The Role of Cadaverine Synthesis on Pneumococcal Capsule and Protein Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, and Growth Conditions
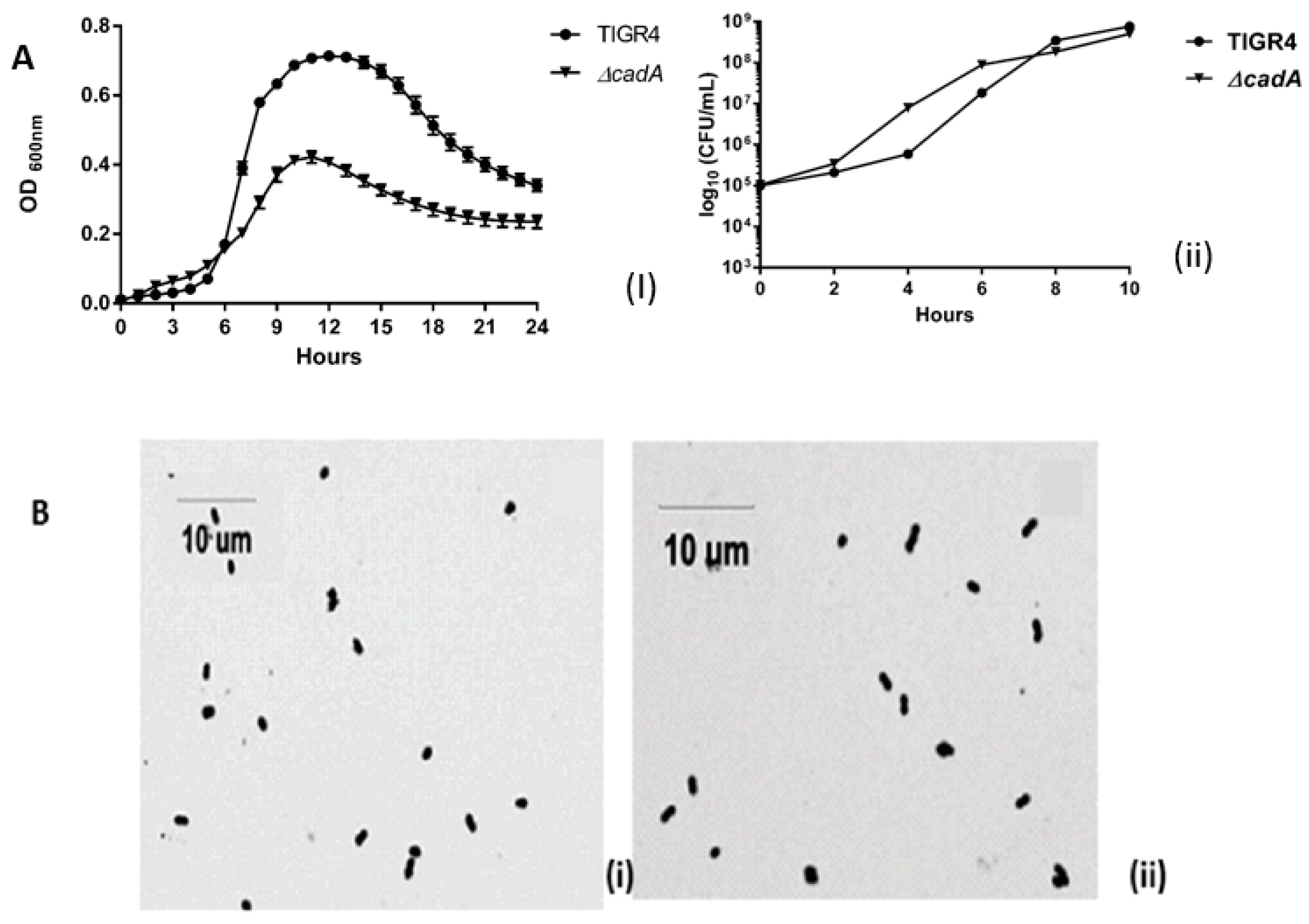
2.2. In Vitro Growth of TIGR4 and ΔcadA
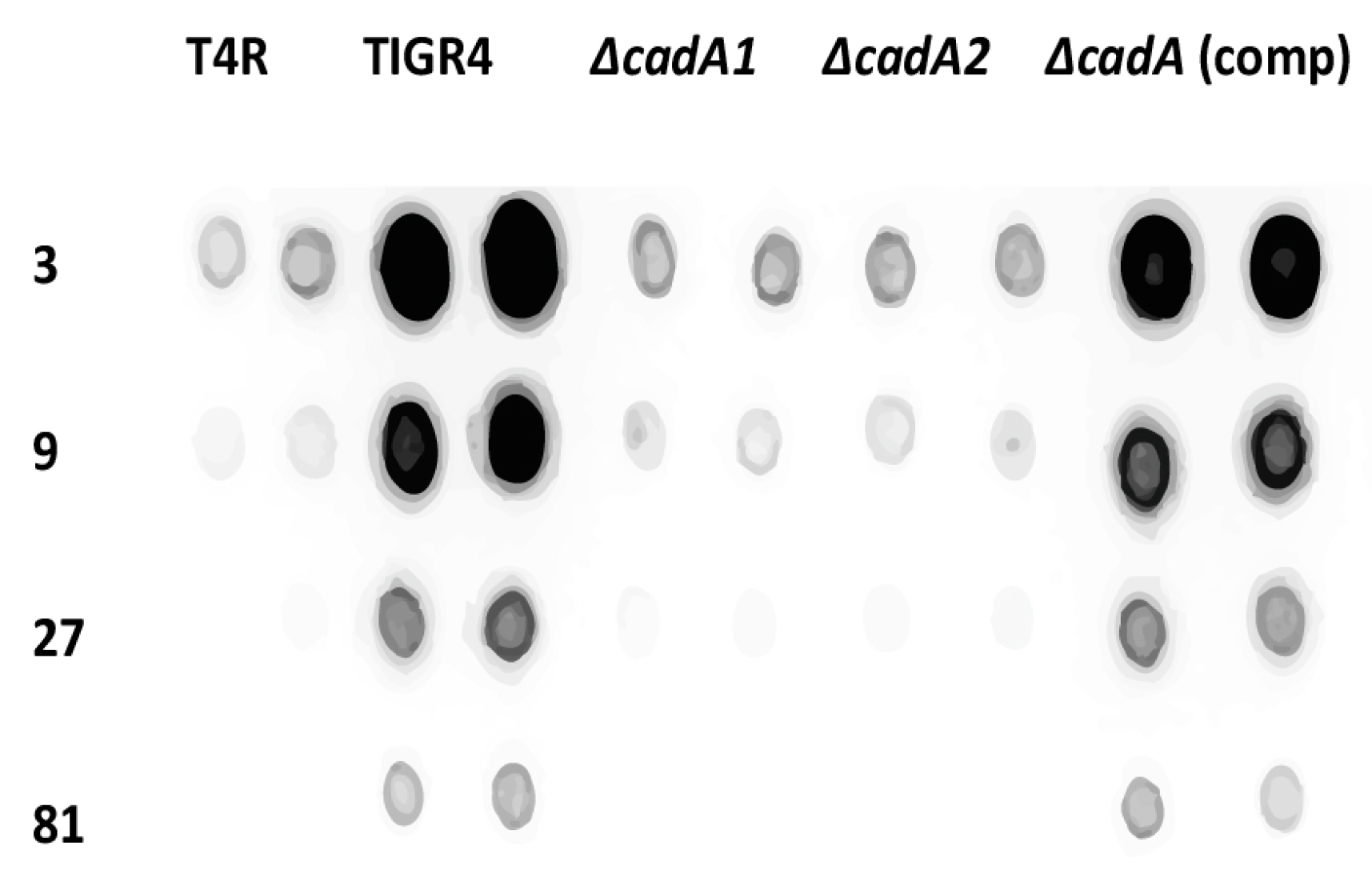
2.3. Measurement of Capsular Polysaccharides
2.4. Proteomics
2.5. Quantitative Real Time PCR
3. Results
3.1. Impact of ∆cadA on Pneumococcal Growth
3.2. Lysine Decarboxylase is Required for Capsule Production in S. pneumoniae
3.3. Lysine Decarboxylase Effects on Pneumococcal Protein Expression
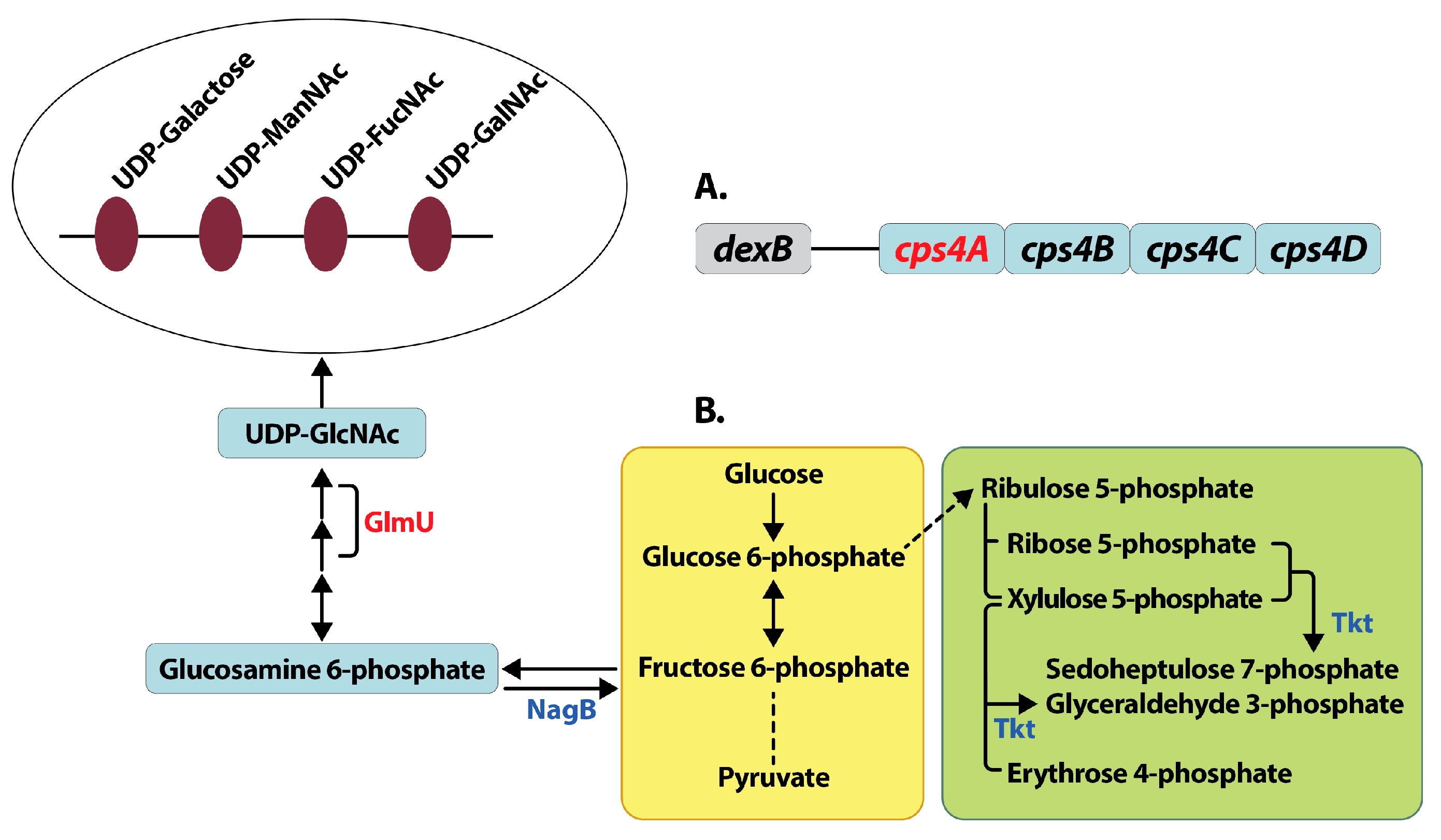
3.3.1. Capsule Biosynthesis
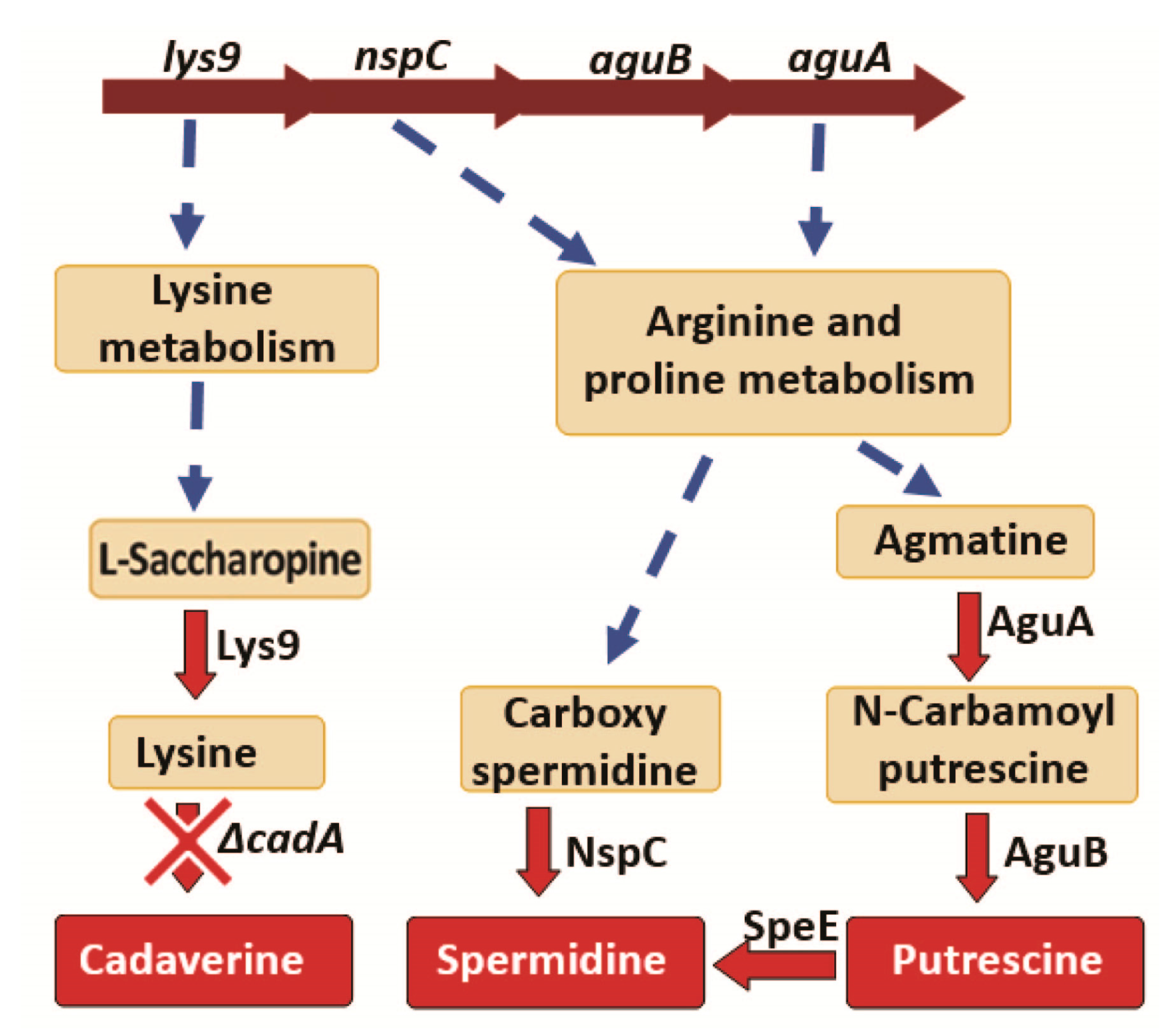
3.3.2. Polyamine Biosynthesis
3.3.3. Peptidoglycan
3.3.4. ABC Transporters
3.3.5. Pentose Phosphate Pathway
3.3.6. Carbohydrate Metabolism
3.4. Measurement of Gene Expression in ΔcadA
3.4.1. Capsule Biosynthesis
