Development of an Anthropomorphic Heterogeneous Female Pelvic Phantom and Its Comparison with a Homogeneous Phantom in Advance Radiation Therapy: Dosimetry Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phantom Design
2.2. Fabrication of Phantom
2.3. Comparison of the Hounsfield Units and the Relative Electron Densities of the Organs
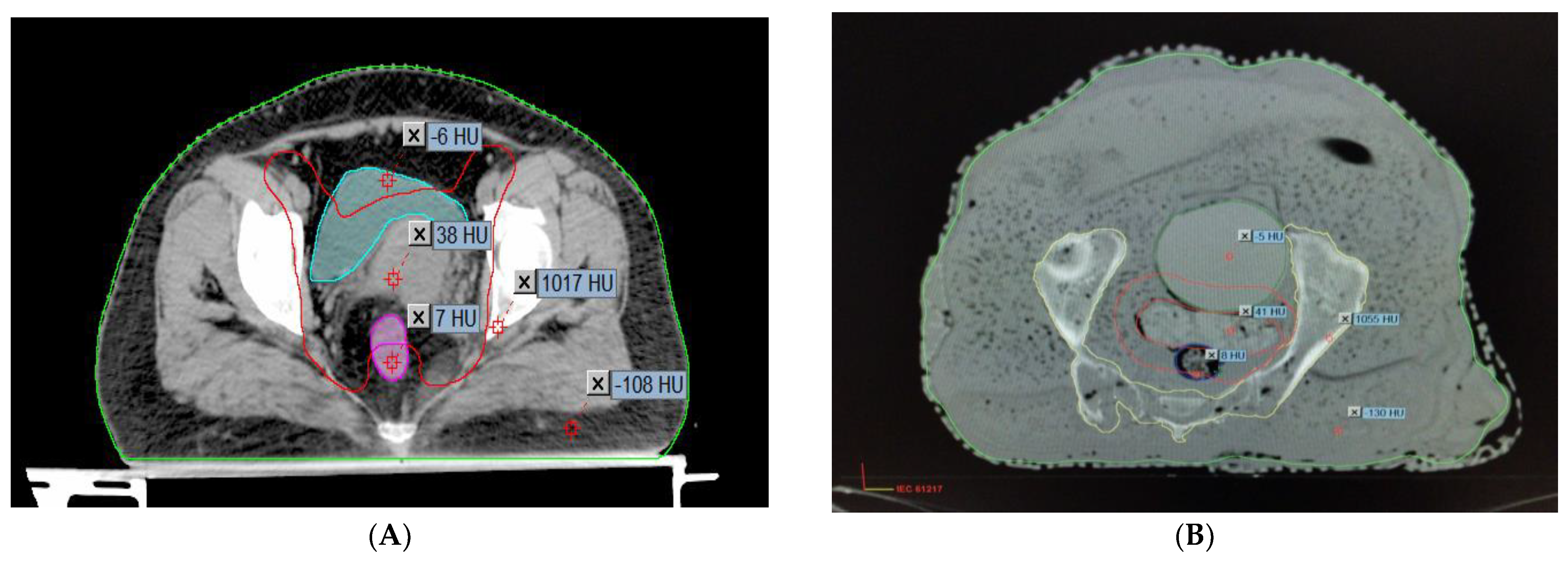
2.4. Anatomical and Measuring Point Identification
2.5. Pretreatment Plan Verification
2.5.1. Patient-Specific Absolute Dosimetry
2.5.2. Relative Dosimetry
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Khan, F.M. The Physics of Radiation Therapy, 4th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2012. [Google Scholar]
- Fraass, B.A.; Doppke, K.P.; Hunt, M.A.; Kutcher, G.; Starkschall, G.; Stern, R.; Van Dyke, J. American Association of Physicists in Medicine Radiation Therapy Committee Task Group 53: Quality assurance for clinical radiotherapy treatment planning. Med. Phys. 1998, 25, 1773–1829. [Google Scholar] [CrossRef]
- Van Dyk, J.; Barnett, R.B.; Cygler, J.E.; Shragge, P.C. Commissioning and quality assurance of treatment planning computers. Int. J. Radiat. Oncol. Biol. Phys. 1993, 26, 261–273. [Google Scholar]
- American Association of Physicists in Medicine Radiation Therapy Committee Task Group 40. Comprehensive QA for Radiation Oncology, Report of the AAPM Radiation Therapy Committee Task Group 40. Med. Phys. 1994, 21, 581–618. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). Absorbed Dose Determination in External Beam Radiotherapy: An International Code of Practice for Dosimetry based on Absorbed Dose to Water; IAEA: Vienna, Austria, 2000. [Google Scholar]
- International Commission on Radiation Units and Measurements (ICRU). The ICRU Report 83, prescribing, recording, and reporting photon beam intensity-modulated radiation therapy (IMRT). Cancer/Radiotherapy 2011, 15, 555–559. [Google Scholar] [CrossRef]
