MicroRNA-30a-3p Influences Milk Fat Metabolism by Targeting PTEN in Mammary Epithelial Cells of Sheep
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Collection of Ovine Tissue Samples
2.3. Isolation and Culture of Ovine MECs
2.4. Cell Viability, Proliferation, and Triglyceride Content Detection
2.5. Target Gene Prediction and Validation
2.6. Relative Expression Analysis
2.7. Statistical Analysis
3. Results
3.1. MiR-30a-3p Is Associated with the Development and Lactation of the Mammary Gland
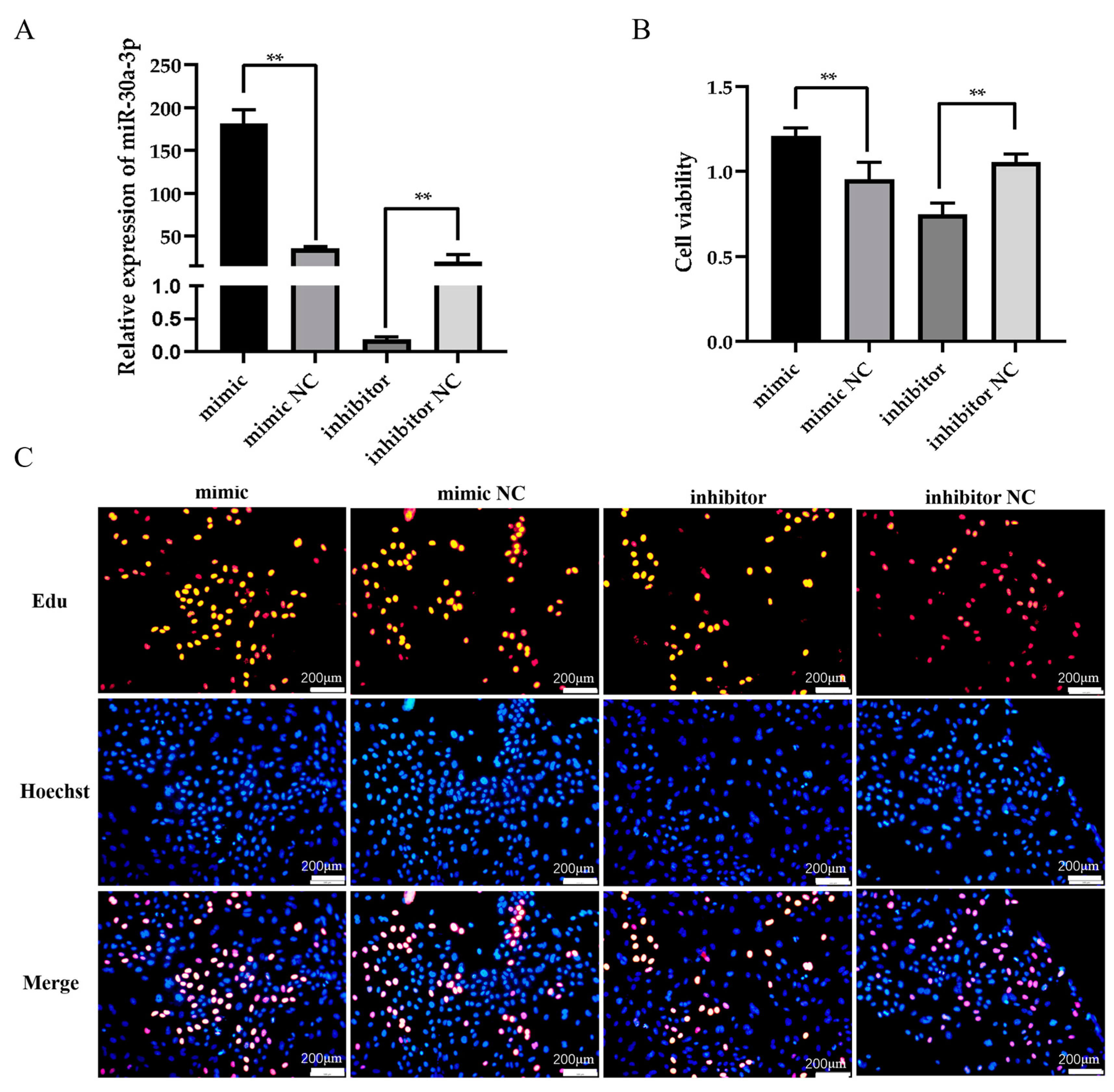
3.2. miR-30a-3p Affects the Proliferation of Ovine MECs
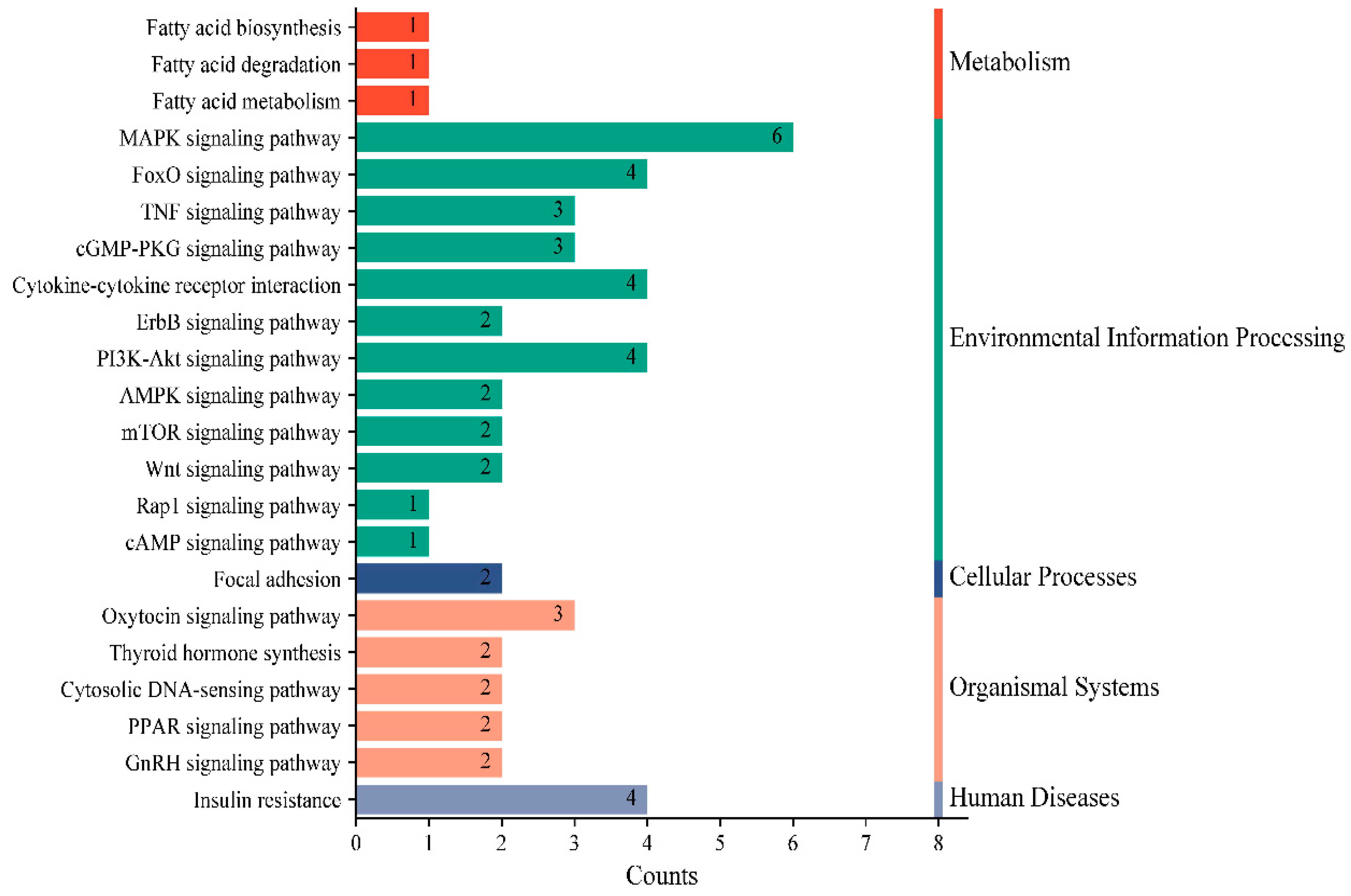
3.3. miR-30a-3p Functional Enrichment Analysis and Target Gene Prediction
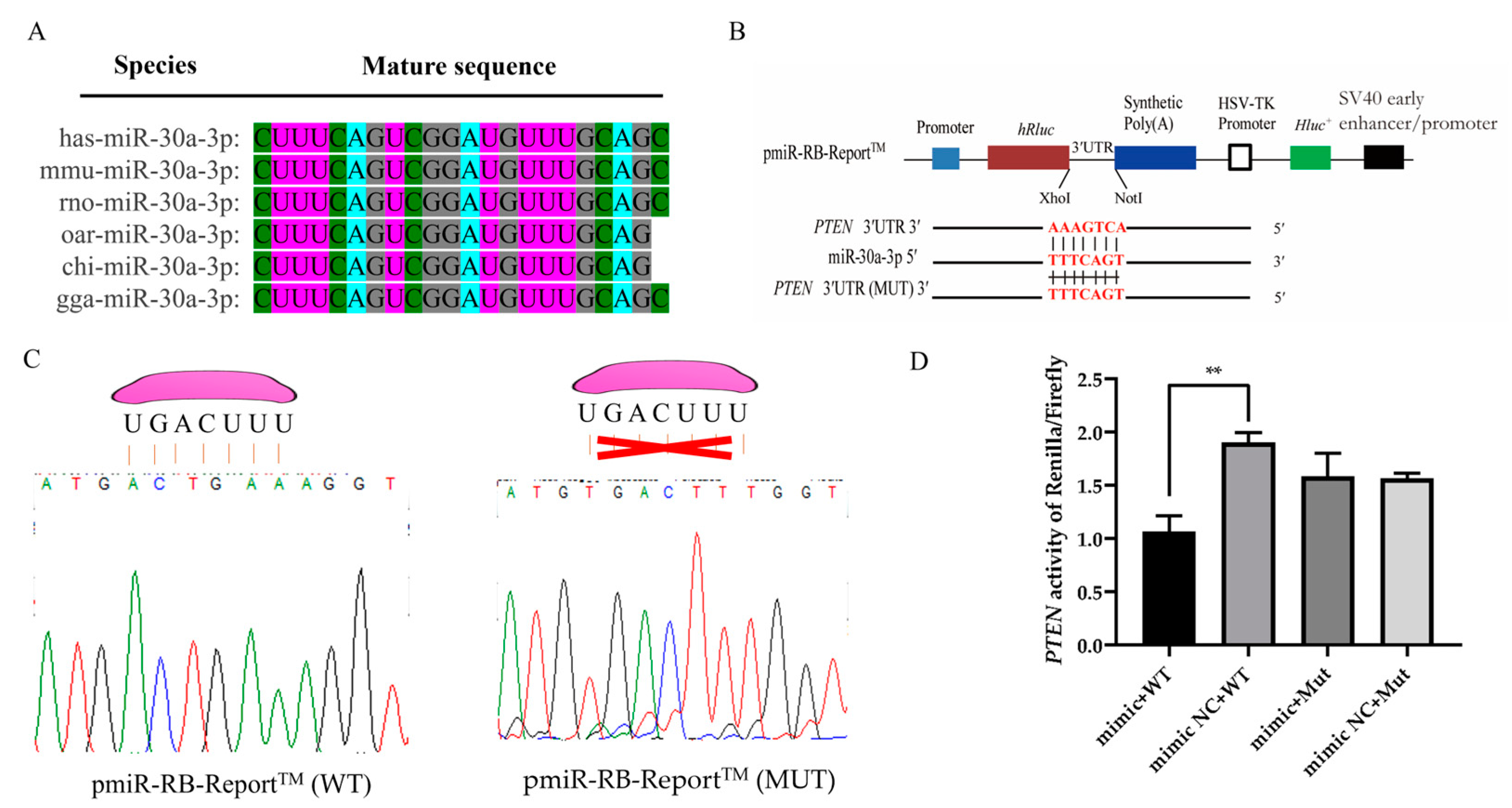
3.4. PTEN Is a Direct Target of miR-30a-3p
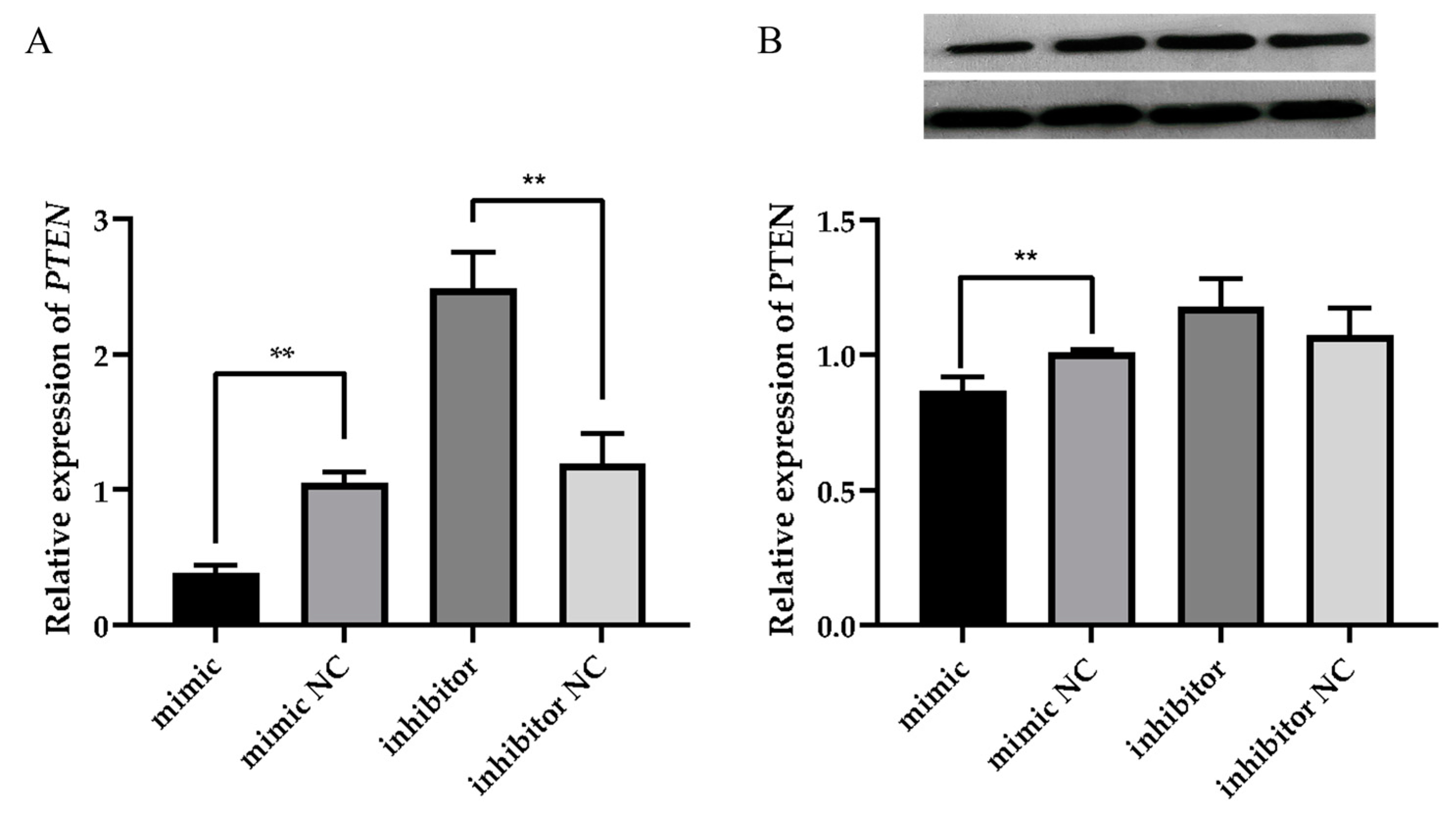
3.5. miR-30a-3p Affects the Milk Fat Synthesis Process by Regulating the Expression of PTEN
4. Discussion
4.1. miR-30a-3p Expression Exhibits Tissue-Specific and Spatiotemporal Specificity
4.2. miR-30a-3p Promotes the Viability and Number of Ovine MECs
4.3. PTEN Is a Direct Target Gene of miR-30a-3p
4.4. MiR-30a-3p Regulates Milk Fat Synthesis by Targeting PTEN in Ovine MECs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.; Zhang, S.S.; Aili, T.; Yang, J.; Jia, Z.H.; Wang, J.J.; Li, H.Y.; Bai, L.L.; Lv, X.Y.; Huang, X.H. Dual signal light detection of beta-lactoglobulin based on a porous silicon bragg mirror. Biosens. Bioelectron. 2022, 204, 114035. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.S.; Wu, H.; Wang, Z.X.; Zhu, J.P.; Wang, W.J.; Wang, J.H.; Wang, Y.M.; Wang, C. Rumen-protected lysine supplementation improved amino acid balance, nitrogen utilization and altered hindgut microbiota of dairy cows. Anim. Nutr. 2023, 15, 320–331. [Google Scholar] [CrossRef]
