Abstract
Toxoplasma gondii is a protozoan parasite widespread worldwide, with over 40 million individuals in the United States. It may infect vital organs such as the heart, kidneys, and liver, resulting in chronic infections. The main objective of this study is to investigate the association of Toxoplasma infection with the combination of cardiovascular disease, chronic kidney disease (CKD), or chronic liver disease (CLD). The National Health and Nutrition Examination Survey (NHANES 2009–2010) data were used, and the association of infection with chronic disease was assessed with biomarkers and indexes using statistical modeling. The percentage of participants with a combination of CLD and CKD was higher among Toxoplasma positive participants compared to the negative participants (2.76 vs. 1.26). Furthermore, exposure to T. gondii may increase the odds of cardiovascular disease, CKD, or CLD, or vice versa.
1. Introduction
Toxoplasma gondii is a parasitic protozoan that infects humans and other warm-blooded animals [1]. It is a common parasite that can cause a variety of diseases in humans, ranging from mild flu-like symptoms to more severe illnesses [2]. While toxoplasmosis is generally not a serious health problem in healthy individuals, it can cause complications in people with weakened immune systems, as well as in pregnant women and their fetuses [3]. In recent years, there has been increasing evidence linking toxoplasmosis to several chronic diseases, including cardiovascular disease, chronic kidney disease, and chronic liver disease.
Cardiovascular disease is a leading cause of death worldwide [4]. The Centers for Disease Control and Prevention indicated that one person dies every 33 s in the U.S. It is a broad term that encompasses a range of conditions affecting the heart and blood vessels, including coronary artery disease, heart failure, and stroke. There is growing evidence suggesting that toxoplasmosis may contribute to the development of cardiovascular disease [5,6]. Studies have shown that individuals infected with T. gondii have a higher risk of developing atherosclerosis, a condition in which fatty deposits build up in the arteries, leading to restricted blood flow and an increased risk of heart attack and stroke [7]. Additionally, toxoplasmosis may lead to chronic inflammation, which is known to contribute to the development of cardiovascular disease [8].
Chronic kidney disease (CKD) is a condition in which the kidneys gradually lose function over time [9]. It is a common condition that affects millions of people worldwide, and it can lead to a range of complications, including anemia, bone disease, and cardiovascular disease [10]. There is some evidence to suggest that toxoplasmosis may contribute to the development of CKD. Studies have shown that individuals with CKD are more likely to have antibodies to T. gondii, indicating previous exposure to the parasite [11]. Additionally, toxoplasmosis may contribute to the development of chronic inflammation, which is known to be a risk factor for CKD.
Chronic liver disease CLD is a condition in which the liver is damaged and cannot function properly. It can be caused by a range of factors, including alcohol abuse, viral infections, and autoimmune disorders [12]. Some studies have suggested that T. gondii may contribute to liver damage in individuals [13]. For example, one study found that those infected with T. gondii may have higher odds of nonalcoholic fatty liver disease (NAFLD) [14] with age serving as a critical factor in this association.
The cardiovascular system, hepatic system, and renal system are all critical components of human physiology, and they are intimately linked to one another [15,16]. The cardiovascular system, which includes the heart and blood vessels, is responsible for delivering oxygen and nutrients to all parts of the body [17]. The hepatic system, which includes the liver, is responsible for filtering blood, producing bile, and regulating a variety of metabolic functions [18]. The renal system, which includes the kidneys, is responsible for filtering blood, maintaining fluid and electrolyte balance, and regulating blood pressure [19].
The hepatic system assumes a pivotal role in upholding cardiovascular well-being through the synthesis of essential coagulation proteins, notably fibrinogen and prothrombin. In the absence of these proteins, hemostasis is compromised, culminating in uncontrollable hemorrhage and fatal outcomes [20]. The liver also produces albumin, a protein that helps to maintain fluid balance in the body [21]. Albumin mitigates edema by regulating tissue fluid accumulation, averting swelling, and preserving organ function. Additionally, the liver is responsible for the metabolism of drugs and toxins that may adversely impact the cardiovascular system [22]. When the liver is not functioning properly, these drugs and toxins can accumulate in the body and cause damage to the heart and blood vessels.
