |
| Na (%) | 0.180 | 0.150 |
| Index | Con | TM | LPS-CON | LPS-TM | p-Value |
|---|---|---|---|---|---|
| Chao1 | 211.56 ± 41.6 | 188.5 ± 47.2 | 197.23 ± 62.96 | 222.1 ± 37.97 | 0.84 |
| Shannon | 3.75 ± 0.25 | 3.76 ± 0.31 | 3.77 ± 0.54 | 3.91 ± 0.34 | 0.43 |
| Pair | F-Value | p-Value |
|---|---|---|
| TM vs. CON | 2.033 | 0.041 |
| TM vs. LPS-CON | 3.4913 | 0.012 |
| TM vs. LPS-TM | 1.5514 | 0.08 |
| CON vs. LPS-CON | 1.0491 | 0.398 |
| CON vs. LPS-TM | 1.3863 | 0.143 |
| LPS-CON vs. LPS-TM | 1.6511 | 0.087 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hundam, S.; Al-Zghoul, M.B.; Ababneh, M.; Alanagreh, L.; Dahadha, R.; Mayyas, M.; Alghizzawi, D.; Mustafa, M.A.; Gerrard, D.E.; Dalloul, R.A. Effects of Embryonic Thermal Manipulation on Body Performance and Cecum Microbiome in Broiler Chickens Following a Post-Hatch Lipopolysaccharide Challenge. Animals 2025, 15, 1149. https://doi.org/10.3390/ani15081149
Hundam S, Al-Zghoul MB, Ababneh M, Alanagreh L, Dahadha R, Mayyas M, Alghizzawi D, Mustafa MA, Gerrard DE, Dalloul RA. Effects of Embryonic Thermal Manipulation on Body Performance and Cecum Microbiome in Broiler Chickens Following a Post-Hatch Lipopolysaccharide Challenge. Animals. 2025; 15(8):1149. https://doi.org/10.3390/ani15081149
Chicago/Turabian StyleHundam, Seif, Mohammad Borhan Al-Zghoul, Mustafa Ababneh, Lo’ai Alanagreh, Rahmeh Dahadha, Mohammad Mayyas, Daoud Alghizzawi, Minas A. Mustafa, David E. Gerrard, and Rami A. Dalloul. 2025. "Effects of Embryonic Thermal Manipulation on Body Performance and Cecum Microbiome in Broiler Chickens Following a Post-Hatch Lipopolysaccharide Challenge" Animals 15, no. 8: 1149. https://doi.org/10.3390/ani15081149
APA StyleHundam, S., Al-Zghoul, M. B., Ababneh, M., Alanagreh, L., Dahadha, R., Mayyas, M., Alghizzawi, D., Mustafa, M. A., Gerrard, D. E., & Dalloul, R. A. (2025). Effects of Embryonic Thermal Manipulation on Body Performance and Cecum Microbiome in Broiler Chickens Following a Post-Hatch Lipopolysaccharide Challenge. Animals, 15(8), 1149. https://doi.org/10.3390/ani15081149