- International Commission on Radiation Units and Measurements (ICRU). Tissue Substitutes in Radiation Dosimetry and Measurement; Report No. 44; ICRU: Bethesda, MD, USA, 1998. [Google Scholar]
- Bagdare, P.; Dubey, S.; Ghosh, S.K.; Bhandigare, S. Analysis of gamma index using in-house developed heterogeneous thorax phantom. Int. J. Radiol. Radiat. Ther. 2021, 8, 9–11. [Google Scholar] [CrossRef]
- Svenesson, G.K. Quality assurance in radiation therapy: Physical effort. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Brahme, A.; Chavaudra, J.; Landberg, T. Accuracy requirements and quality assurance of external beam therapy with photons and electrons. Acta Oncol. 1988, 27, 1–76. [Google Scholar]
- Kry, S.F.; Salehpour, M.; Followill, D.S.; Stovall, M.; Kuban, D.A.; White, R.A.; Rosen, I.I. The calculated risk of fatal secondary malignancies from intensity-modulated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, A.; Sekaran, S.C.; Sarkar, B.; Manikandan, S. A homogeneous water-equivalent anthropomorphic phantom for dosimetric verification of radiotherapy plans. J. Med. Phys. 2018, 43, 100–105. [Google Scholar]
- Letourneau, D.; Publicover, J.; Kozelka, J.; Moseley, D.J.; Jaffray, D.A. Novel dosimetric phantom for quality assurance of volumetric modulated arc therapy. Med. Phys. 2009, 36, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Gurjar, O.P.; Mishra, S.P. A comparative study on patient–specific absolute dosimetry using slab phantom, acrylic body phantom, and goat head phantom. Int. J. Cancer Ther. Oncol. 2015, 3, 3213. [Google Scholar] [CrossRef]
- Yasui, K.; Toshito, T.; Omachi, C.; Hayashi, K.; Kinou, H.; Katsurada, M.; Hayashi, N.; Ogino, H. Dosimetric verification of IMPT using a commercial heterogeneous phantom. J. Appl. Clin. Med. Phys. 2019, 20, 114–120. [Google Scholar] [CrossRef]