The renal system also plays an important role in maintaining cardiovascular health. The renal system is tasked with the filtration of metabolic waste substances from the bloodstream and the elimination of surplus bodily fluids [23]. When the kidneys are not functioning properly, waste products and excess fluid can accumulate in the body, leading to a condition called uremia [24]. Uremia can cause a variety of symptoms, including fatigue, nausea, and shortness of breath. In addition to these symptoms, uremia can also have negative effects on the cardiovascular system [24]. Uremic toxins can cause inflammation in the blood vessels, increasing the risk of developing atherosclerosis, a condition in which plaques build up on the walls of the arteries. Atherosclerosis can increase the risk of heart attack and stroke.
The cardiovascular system, in turn, can have a significant impact on the hepatic and renal systems. The heart is responsible for pumping blood to the liver and kidneys, providing them with the oxygen and nutrients they need to function properly. When the heart is not functioning properly, blood flow to the liver and kidneys can be impaired, which can damage these organs [25].
This study sought to explore the role of T. gondii on the cardiovascular, renal, and hepatic systems simultaneously using data from the National Health and Nutrition Examination Survey. The direction through which the damage occurred was unclear; therefore, we explored both directions. The first direction is the association between these chronic diseases and susceptibility to Toxoplasma infection. The second direction was the association of Toxoplasma in the presence of a chronic disease with the other chronic diseases investigated in this study.
2. Materials and Methods
2.1. Hypothesis
This study hypothesized that exposure to Toxoplasma gondii infection has a negative effect on the cardiovascular system, renal system, and hepatic system simultaneously by causing inflammation, oxidative stress, and tissue damage, leading to an increased risk of cardiovascular disease, chronic kidney disease, and chronic liver disease. To test this hypothesis, we examined the effect of T. gondii on an overall cardiovascular biomarker index (OCBI) developed in our prior work [5], a chronic liver disease index, and a chronic kidney disease index used in our prior work [11,13].
2.2. Study Population
The National Health and Nutrition Examination Survey (NHANES) is a program conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) in the United States. NHANES collects health and nutrition data from a representative sample of the US population through a complex, multistage probability sampling design. The NHANES 2009–2010 study population consisted of 10,149 individuals, ages 0–85 years, who were selected from households located in counties across the US. However, only 2061 individuals were included in this study who were older than 19 years and had either a Toxoplasma infection, chronic cardiovascular disease, CKD, or CLD.
The NHANES 2009–2010 study population was diverse in terms of race/ethnicity, with 70% non-Hispanic Whites, 12% non-Hispanic Blacks, 12% Mexican Americans, and 6% other races/ethnicities. This study population was evenly distributed between males and females. The majority of participants (57%) were aged 20–64 years, while 26% were younger than 20 years, and 17% were 65 years or older.
NHANES 2009–2010 collected a wide range of health and nutrition data from the study population, including medical history, physical examination, laboratory testing, and dietary assessments. The data collected in this study has been used to monitor the health and nutritional status of the US population, as well as to inform public health policies, guide healthcare interventions, and identify trends and disparities in health outcomes.
2.3. Study Indicators for Cardiovascular, Kidney, and Liver
In this study, cardiovascular, kidney, and liver health were evaluated using biomarker indexes developed in our previous studies [5,11,13].
2.3.1. Cardiovascular Disease
An overall cardiovascular biomarker index (OCBI) had been developed using eight biomarkers (Table 1). Each biomarker was assigned an index value (0 or 1) based on the threshold recommended clinical practices, giving a value of 0 to the undesirable values. The sum of the index values of all the biomarkers is the OCBI.

Table 1.
Overall Cardiovascular Biomarkers Index (OCBI).
2.3.2. CKD
CKD was classified into negative and positive (all stages) based on the calculated estimated glomerular filtration rate (eGFR) and persistent albuminuria.
- Stage 1: eGFR ≥ 90 mL/min/1.73 m2 and estimated persistent albuminuria;
- Stage 2: eGFR 60–89 mL/min/1.73 m2 and estimated persistent albuminuria;
- Stage 3: eGFR 30–59 mL/min/1.73 m2;
- Stage 4: eGFR 15–29 mL/min/1.73 m2;
- Stage 5: eGFR < 15 mL/min/1.73 m2.