3.4.2. Polyamine Synthesis and Transport
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bridy-Pappas, A.E.; Margolis, M.B.; Center, K.J.; Isaacman, D.J. Streptococcus pneumoniae: Description of the pathogen, disease epidemiology, treatment, and prevention. Pharmacotherapy 2005, 25, 1193–1212. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Johnson, K.M.; Ray, G.T.; Wroe, P.; Lieu, T.A.; Moore, M.R.; Zell, E.R.; Linder, J.A.; Grijalva, C.G.; Metlay, J.P.; et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine 2011, 29, 3398–3412. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, D.M.; Trzcinski, K.; Lu, Y.J.; Bogaert, D.; Brandes, A.; Galagan, J.; Anderson, P.W.; Malley, R.; Lipsitch, M. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathog. 2009, 5, e1000476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayward, S.; Thompson, L.A.; McEachern, A. Is 13-Valent Pneumococcal Conjugate Vaccine (PCV13) Combined With 23-Valent Pneumococcal Polysaccharide Vaccine (PPSV23) Superior to PPSV23 alone for reducing incidence or severity of pneumonia in older adults? A Clin-IQ. J. Patient Cent. Res. Rev. 2016, 3, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Felmingham, D. Comparative antimicrobial susceptibility of respiratory tract pathogens. Chemotherapy 2004, 50 (Suppl. 1), 3–10. [Google Scholar] [CrossRef] [PubMed]
- Doern, G.V.; Richter, S.S.; Miller, A.; Miller, N.; Rice, C.; Heilmann, K.; Beekmann, S. Antimicrobial resistance among Streptococcus pneumoniae in the United States: Have we begun to turn the corner on resistance to certain antimicrobial Classes? Clin. Infect. Dis. 2005, 41, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kashiwagi, K. Polyamines: Mysterious modulators of cellular functions. Biochem. Biophys. Res. Commun. 