- Chen, M.W.; Deng, X.W.; Huang, S.M.; Chen, L.; Kang, D.-H. Application of amorphous silicon electronic portal imaging device (a–SiEPID) to dosimetry quality assurance of radiation therapy. AiZheng 2007, 26, 1272–1275. [Google Scholar]
- Litzenberg, D.W.; Amro, H.; Prisciandaro, J.I.; Acosta, E.; Gallagher, I.; Roberts, D.A. Dosimetric impact of density variations in Solid Water 457 water-equivalent slabs. J. Appl. Clin. Med. Phys. 2011, 12, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Artuso, E.; Brambilla, M.G.; Gambarini, G.; Lenardi, C.; Monti, A.F.; Torresin, A.; Pignoli, E.; Veronese, I. Characterization of radiochromic poly(vinyl-alcohol)–glutaraldehyde Fricke gels for dosimetry in external X-ray radiation therapy. J. Phys. D Appl. Phys. 2019, 52, 225601. [Google Scholar] [CrossRef]
- Thomas, S.J. Relative electron density calibration of CT scanners for radiotherapy treatment planning. Br. J. Radiol. 1999, 72, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Johns, H.E.; Cunningham, J.R. The Physics of Radiology, 4th ed.; Charles C. Thomas: Springfield, IL, USA, 1983. [Google Scholar]
- Almond, P.R.; Biggs, P.J.; Coursey, B.M. AAPM’s TG-51 protocol for clinical reference dosimetry of high-energy photon and electron beams. Med. Phys. 1999, 26, 1847–1870. [Google Scholar] [CrossRef]
- Winslow, J.F.; Hyer, D.E.; Fisher, R.F.; Tien, C.J.; Hintenlang, D.E. Construction of anthropomorphic phantoms for use in dosimetry studies. J. Appl. Clin. Med. Phys. 2009, 10, 195–204. [Google Scholar] [CrossRef]
- Trujillo-Bastidas, C.D.; García-Garduño, O.A.; Lárraga-Gutiérrez, J.M.; Martínez-Dávalos, A.; Rodríguez-Villafuerte, M. Effective atomic number and electron density calibration with a dual-energy CT technique. AIP Conf. Proc. 2016, 1747, 080009. [Google Scholar]
- Kanematsu, N. Relationship between mass density, electron density, and elemental composition of body tissues for Monte Carlo simulation in radiation treatment planning. Phys. Med. Biol. 2016, 61, 5037–5050. [Google Scholar] [CrossRef] [PubMed]