- Xiong, J.L.; Chen, F.Y.; Zhang, J.; Ao, W.P.; Zhou, X.L.; Yang, H.; Wu, Z.Y.; Wu, L.Y.; Wang, C.; Qiu, Y.S. Occurrence of aflatoxin m1 in three types of milk from xinjiang, China, and the risk of exposure for milk consumers in different age-sex groups. Foods 2022, 11, 3922. [Google Scholar] [CrossRef]
- Xiong, J.L.; Wen, D.F.; Zhou, H.L.; Chen, R.; Wang, H.; Wang, C.; Wu, Z.Y.; Qiu, Y.S.; Wu, L.Y. Occurrence of aflatoxin M1 in yogurt and milk in central-eastern China and the risk of exposure in milk consumers. Food Control 2022, 137, 108928. [Google Scholar] [CrossRef]
- Akers, R.M. A 100-Year Review: Mammary development and lactation. J. Dairy Sci. 2017, 100, 10332–10352. [Google Scholar] [CrossRef]
- Ames, S.R.; Lotoski, L.C.; Azad, M.B. Comparing early life nutritional sources and human milk feeding practices: Personalized and dynamic nutrition supports infant gut microbiome development and immune system maturation. Gut Microbes 2023, 15, 2190305. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.J.; Stafford, K.J. Animal welfare implications of neonatal mortality and morbidity in farm animals. Vet. J. 2004, 168, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Danso, A.S.; Morel, P.C.; Kenyon, P.R.; Blair, H.T. Relationships between prenatal ewe traits, milk production, and preweaning performance of twin lambs. J. Anim. Sci. 2016, 94, 3527–3539. [Google Scholar] [CrossRef]
- Morgan, J.E.; Fogarty, N.M.; Nielsen, S.; Gilmour, A.R. The relationship of lamb growth from birth to weaning and the milk production of their primiparous crossbred dams. Aust. J. Exp. Agric. 2007, 47, 899–904. [Google Scholar] [CrossRef]
- Miglior, F.; Fleming, A.; Malchiodi, F.; Brito, L.F.; Martin, P.; Baes, C.F. A 100-Year Review: Identification and genetic selection of economically important traits in dairy cattle. J. Dairy Sci. 2017, 100, 10251–10271. [Google Scholar] [CrossRef]