2.3.3. CLD
CLD was categorized in this analysis based on gender and the level of alanine aminotransferase (ALT) or aspartate aminotransferase (AST). Positive CLD was defined as [30]:
- Men: ALT > 40 U/L or AST > 37 U/L
- Women: ALT or AST > 31 U/L
2.4. Statistical Analysis
The association of Toxoplasma with the combination of cardiovascular disease, CKD, and CLD was conducted using logistic regression models. The holistic assessment was conducted in two directions. The first direction was to evaluate the association of OCBI, CLD, and CKD with Toxoplasma infection, where Toxoplasma (positive/negative) was used as the outcome. The second direction was to evaluate the association of Toxoplasma along with OCBI, CLD, or CKD using three individual models where OCBI, CLD, or CKD was used as the outcome.
The analysis was conducted using R (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria). To account for the survey weights and sampling design of NHANES, the statistical analysis utilized the “Survey” package in R, which is specifically designed for analyzing complex survey data. A p-value < 0.05 was considered significant in all our analyses.
3. Results
3.1. Study Participants’ Characteristics
Table 2 provides statistical summaries of the variables utilized in this research, including survey-weighted percentages and means. The total number of participants in this sample was 2061, with 42.1% male and 52.8% female. Among these participants, 15.1% were Toxoplasma-positive. The mean age was 48.12. The participants included 9.4% Mexican American, 5.5% other Hispanic, 69.8% non-Hispanic white, 9.6% non-Hispanic black, and 5.8% other race. The percentage of CLD was 16.2 and CKD was 10.3, while the median of OCBI-Subindex1 was 5.0.

Table 2.
Summary Statistics for the Characteristics of Sample Participants, n = 2061.
3.2. Holistic Assessment
Figure 1 shows that the percentage of participants with a combination of CLD and CKD was higher among Toxoplasma-positive participants compared to the negative participants (2.76 vs. 1.26).
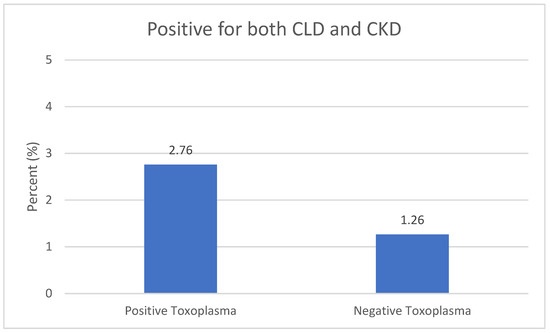
Figure 1.
Infection with a combination of CLD and CKD vs. Toxoplasma Infection. Chronic liver disease (CLD) and chronic kidney disease (CKD).
A holistic assessment was conducted in two directions: the first direction was to evaluate the association of OCBI, CLD, and CKD with Toxoplasma infection; the second direction was to evaluate the association of Toxoplasma along with OCBI, CLD, or CKD with each of these chronic diseases. Table 3 shows that positive CLD increased the odds of having positive Toxoplasma (OR = 1.42, 95% CI = 1.07–1.90, p = 0.0149), and OCBI had a negative association with positive Toxoplasma (OR = 0.92, 95% CI = 0.85–0.99, p = 0.0448). The association of CKD was not apparent in this model; however, adjusting the model for age factor (Table 4) showed that negative CKD decreased the odds of having Toxoplasma infection (OR = 0.64, 95% CI = 0.45–0.90, p = 0.0119). Also, the positive CLD was associated with Toxoplasma infection in this model (OR = 1.72, 95% CI = 1.28–2.31, p = 0.0001). The association of OCBI with Toxoplasma infection was not apparent in this age-adjusted model.

Table 3.
First Direction—Results of Logistic Regression Model for T. gondii (IgG+/IgG−) on OCBI- Subindex1, CLD, and CKD.

Table 4.
First Direction—Results of Logistic Regression Model for T. gondii (IgG+/IgG−) on OCBI-Subindex1, CLD, and CKD—Adjusted for Age.
The first model in the second direction (Table 5) evaluated the association of OCBI, CKD, and Toxoplasma infection with CLD after adjusting for age. The model showed that OCBI-Subindex1 was negatively associated with CLD (OR = 0.63, 95% CI = 0.58–0.68, p = 0.0001), while Toxoplasma infection increased the odds of CLD (OR = 1.68, 95% CI = 1.25–2.26, p = 0.0006). Moreover, CKD was not associated with CLD in this model (OR = 0.84, 95% CI = 0.54–1.30, p = 0.4445).