- Shrotriya, D.; Yadav, R.S.; Srivastava, R.N.L.; Verma, T.R. Design and development of an indigenous in-house tissue-equivalent female pelvic phantom for radiological dosimetric applications. Iran. J. Med. Phys. 2018, 15, 200–205. [Google Scholar]
- Singh, S.; Raina, P.; Gurjar, O.P. Dosimetric Study of an Indigenous and Heterogeneous Pelvic Phantom for Radiotherapy Quality Assurance. Iran. J. Med. Phys. 2020, 17, 120–125. [Google Scholar]
- ICRU Report 50, Prescribing, Recording, and Reporting Photon Beam Therapy; International Commission on Radiation Units and Measurements: Bethesda, MD, USA, 1993.
- Nakao, M.; Ozawa, S.; Miura, H.; Yamada, K.; Habara, K.; Hayata, M.; Kusaba, H.; Kawahara, D.; Miki, K.; Nakashima, T.; et al. Development of a CT number calibration audit phantom in photon radiation therapy: A pilot study. Med. Phys. 2020, 47, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Schaly, B.; Varchena, V.; Au, P.; Pang, G. Evaluation of an anthropomorphic male pelvic phantom for image-guided radiotherapy. Rep. Med. Imaging 2009, 2, 69–78. [Google Scholar]
- Singh, V.P.; Badiger, N. Effective atomic numbers of some tissue substitutes by different methods: A comparative study. J. Med. Phys. 2014, 39, 24–31. [Google Scholar] [CrossRef]
- Srivastava, R.P.; De Wagter, C. Clinical experience using Delta 4 phantom for pretreatment patient-specific quality assurance in modern radiotherapy. J. Radiother. Pract. 2019, 18, 210–214. [Google Scholar] [CrossRef]
- Gurjar, O.; Mishra, S.; Bhandari, V.; Pathak, P.; Patel, P.; Shrivastav, G. Radiation dose verification using real tissue phantom in modern radiotherapy techniques. J. Med. Phys. 2014, 39, 44–49. [Google Scholar] [CrossRef]
- Zain, N.E.M.; Jais, U.; Abdullah, R.; Rahman, W.N.W. Dosimetric Characterization of Customized PLA Phantom for Radiotherapy. J. Sains Nukl. Malays. 2019, 31, 1–6. [Google Scholar]