- Ivanova, E.; Le Guillou, S.; Hue-Beauvais, C.; Le Provost, F. Epigenetics: New insights into mammary gland biology. Genes 2021, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Weiss, W.P. A 100-Year Review: From ascorbic acid to zinc-Mineral and vitamin nutrition of dairy cows. J. Dairy Sci. 2017, 100, 10045–10060. [Google Scholar] [CrossRef]
- Chen, W.H.; Gu, X.Y.; Lv, X.Y.; Cao, X.K.; Yuan, Z.H.; Wang, S.H.; Sun, W. Non-coding transcriptomic profiles in the sheep mammary gland during different lactation periods. Front. Vet. Sci. 2022, 9, 983562. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Kim, V.N. Small and long non-coding RNAs: Past, present, and future. Cell 2024, 187, 6451–6485. [Google Scholar] [CrossRef]
- Wang, H.; Shi, H.P.; Luo, J.; Yi, Y.Q.; Yao, D.W.; Zhang, X.Y.; Ma, G.Z.; Loor, J.J. MiR-145 regulates lipogenesis in goat mammary cells via targeting INSIG1 and epigenetic regulation of lipid-related genes. J. Cell Physiol. 2017, 232, 1030–1040. [Google Scholar] [CrossRef]
- Lu, Q.Y.; Chen, Z.; Ji, D.J.; Mao, Y.J.; Jiang, Q.M.; Yang, Z.P.; Loor, J.J. Progress on the regulation of ruminant milk fat by noncoding RNAs and ceRNAs. Front. Genet. 2021, 12, 733925. [Google Scholar] [CrossRef]
- Duman, E.; Özmen, Ö.; Kul, S. Oar-miR-16b and oar-miR-27a: Negatively correlated with milk yield and milk protein in sheep. Anim. Biotechnol. 2022, 33, 1466–1479. [Google Scholar] [CrossRef]
- Hao, Z.; Wang, J.; Luo, Y.; Hu, J.; Liu, X.; Li, S.; Li, M.; Shi, B.; Hu, L.; Liu, Y.; et al. MicroRNA-200c affects milk fat synthesis by targeting PANK3 in ovine mammary epithelial cells. Int. J. Mol. Sci. 2022, 23, 15601. [Google Scholar] [CrossRef]