Table 5.
Second Direction—Results of Logistic Regression Model for CLD on T. gondii (IgG+/IgG−), OCBI-Subindex1, and CKD—Adjusted for Age.
The second model in the second direction (Table 6) evaluated the association of OCBI, CLD, and Toxoplasma infection with CKD after adjusting for age. The model showed that OCBI was negatively associated with CKD (OR = 0.90, 95% CI = 0.80–0.91, p = 0.0353), while Toxoplasma infection increased the odds of CKD (OR = 1.50, 95% CI = 1.60–2.13, p = 0.0221). However, CLD was not associated with CKD in this model (OR = 1.01, 95% CI = 0.67–1.56, p = 0.9557).

Table 6.
Second Direction—Results of Logistic Regression Model for CKD on T. gondii (IgG+/IgG−), CLD, and OCBI-Subindex1—Adjusted for Age.
The third model in the second direction (Table 7) evaluated the association of CKD, CLD, and Toxoplasma infection with OCBI after adjusting for age. The model showed that negative CKD decreased the odds of having low OCBI (OR = 0.20, 95% CI = 0.08–0.50, p = 0.0006). While age was significant in this model, Toxoplasma was not associated with OCBI in this model. In this holistic evaluation, the model with the highest R square was the CKD model (0.22).

Table 7.
Second Direction—Results of Logistic Regression Model for OCBI-Subindex1 on T. gondii (IgG+/IgG−), CLD, and CKD—Adjusted for Age.
4. Discussion
Our results showed that Toxoplasma infection was associated with an increased level of cardiovascular biomarkers and lower OCBI, indicating its negative effect on the cardiovascular system. The holistic analysis revealed individuals with higher OCBI had lower odds of Toxoplasma (OR = 0.92, 95% CI = 0.85–0.99, p = 0.0448), while positive CLD increased the risk of Toxoplasma (OR = 1.42, 95% CI = 1.07–1.90, p = 0.0149). This study is in concordance with previous research that has consistently elucidated the deleterious consequences of Toxoplasma infection on the cardiovascular and hepatic systems. Multiple investigations have documented an association between Toxoplasma exposure and an elevated risk of cardiovascular disorders, encompassing coronary heart disease and cerebrovascular events, alongside hepatic conditions, notably chronic liver disease. These findings, taken together, underscore the imperative of comprehending and mitigating the plausible health ramifications linked to Toxoplasma infection, especially within the framework of these pivotal physiological organ systems [5,6,13,31,32]. The immune system plays an important role in susceptibility to Toxoplasma; however, the cardiovascular system works with the immune system through hormones, cytokines, and neurotransmitters and the health of this system is essential in this connection [33]. The efficiency of this communication is affected by several factors, such as physical and phycological stressors. Degradation of cardiovascular health may unbalance this communication and increase the risk of Toxoplasma infection.
The immune system provides three types of immunity: innate, adaptive, and passive immunity [34]. The innate immunity is the natural immunity that a person is born with, and it consists of several elements, including antimicrobial peptides, immune cells, and pattern recognition receptors (PRRs). The liver is the main provider of PRRS in the human body, such as CRP, lipopolysaccharide binding protein, soluble CD14,17–19, peptidoglycan recognition protein, nucleotide binding oligomerization (NOD)-like receptors, RNA helicases, and toll-like receptors [35]. Deterioration of liver function may reduce the production of PRRS, weaken the immune system, and increase the risk of Toxoplasma infection. This supports the findings of the holistic analysis, which showed CLD increased the risk of Toxoplasma infection.
Studies have highlighted the complex interaction between cardiovascular health and hepatic system health. Several mechanisms were suggested, including inflammation, insulin resistance, endothelial dysfunction, atherosclerosis, and oxidative stress [36]. The holistic analysis showed that cardiovascular disease and Toxoplasma infection significantly increased the odds of CLD (OR = 1.68, 95% CI = 1.25–2.26, p = 0.0006).