- Zhang, F.; Zhang, H.; Zhao, H.; He, Z.; Shi, L.; He, Y.; Ju, N.; Rong, Y.; Qiu, J. Design and fabrication of a personalized anthropomorphic phantom using 3D printing and tissue equivalent materials. Quant. Imaging Med. Surg. 2019, 9, 94–100. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, S.; Hu, W.; Yu, X.; Li, J.; Liu, T.; Zhang, X. Dosimetric validation and accuracy assessment of an in-house developed anthropomorphic heterogeneous female pelvic phantom for radiotherapy quality assurance. Med. Dosim. 2021, 46, 55–63. [Google Scholar]
- Lee, J.; Kim, S.; Park, H.; Lee, S.; Cho, S.; Kim, D. Evaluation of a homemade anthropomorphic heterogeneous pelvic phantom for patient-specific quality assurance in intensity-modulated radiation therapy. Radiat. Oncol. J. 2023, 41, 32–40. [Google Scholar]
- Smith, A.; Johnson, R.; Williams, C.; Brown, K.; Wilson, M. Investigation of the dosimetric accuracy of treatment plans using an anthropomorphic heterogeneous female pelvic phantom and gamma index analysis. J. Appl. Clin. Med. Phys. 2022, 23, 167–176. [Google Scholar]
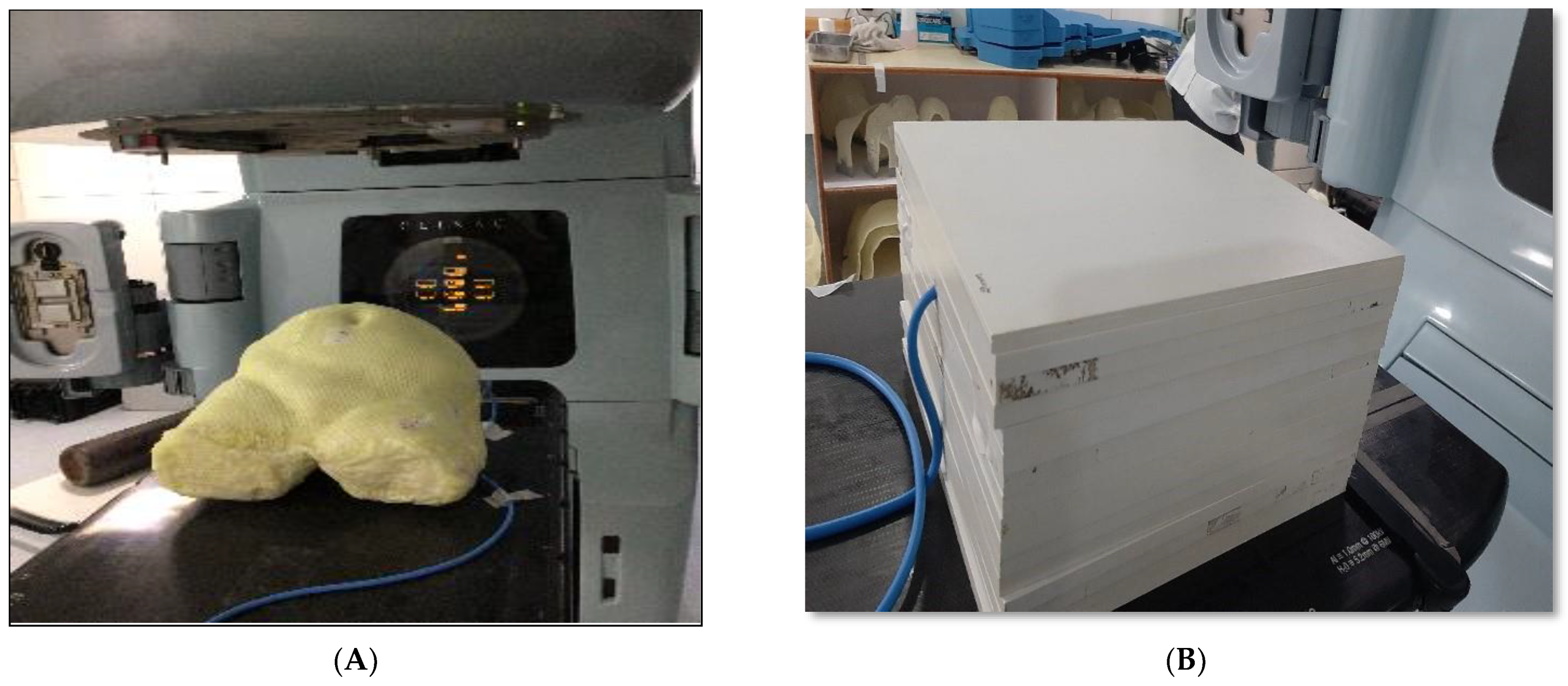


| AHFP Phantom | Composition of Materials |
|---|---|
| Fat and skin | Paraffin wax (13 kg) and NaCl (50 g) |
| Muscles | Silicon sealant (100 g) and paraffin wax (200 g) |
| Bladder | Balloon filled with 220 mL of water |
| Rectum | Polyvinyl chloride, gauze, and paraffin wax (10 g) |
| Uterus | Polymerized siloxanes (150 g) and wax (50 g) |
| Bone | Pelvic bone: human equivalent |
| S.N. | Pelvic Organs | Actual Female Patients | AHFP Phantom | ||
|---|---|---|---|---|---|
| HU ± SD | RED | HU ± SD | RED | ||
| 1 | Uterus | 45 ± 20 | 1.031 | 50 ± 21 | 1.07 |
| 2 | Bladder | 12 ± 6 | 1.02 | −4.0 ± 17 | 1.015 |
| 3 | Rectum | 42 ± 17 | 1.040 | 43 ± 26 | 1.069 |
| 4 | Muscle | 70 ± 12 | 1.08 | 72 ± 33 | 1.105 |
| 5 | Fat | −120 ± 8 | 0.955 | −170 ± 79 | 0.909 |
| 6 | Bone | 965 ± 110 | 1.489 | 947 ± 277 | 1.628 |