- Tian, L.; Li, Y.; Wang, C.; Li, Q. Let-7g-5p regulates mouse mammary cells differentiation and function by targeting PRKCA. J. Cell Physiol. 2019, 234, 10101–10110. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, L.; Cui, Y.; Li, H.; Xie, X.; Li, Y.; Wang, C. miR-142-3p regulates milk synthesis and structure of murine mammary glands via PRLR-mediated multiple signaling pathways. J. Agric. Food Chem. 2019, 67, 9532–9542. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.Y.; Zhou, H.T.; Hickford, J.G.H.; Gong, H.; Wang, J.Q.; Hu, J.; Liu, X.; Li, S.B.; Zhao, M.L.; Luo, Y.Z. Transcriptome profile analysis of mammary gland tissue from two breeds of lactating sheep. Genes 2019, 10, 781. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Zhou, H.T.; Hickford, J.G.H.; Hao, Z.Y.; Shen, J.Y.; Luo, Y.Z.; Hu, J.; Liu, X.; Li, S.B. Comparison of the transcriptome of the ovine mammary gland in lactating and non-lactating small-tailed Han sheep. Front. Genet. 2020, 21, 472–483. [Google Scholar] [CrossRef]
- Wang, J.Q.; Hao, Z.Y.; Hu, J.; Liu, X.; Li, S.B.; Shen, J.Y.; Song, Y.Z.; Ke, N.; Luo, Y.Z. Small RNA deep sequencing reveals the expressions of microRNAs in ovine mammary gland development at peak-lactation and during the non-lactating period. Genomics 2021, 113, 637–646. [Google Scholar] [CrossRef]
- Tian, L.; Guo, S.; Zhao, Z.; Chen, Y.; Wang, C.; Li, Q.; Li, Y. miR-30a-3p regulates autophagy in the involution of mice mammary glands. Int. J. Mol. Sci. 2023, 24, 14352. [Google Scholar] [CrossRef]
- Cai, X.; Liu, Q.; Zhang, X.; Ren, Y.; Lei, X.; Li, S.; Chen, Q.; Deng, K.; Wang, P.; Zhang, H.; et al. Identification and analysis of the expression of microRNA from lactating and nonlactating mammary glands of the Chinese swamp buffalo. J. Dairy Sci. 2017, 100, 1971–1986. [Google Scholar] [CrossRef] [PubMed]