On the other hand, several studies indicated an association between chronic cardiovascular disease and CKD; people with low eGFR and elevated albuminuria had an increased incidence of cardiovascular disease [37]. Furthermore, CKD was suggested to increase the risk of cardiovascular disease more than other risk factors such as diabetes mellitus hypertension [37]. The holistic model in this study supported this finding and showed the association of OCBI and CKD (OR = 0.90, 95% CI = 0.80–0.91, p = 0.0353). Furthermore, besides cardiovascular disease, Toxoplasma infection increased the odds of CKD in this model (OR = 1.50, 95% CI = 1.60–2.13, p = 0.0221). The immune system can also play a role in mediating the association between CKD, cardiovascular disease, and Toxoplasma, since the accumulation of uremic toxins such as protein-bound uremic retention solutes during the progression of CKD weakens the innate and adaptive immunity [38]. This was indicated in the association of CKD with Toxoplasma infection in the holistic model, where negative CKD decreased the odds of Toxoplasma (OR = 0.64, 95% CI = 0.45–0.90, p = 0.0119). This corresponds with previous investigations that have demonstrated the adverse impact of Toxoplasma on the renal system. Several studies have reported a clear association between Toxoplasma exposure and an increased risk of renal disorders, including CKD. These collective findings underscore the significance of comprehending and addressing the potential health implications associated with Toxoplasma infection, particularly in the context of the renal system, which plays a critical role in maintaining physiological homeostasis [11,39].
The health of the human system, including the immune system, is essential to minimizing the risk of Toxoplasma infection and its health complications. A healthy human body reacts to T. gondii infection by activating innate immunity and triggering acquired immune responses, which induce the production of the proinflammatory cytokine interferon-γ (IFN-γ) and activate IFN-γ-inducible proteins to accumulate in parasitophorous vacuole membranes in order to inactivate or inhibit T. gondii [40]. Thus, chronic diseases such as CLD and CKD may diminish the immune response to T. gondii infection.
Determining the causal relationship between exposure to T. gondii and the development of chronic diseases, including CVD, CLD, and CKD, continues to pose a formidable challenge, especially when limited to the confines of cross-sectional data analysis. While the complexity of disentangling causality from correlation in such studies cannot be overstated, emerging evidence underscores the undeniable impact of T. gondii on these organ systems. This recognition, in turn, supports a compelling argument that, irrespective of the precise directionality of the association, preventative measures aimed at reducing T. gondii infection can significantly mitigate the risk or severity of these debilitating diseases [41,42,43].
Limitaitons
This study was a cross-sectional study using data gathered at a particular point in time. Because the temporality of association has a strong cause-and-effect criterion, this study cannot demonstrate causation; however, it makes it possible to generate a causal hypothesis of T. gondii’s role in chronic diseases, including cardiovascular, kidney, and liver chronic diseases. A future longitudinal study will be ideally suited to confirm the associations identified in this study.
5. Conclusions
Toxoplasmosis is an issue of significant public health importance and can contribute to the development of several chronic diseases, including cardiovascular disease, chronic kidney disease, and chronic liver disease. More research is needed to fully understand the relationship between Toxoplasma gondii infection and these conditions and to develop effective strategies for preventing and treating toxoplasmosis-related complications. The growing evidence linking toxoplasmosis to chronic diseases underscores the importance of taking steps to prevent exposure to the parasite, particularly for individuals with weakened immune systems or other underlying health conditions. Our findings suggested that exposure to Toxoplasma gondii may increase the odds of cardiovascular disease, CKD, or CLD, or vice versa.
This study highlights the implications of parasitic infection on the biomarkers of chronic diseases. In addition, the results of this study have the potential to increase awareness of the health complications of Toxoplasma and the need for increased education efforts among the most vulnerable populations and occupations about the silent infection of Toxoplasma.
Author Contributions
Conceptualization, E.O.-G.; methodology, A.B., E.O.-G. and S.M.; formal analysis, A.B., E.O.-G. and S.M.; investigation, A.B., E.O.-G. and S.M.; resources, E.O.-G.; data curation, E.O.-G.; writing—original draft preparation, A.B. and E.O.-G.; writing—review and editing, A.B., E.O.-G. and S.M.; supervision, E.O.-G. and S.M.; project administration, E.O.-G.; funding acquisition, E.O.-G. All authors have read and agreed to the published version of the manuscript.
