4.1. Dietary Supply of Indispensable Amino Acids
In the current experiment, the pigs were fed controlled amounts of feed from 60 to 145 kg BW in order to improve feed efficiency and carcass uniformity at slaughter [
19]. Growth performances were good, the daily gain averaged 0.95 kg/d and the gain:feed ratio was 0.357, in agreement with previous studies conducted under similar conditions [
20]. In a study with pigs fed
ad libitum and with lighter BW ranges (30–90 kg), Ball et al. (2013) [
21] reported a growth rate of 0.94 kg/d and a gain:feed ratio of 0.406, while similar figures were reported by Morales et al. (2011) [
22].
Protein retention, estimated from in vivo measurements of body weight and of backfat depth by ultrasound, averaged 182 g/d during growing and 102 g/d during finishing. The former value is greater than and the latter lower than those recommended by the NRC (2012) [
10] for high-medium lean growth rates, which are 147, 141 and 122 g/d in the BW ranges of 50–75, 75–100 and 100–135 kg BW, respectively. Ruiz-Ascacibar et al. (2017) [
23] reported mean protein deposition rates in barrows of 142, 153, 147, 141 g/d at BWs of 80, 100, 120 and 140 kg, respectively. The estimated N retention averaged 23.3 g/kg of body gain, slightly lower than the value (25 g/kg) calculated elsewhere [
24] for pigs slaughtered at lighter weights (90–120 kg), and consistent with the value of 24 g/kg calculated by others for heavy pigs of about 160 kg body weight [
25,
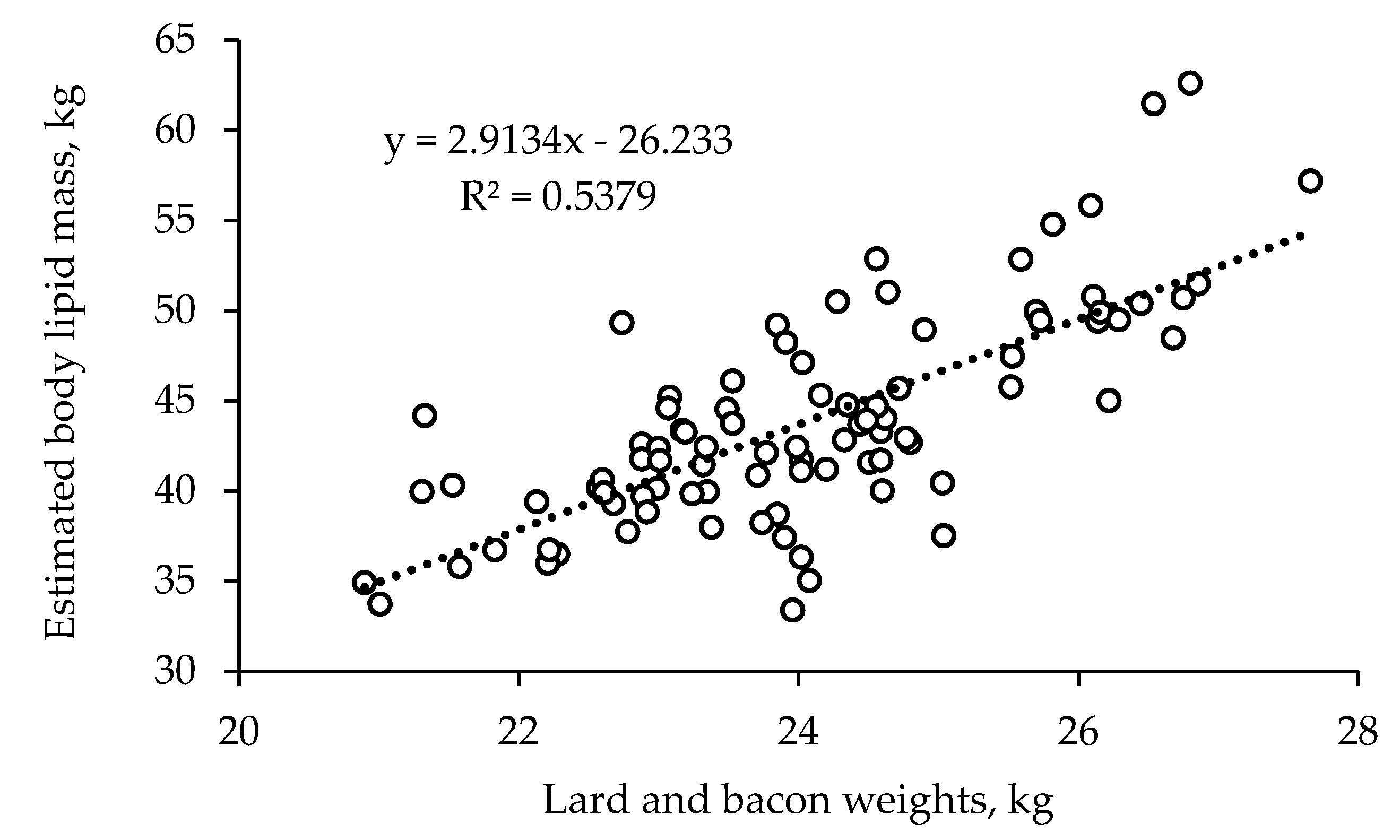
26]. There was also good correspondence between the body lipid mass estimated from BW and BF depth measured by ultrasound and the weights of the lard and bacon, the main fat components of the carcasses. These figures suggest that the pigs in the current experiment had a high potential for lean growth at early BWs, particularly between 60 and 100 kg.