- Norgaard, J.V.; Nielsen, M.O.; Theil, P.K.; Sorensen, M.T.; Safayi, S.; Sejrsen, K. Development of mammary glands of fat sheep submitted to restricted feeding during late pregnancy. Small Rumin. Res. 2008, 76, 155–165. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR. Methods 2002, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, J.; Sun, S.; Cao, D.; Shi, H.B.; Loor, J.J. miR-148a and miR-17-5p synergistically regulate milk TAG synthesis via PPARGC1A and PPARA in goat mammary epithelial cells. RNA Biol. 2017, 14, 326–338. [Google Scholar] [CrossRef]
- Wang, H.; Luo, J.; Zhang, T.; Tian, H.; Ma, Y.; Xu, H.; Yao, D.; Loor, J.J. MicroRNA-26a/b and their host genes synergistically regulate triacylglycerol synthesis by targeting the INSIG1 gene. RNA Biol. 2016, 13, 500–510. [Google Scholar] [CrossRef]
- Cataldo, A.; Cheung, D.G.; Hagan, J.P.; Fassan, M.; Sandhu-Deol, S.; Croce, C.M.; Di Leva, G.; Iorio, M.V. Genetic loss of miR-205 causes increased mammary gland development. Non-Coding RNA 2023, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Berezikov, E.; Guryev, V.; van de Belt, J.; Wienholds, E.; Plasterk, R.H.; Cuppen, E. Phylogenetic shadowing and computational identification of human microRNA genes. Cell 2005, 120, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Dhaoui, A.; Chniter, M.; Atigui, M.; Dbara, M.; Seddik, M.M.; Hammadi, M. Factors affecting the milk yield and composition over lactation of prolific D’man ewes in Tunisian oases. Trop. Anim. Health Prod. 2019, 51, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, T.D.; Nikapitiya, C.; Lee, J.; De Zoysa, M. Molecular insight into regulation of miRNAs in the spleen of zebrafish (Danio rerio) upon pathogenic Streptococcus parauberis infection. Fish. Shellfish. Immunol. 2020, 106, 898–909. [Google Scholar] [CrossRef]