Funding
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R16GM149473. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The NHANES dataset is publicly available online, accessible at cdc.gov/nchs/nhanes/index.htm (accessed on 29 September 2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Innes, E. A brief history and overview of Toxoplasma gondii. Zoonoses Public Health 2010, 57, 1–7. [Google Scholar] [CrossRef]
- Montoya, J.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Robert-Gangneux, F.; Dardé, M.-L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef] [PubMed]
- Cardiovascular Diseases-PAHO/WHO|Pan American Health Organization. 2023. Available online: https://www.paho.org/en/topics/cardiovascular-diseases (accessed on 29 September 2023).
- Babekir, A.; Mostafa, S.; Obeng-Gyasi, E. The Association of Toxoplasma gondii IgG and Cardiovascular Biomarkers. Int. J. Environ. Res. Public Health 2021, 18, 4908. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lopez, H.I.A.O.; Pérez, G.E.; Burgos, L.M.; Farina, J.M.; Saldarriaga, C.; Lopez-Santi, R.; Cotella, J.I.; Pérez, A.L.S.; Baranchuk, A. Toxoplasmosis and the Heart. Curr. Probl. Cardiol. 2021, 46, 100741. [Google Scholar] [CrossRef]
- Egorov, A.I.; Converse, R.R.; Griffin, S.M.; Styles, J.N.; Sams, E.; Hudgens, E.; Wade, T.J. Latent Toxoplasma gondii infections are associated with elevated biomarkers of inflammation and vascular injury. BMC Infect. Dis. 2021, 21, 188. [Google Scholar] [CrossRef]
- Melo, M.B.; Jensen, K.D.; Saeij, J.P. Toxoplasma gondii effectors are master regulators of the inflammatory response. Trends Parasitol. 2011, 27, 487–495. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, J. Chronic kidney disease: Overview. In Chronic Kidney Disease; Springer: Singapore, 2020; pp. 3–12. [Google Scholar]
- Thomas, R.; Kanso, A.; Sedor, J.R. Chronic kidney disease and its complications. Prim. Care Clin. Off. Pract. 2008, 35, 329–344. [Google Scholar] [CrossRef]
- Babekir, A.; Mostafa, S.; Obeng-Gyasi, E. The Association of Toxoplasma gondii IgG Antibody and Chronic Kidney Disease Biomarkers. Microorganisms 2022, 10, 115. [Google Scholar] [CrossRef]
- Adams, L.A.; Angulo, P.; Lindor, K.D. Nonalcoholic fatty liver disease. CMAJ Can. Med. Assoc. J. 2005, 172, 899–905. [Google Scholar] [CrossRef]
- Babekir, A.; Mostafa, S.; Minor, R.C.; Williams, L.L.; Harrison, S.H.; Obeng-Gyasi, E. The Association of Toxoplasma gondii IgG and Liver Injury in US Adults. Int. J. Environ. Res. Public Health 2022, 19, 7515. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, H.; Liu, S.; Wang, M.; Wan, B.; Velani, B.; Zhu, Y.; Lin, S. Is Toxoplasma gondii infection correlated with nonalcoholic fatty liver disease?—A population-based study. BMC Infect. Dis. 2018, 18, 629. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, V.P.; Wright, G.A.; Banaji, M.; Mukhopadhya, A.; Mookerjee, R.; Moore, K.; Jalan, R. Relationship between activation of the sympathetic nervous system and renal blood flow autoregulation in cirrhosis. Gastroenterology 2008, 134, 111–119.e2. [Google Scholar] [CrossRef]
- Adams, L.A.; Anstee, Q.M.; Tilg, H.; Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017, 66, 1138–1153. [Google Scholar] [CrossRef]
- Wuche, C. The cardiovascular system and associated disorders. Br. J. Nurs. 2022, 31, 886–892. [Google Scholar] [CrossRef]
- Ozougwu, J.C. Physiology of the liver. Int. J. Res. Pharm. Biosci. 2017, 4, 13–24. [Google Scholar]
- Wadei, H.M.; Textor, S.C. The role of the kidney in regulating arterial blood pressure. Nat. Rev. Nephrol. 2012, 8, 602–609. [Google Scholar] [CrossRef]
- Tennant, B.C. Hepatic function. In Clinical Biochemistry of Domestic Animals; Elsevier: Amsterdam, The Netherlands, 1997; pp. 327–352. [Google Scholar]
- Hankins, J. The role of albumin in fluid and electrolyte balance. J. Infus. Nurs. 2006, 29, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, H.J. Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 1999. [Google Scholar]
- US Department of Health and Human Services, Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States, 2019; US Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019. Available online: https://www.cdc.gov/kidneydisease/pdf/2019_National-Chronic-Kidney-Disease-Fact-Sheet.pdf (accessed on 29 September 2023).