In the current experiment, the indispensable AA allowance, expressed per kg of feed, was greater (HAA) or similar (LAA) to that recommended by the NRC (2012) [
10] for pigs with high-medium lean growth rates. The higher AA content was tested to avoid limiting conditions with respect to the indispensable AA daily allowance, given the pigs’ high potential for lean growth and the possibility of the feed intake being lower than that recommended by the NRC (2012) [
10]. Based on the actual feed intakes, we calculated the average SID Lys intake as 24.7 g/d (growing) and 22.3 g/d (finishing) for the HAA pigs, and 22.3 g/d (growing) and 18.0 g/d (finishing) for the LAA pigs. According to the NRC (1998) [
27], 0.123 g of true digestible lysine per g of protein accretion is required above maintenance. On the basis of this recommendation, we calculated the total Lys requirements for maintenance and protein accretion to be about 26 g/d during growing and 15 g/d during finishing. The HAA feed provided 27.4 g/d (growing) and 24.9 g/d (finishing) of total lysine, the LAA feed 24.7 and 20.0 g/d, respectively. In the current experiment, the indispensable AA allowance had little or no influence on growth performance, body protein (or N) retention, feed efficiency, carcass characteristics and meat quality parameters. As the highest dietary AA contents produced no benefits, we suggest that they were greater than requirement and that, therefore, the lowest dietary AA content can be used with pigs similar to those tested in the current experiment to reduce feed costs and lower N excretion into the environment. Given the nature of the estimated protein growth, a further reduction in indispensable AA contents may be possible, particularly in the 104–145 kg BW range.
4.2. Feed × Genotype Interaction
The current experiment did not aim to compare the two genetic lines, but rather to explore their possible interaction in response to a variation in the indispensable AA content of the feed. There are very few comparisons between pig genetic lines in the literature [
28], but the potential for lean growth is known to largely differ among genotypes [
6]. Emphasis on traits such as growth rate and subcutaneous fat affects the mass of metabolically active organs, feed intake, protein retention, activity of lipogenic enzymes, plasma concentrations of hormones and metabolites, and metabolism of adipose tissues [
8]. The two genetic lines used in the current experiment were selected for the production of both fresh meat and high-quality cooked hams [
29,
30], which requires fast growing pigs slaughtered at about 135–145 kg BW to obtain untrimmed thighs of about 14–16 kg [
20]. The Hypor Maxter pigs used in the current experiment were selected for fast growth, carcass leanness, and feed efficiency [
31], while the PIC 337 were selected for fast growth, feed efficiency, lower P2 backfat depth, carcass yield and conformation [
32]. There is little information in the scientific literature on these two genetic lines.
The two GLs used in the current study differed significantly in growth performances, although the difference was slight-close to 30 g/d, and arose under conditions of controlled feeding, likely reflecting a difference in feed efficiency, which was 2% better in the PIC 337 than in the Hypor Maxter pigs. Conversely, the carcass yield of the Hypor Maxter was greater than that of the PIC 337 pigs. The carcasses of the two genetic lines were very similar in the composition of the anatomical cuts and in their meat quality characteristics.
Kyriazakis (2011) [
6] suggested that when the nutrient supplies are limiting, their partition among body constituents and functions tends to be similar among genotypes, so that high, non-limiting protein supplies are required to point up the differences among genotypes. Taylor et al. (2015) found that Hampshire piglets grew faster than Large White pigs when fed a non-lysine-limiting diet but grew at similar rates when fed a lysine-limiting diet, indicating that dietary lysine level rather than genotype determined their growth performance. Chiba et al. (2002) [
33] observed some diet × genotype interactions, suggesting that fast-growing, lean pigs need to be provided with adequate indispensable AA concentrations. They also concluded that compensatory growth responses are influenced by pig genotype.
Ruiz-Ascacibar et al. (2017) [
23] found a significant diet × sex interaction in a trial involving entire and castrated males and entire females fed ad libitum diets that differed in protein content. In particular, in the 100 to 140 kg BW interval entire males fed a low protein diet increased their feed intake by 20% compared with entire males fed the control diet. In contrast, castrated males and females reduced their feed intake when fed the low protein diet. This interaction may be due to the different shapes of the protein deposition curves, and the authors also attributed it to the extra energy required for castrated males and females to eliminate the excess protein. They also reported that the highest protein deposition rates were found with BWs heavier than those indicated by the NRC (2012), namely 168 (at 140 kg BW) for entire males, 153 (at 100 kg BW) for castrated males and 148 g/d (at 120 kg BW) for gilts. These results would suggest that different response to a given feed would be possible among entire males, castrated males and females of the same genotype. However, the pigs of two strains studied by De Greef (2002) [
34] responded similarly to protein deficiency from 28 to 65 kg BW and re-alimentation from 66 to 105 kg BW. These authors suggested that the two strains of pigs did not differ sufficiently in leanness to respond differently, a finding similar to ours.
In fact, in the current experiment, the HAA diet was formulated to contain an amount of indispensable AA in excess of the NRC’s (2012) [
10] recommendation, so that the potential for protein growth could have been expressed. The growth performances and carcass characteristics of the pigs of the two genetic lines were very similar whether the pigs were on the HAA or the LAA diet, and little or no interaction was found. However, unlike the previous experiments, the pigs of the current trial were fed controlled amount of feed, a feeding regime that may have limited their energy allowance and, in turn, limited their growth rate. Actual performance shows that when BW was over 104 kg the indispensable AA supply was probably in excess of requirements with both the HAA and the LAA diets, particularly when BW was in the range of 104–145 kg. This might have prevented a diet × genotype interaction emerging. However, it also suggests that the dietary indispensable AA content can probably be further reduced in the 104–145 BW interval.