- Ding, D.; Zhang, Y.; Zhang, X.; Shi, K.; Shang, W.; Ying, J.; Wang, L.; Chen, Z.; Hong, H. MiR-30a-3p suppresses the growth and development of lung adenocarcinoma cells through modulating GOLM1/JAK-STAT signaling. Mol. Biotechnol. 2022, 64, 1143–1151. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, P.; Fan, S.; Zhai, B.; Li, S.; Li, H.; Zhang, Y.; Li, W.; Sun, G.; Han, R.; et al. miR-30a-3p can inhibit the proliferation and promote the differentiation of chicken primary myoblasts. Br. Poult. Sci. 2022, 63, 475–483. [Google Scholar] [CrossRef]
- Su, J.; Wang, Y.; Xie, J.; Chen, L.; Lin, X.; Lin, J.; Xiao, X. MicroRNA-30a inhibits cell proliferation in a sepsis-induced acute kidney injury model by targeting the YAP-TEAD complex. J. Intensive Med. 2023, 4, 231–239. [Google Scholar] [CrossRef]
- Boutinaud, M.; Guinard-Flamenta, J.; Jammes, H. The number and activity of mammary epithelial cells, determining factors for milk production. Reprod. Nutr. Dev. 2004, 44, 499–508. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. MicroRNAs: Milk’s epigenetic regulators. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 427–442. [Google Scholar] [CrossRef]
- Dysin, A.P.; Barkova, O.Y.; Pozovnikova, M.V. The role of microRNAs in the mammary gland development, health, and function of cattle, goats, and sheep. Non-Coding RNA 2021, 7, 78. [Google Scholar] [CrossRef]
- Yamada, K.M.; Araki, M. Tumor suppressor PTEN: Modulator of cell signaling, growth, migration and apoptosis. J. Cell Sci. 2001, 114, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hou, X.; Qu, B.; Wang, J.; Gao, X.; Li, Q. Pten regulates development and lactation in the mammary glands of dairy cows. PLoS ONE 2014, 9, e102118. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.W.; Ma, J.; Yang, C.L.; Chen, L.L.; He, Q.Y.; Coleman, D.N.; Wang, T.Z.; Jiang, X.L.; Luo, J.; Ma, Y.; et al. Phosphatase and tensin homolog (PTEN) suppresses triacylglycerol accumulation and monounsaturated fatty acid synthesis in goat mammary epithelial cells. J. Dairy Sci. 2021, 104, 7283–7294. [Google Scholar] [CrossRef] [PubMed]
- Shore, A.N.; Chang, C.H.; Kwon, O.J.; Weston, M.C.; Zhang, M.; Xin, L.; Rosen, J.M. PTEN is required to maintain luminal epithelial homeostasis and integrity in the adult mammary gland. Dev. Biol. 2016, 409, 202–217. [Google Scholar] [CrossRef]
- Dupont, J.; Renou, J.P.; Shani, M.; Hennighausen, L.; LeRoith, D. PTEN overexpression suppresses proliferation and differentiation and enhances apoptosis of the mouse mammary epithelium. J. Clin. Investig. 2002, 110, 815–825. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Xu, T.; Li, C.; Wu, J.; He, Q.; Wang, G.; Ding, C.; Liu, K.; Tang, H.; et al. Increased expression of microRNA-148a in osteosarcoma promotes cancer cell growth by targeting PTEN. Oncol. Lett. 2016, 12, 3208–3214. [Google Scholar] [CrossRef]
- Jin, X.; Hao, Z.; Zhao, M.; Shen, J.; Ke, N.; Song, Y.; Qiao, L.; Lu, Y.; Hu, L.; Wu, X.; et al. MicroRNA-148a regulates the proliferation and differentiation of ovine preadipocytes by targeting PTEN. Animals 2021, 11, 820. [Google Scholar] [CrossRef]
- Do, D.N.; Dudemaine, P.L.; Li, R.; Ibeagha-Awemu, E.M. Co-Expression network and pathway analyses reveal important modules of miRNAs regulating milk yield and component traits. Int. J. Mol. Sci. 2017, 18, 1560. [Google Scholar] [CrossRef]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genomics 2008, 9, 366. [Google Scholar] [CrossRef]