- Himmelfarb, J.; Stenvinkel, P.; Ikizler, T.A.; Hakim, R.M. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002, 62, 1524–1538. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Ischemia/reperfusion. Compr. Physiol. 2016, 7, 113. [Google Scholar] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Khera, A.V.; Plutzky, J. Management of Low Levels of High-Density Lipoprotein-Cholesterol. Circulation 2013, 128, 72–78. [Google Scholar] [CrossRef][Green Version]
- Ridker, P.M. Cardiology Patient Page. C-reactive protein: A simple test to help predict risk of heart attack and stroke. Circulation 2003, 108, e81–e85. [Google Scholar] [CrossRef] [PubMed]
- Ruttmann, E.; Brant, L.J.; Concin, H.; Diem, G.; Rapp, K.; Ulmer, H. γ-Glutamyltransferase as a Risk Factor for Cardiovascular Disease Mortality. Circulation 2005, 112, 2130–2137. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Younossi, Y.; Golabi, P.; Mishra, A.; Rafiq, N.; Henry, L. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020, 69, 564–568. [Google Scholar] [CrossRef]
- Dragomir, A.; Lupu, M.A.; Lighezan, R.; Paduraru, A.A.; Olariu, T.R. Toxoplasma gondii Infection in Patients with Cardiovascular Diseases from Western Romania: A Case–Control Study. Life 2023, 13, 1575. [Google Scholar] [CrossRef]
- El-Sayed, N.M.; Ramadan, M.E.; Ramadan, M.E. Toxoplasma gondii infection and chronic liver diseases: Evidence of an association. Trop. Med. Infect. Dis. 2016, 1, 7. [Google Scholar] [CrossRef]
- Dal Lin, C.; Tona, F.; Osto, E. The crosstalk between the cardiovascular and the immune system. Vasc. Biol. 2019, 1, H83–H88. [Google Scholar] [CrossRef] [PubMed]
- The Innate and Adaptive Immune Systems; Institute for Quality and Efficiency in Health Care (IQWiG): Cologne, Germany, 2020.
- Noor, M.T.; Manoria, P. Immune Dysfunction in Cirrhosis. J. Clin. Transl. Hepatol. 2017, 5, 50–58. [Google Scholar] [CrossRef]
- Chang, W.H.; Mueller, S.H.; Chung, S.-C.; Foster, G.R.; Lai, A.G. Increased burden of cardiovascular disease in people with liver disease: Unequal geographical variations, risk factors and excess years of life lost. J. Transl. Med. 2022, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Said, S.; Hernandez, G.T. The link between chronic kidney disease and cardiovascular disease. J. Nephropathol. 2014, 3, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Espi, M.; Koppe, L.; Fouque, D.; Thaunat, O. Chronic Kidney Disease-Associated Immune Dysfunctions: Impact of Protein-Bound Uremic Retention Solutes on Immune Cells. Toxins 2020, 12, 300. [Google Scholar] [CrossRef]
- Renoult, E.; Georges, E.; Biava, M.-F.; Hulin, C.; Frimat, L.; Hestin, D.; Kessler, M. Toxoplasmosis in kidney transplant recipients: Report of six cases and review. Clin. Infect. Dis. 1997, 24, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Sasai, M.; Yamamoto, M. Innate, adaptive, and cell-autonomous immunity against Toxoplasma gondii infection. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Jones, J.L.; Parise, M.E.; Fiore, A.E. Neglected parasitic infections in the United States: Toxoplasmosis. Am. J. Trop. Med. Hyg. 2014, 90, 794. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Khanna, P. Development of Toxoplasma gondii vaccine: A global challenge. Hum. Vaccines Immunother. 2013, 9, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Opsteegh, M.; Kortbeek, T.M.; Havelaar, A.H.; van der Giessen, J.W. Intervention strategies to reduce human Toxoplasma gondii disease burden. Clin. Infect. Dis. 2015, 60, 101–107. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).