| Homogeneous Phantom (RW3 Phantom) | Heterogeneous Phantom (AHFP) | |||||
|---|---|---|---|---|---|---|
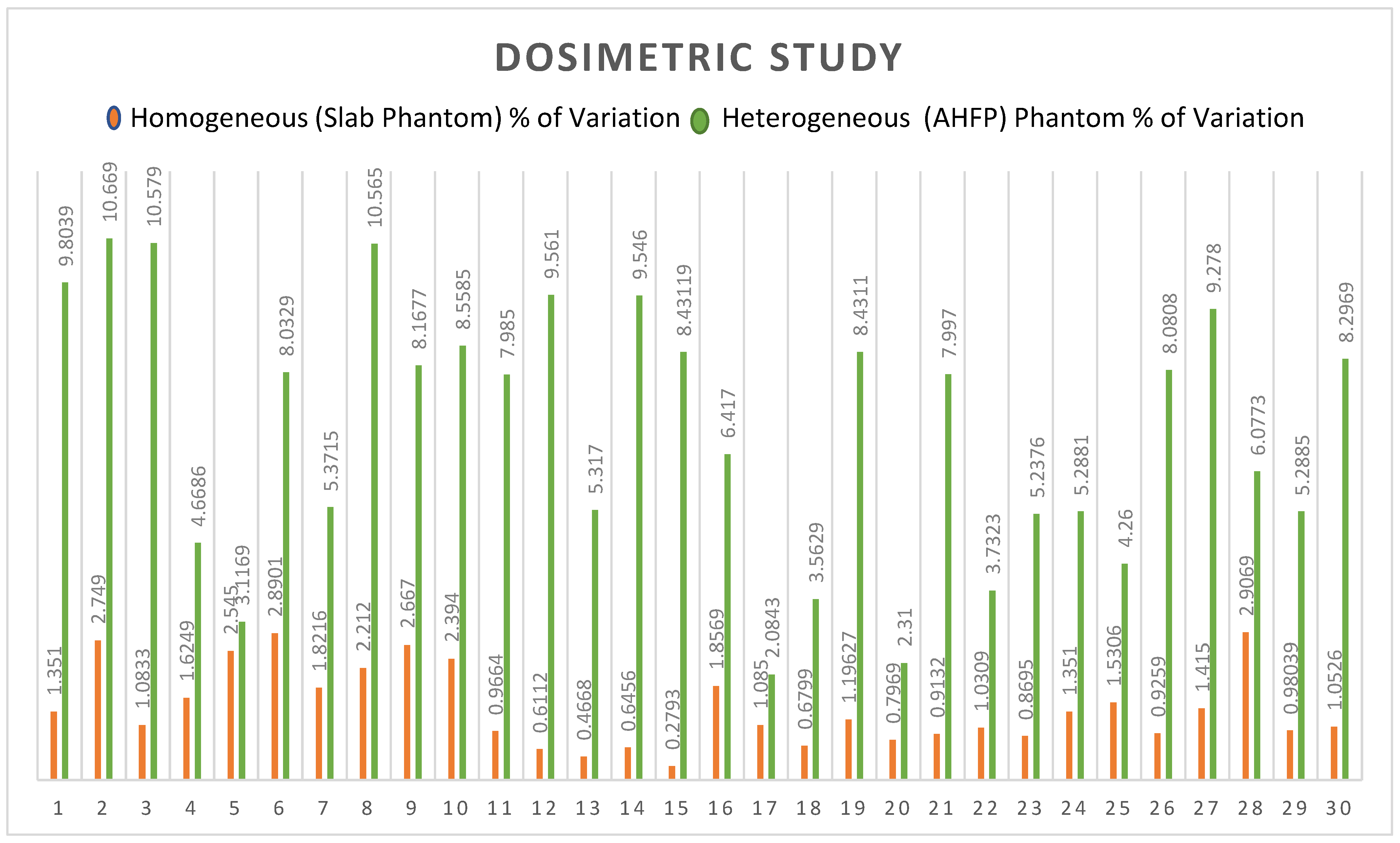
| Sr. No. | Planned Dose of TPS (cGy) | Measured Dose of LA (cGy) | % of Variation | Planned Dose of TPS (cGy) | Measured Dose of LA (cGy) | % of Variation |
| 1 | 199.01 | 196.32 | −1.352 | 204 | 184 | −9.804 |
| 2 | 200.4 | 194.89 | −2.749 | 210.6 | 188.13 | −10.669 |
| 3 | 192 | 189.92 | −1.083 | 214 | 191.36 | −10.579 |
| 4 | 230.16 | 233.9 | 1.625 | 203.27 | 193.78 | −4.669 |
| 5 | 200.38 | 205.48 | 2.545 | 194.1 | 188.05 | −3.117 |
| 6 | 216.25 | 210 | −2.890 | 212.5 | 195.43 | −8.033 |
| 7 | 185 | 181.63 | −1.822 | 205.9 | 194.84 | −5.372 |
| 8 | 220.13 | 225 | 2.212 | 192.6 | 172.25 | −10.566 |
| 9 | 205.09 | 199.62 | −2.667 | 226.5 | 208 | −8.168 |
| 10 | 205.09 | 210 | 2.394 | 210.9 | 192.85 | −8.558 |
| 11 | 172.8 | 171.13 | −0.966 | 200 | 184.03 | −7.985 |
| 12 | 196.3 | 197.5 | 0.611 | 210 | 189.92 | −9.562 |
| 13 | 192.8 | 193.7 | 0.467 | 230 | 217.77 | −5.317 |
| 14 | 196.7 | 195.43 | −0.646 | 216 | 195.38 | −9.546 |
| 15 | 200.5 | 199.94 | −0.279 | 218 | 199.62 | −8.431 |
| 16 | 187.4 | 183.92 | −1.857 | 199 | 186.23 | −6.417 |
| 17 | 202.7 | 200.5 | −1.085 | 220.7 | 225.3 | 2.084 |
| 18 | 205.9 | 204.5 | −0.680 | 210.5 | 218 | 3.563 |
| 19 | 225.7 | 228.4 | 1.196 | 218 | 199.62 | −8.431 |
| 20 | 197 | 195.43 | −0.797 | 200 | 195.38 | −2.31 |
| 21 | 219 | 221 | 0.913 | 172.8 | 158.98 | −7.997 |
| 22 | 194 | 192 | −1.031 | 191.3 | 198.44 | 3.732 |
| 23 | 230 | 228 | −0.869 | 197.8 | 187.44 | −5.238 |
| 24 | 185 | 187.5 | 1.351 | 189.48 | 199.5 | 5.288 |
| 25 | 196 | 199 | 1.531 | 223 | 213.5 | −4.260 |
| 26 | 216 | 218 | 0.926 | 198 | 182 | −8.081 |
| 27 | 212 | 215 | 1.415 | 194 | 176 | −9.278 |
| 28 | 172 | 177 | 2.907 | 181 | 192 | 6.077 |
| 29 | 204 | 206 | 0.980 | 208 | 197 | −5.288 |
| 30 | 190 | 188 | −1.053 | 229 | 210 | −8.297 |