- Fan, Y.L.; Han, Z.Y.; Lu, X.B.; Zhang, H.M.; Arbab, A.A.I.; Loor, J.J.; Yang, Y.; Yang, Z.P. Identification of milk fat metabolism-related pathways of the bovine mammary gland during mid and late lactation and functional verification of the ACSL4 Gene. Genes 2020, 11, 1357. [Google Scholar] [CrossRef]
- Wu, H.Y.; Ji, Z.H.; Xie, W.Y.; Guo, H.X.; Zheng, Y.; Gao, W.; Yuan, B. KLF4 promotes milk fat synthesis by regulating the PI3K-AKT-mTOR pathway and targeting FASN activation in bovine mammary epithelial cells. iScience 2024, 27, 109850. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.F.; Li, Q.; Xu, H.M.; Li, Y.Y.; Wang, S.J.; Xiong, Y.; Lan, D.L.; Li, J.; Xiong, X.R.; Fu, W. Zearalenone triggers programmed cell death and impairs milk fat synthesis via the AKT-mTOR-PPARγ-ACSL4 pathway in bovine mammary epithelial cells. J. Anim. Sci. 2024, 102, skae276. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Wu, X.; Zhen, H.; Feng, Y.; Li, M.; Ren, C.; Wang, J.; Hao, Z. MicroRNA-30a-3p Influences Milk Fat Metabolism by Targeting PTEN in Mammary Epithelial Cells of Sheep. Animals 2025, 15, 1180. https://doi.org/10.3390/ani15081180
Guo Y, Wu X, Zhen H, Feng Y, Li M, Ren C, Wang J, Hao Z. MicroRNA-30a-3p Influences Milk Fat Metabolism by Targeting PTEN in Mammary Epithelial Cells of Sheep. Animals. 2025; 15(8):1180. https://doi.org/10.3390/ani15081180
Chicago/Turabian StyleGuo, Yamin, Xinmiao Wu, Huimin Zhen, Yuxin Feng, Mingna Li, Chunyan Ren, Jiqing Wang, and Zhiyun Hao. 2025. "MicroRNA-30a-3p Influences Milk Fat Metabolism by Targeting PTEN in Mammary Epithelial Cells of Sheep" Animals 15, no. 8: 1180. https://doi.org/10.3390/ani15081180
APA StyleGuo, Y., Wu, X., Zhen, H., Feng, Y., Li, M., Ren, C., Wang, J., & Hao, Z. (2025). MicroRNA-30a-3p Influences Milk Fat Metabolism by Targeting PTEN in Mammary Epithelial Cells of Sheep. Animals, 15(8), 1180. https://doi.org/10.3390/ani15081180