| Sr. No. | Statistical Parameters | Homogeneous (RW3) Phantom | AHFP Phantom |
|---|---|---|---|
| 1 | N | 30 | 30 |
| 2 | ∑X | 42.898 | 206.715 |
| 3 | Mean | 1.429 | 6.890 |
| 4 | ∑X2 | 78.454 | 1615.167 |
| 5 | SD | 0.768 | 2.565 |
| 6 | t-value | 0.005 | 3.216 |
| 7 | ρ | 0.498 | 0.001 |
| Target Location | Treatment Technique | Area Gamma | Maximum Dose Difference | Average Dose Difference |
|---|---|---|---|---|
| Target 1 | RapidArc | 99.8% | 18.7% | 1.1% |
| Target 2 | RapidArc | 97.9% | 25.6% | 1.2% |
| Target 3 | RapidArc | 98.8% | 20.2% | 1.3% |
| Target 1 | IMRT | 99.5% | 26.5% | 1.95% |
| Target 2 | IMRT | 99.6% | 39.4.0% | 2.5% |
| Target 3 | IMRT | 98.6% | 20.2% | 2.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, N.; Singh, M.; Mishra, S.P.; Ansari, S. Development of an Anthropomorphic Heterogeneous Female Pelvic Phantom and Its Comparison with a Homogeneous Phantom in Advance Radiation Therapy: Dosimetry Analysis. Med. Sci. 2023, 11, 59. https://doi.org/10.3390/medsci11030059
Yadav N, Singh M, Mishra SP, Ansari S. Development of an Anthropomorphic Heterogeneous Female Pelvic Phantom and Its Comparison with a Homogeneous Phantom in Advance Radiation Therapy: Dosimetry Analysis. Medical Sciences. 2023; 11(3):59. https://doi.org/10.3390/medsci11030059
Chicago/Turabian StyleYadav, Neha, Manisha Singh, Surendra P. Mishra, and Shahnawaz Ansari. 2023. "Development of an Anthropomorphic Heterogeneous Female Pelvic Phantom and Its Comparison with a Homogeneous Phantom in Advance Radiation Therapy: Dosimetry Analysis" Medical Sciences 11, no. 3: 59. https://doi.org/10.3390/medsci11030059
APA StyleYadav, N., Singh, M., Mishra, S. P., & Ansari, S. (2023). Development of an Anthropomorphic Heterogeneous Female Pelvic Phantom and Its Comparison with a Homogeneous Phantom in Advance Radiation Therapy: Dosimetry Analysis. Medical Sciences, 11(3), 59. https://doi.org/10.3390/medsci11030059