2000, 271, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kashiwagi, K. Characteristics of cellular polyamine transport in prokaryotes and eukaryotes. Plant Physiol. Biochem. 2010, 48, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.J. Exploring polyamine biosynthetic diversity through comparative and functional genomics. Methods Mol. Biol. 2011, 720, 39–50. [Google Scholar] [PubMed]
- Michael, A.J. Biosynthesis of polyamines and polyamine-containing molecules. Biochem. J. 2016, 473, 2315–2329. [Google Scholar] [CrossRef] [PubMed]
- Tabor, C.W.; Tabor, H. Polyamines in microorganisms. Microbiol. Rev. 1985, 49, 81–99. [Google Scholar] [PubMed]
- Di Martino, M.L.; Campilongo, R.; Casalino, M.; Micheli, G.; Colonna, B.; Prosseda, G. Polyamines: Emerging players in bacteria-host interactions. Int. J. Med. Microbiol. 2013, 303, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Goytia, M.; Dhulipala, V.L.; Shafer, W.M. Spermine impairs biofilm formation by Neisseria gonorrhoeae. FEMS Microbiol. Lett. 2013, 343, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Burrell, M.; Hanfrey, C.C.; Murray, E.J.; Stanley-Wall, N.R.; Michael, A.J. Evolution and multiplicity of arginine decarboxylases in polyamine biosynthesis and essential role in Bacillus subtilis biofilm formation. J. Biol. Chem. 2010, 285, 39224–39238. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Terui, Y.; Yamamoto, T.; Kasahara, T.; Nakamura, M.; Tomitori, H.; Yamamoto, K.; Ishihama, A.; Michael, A.J.; Igarashi, K.; et al. Enhanced biofilm formation and/or cell viability by polyamines through stimulation of response regulators UvrY and CpxR in the two-component signal transducing systems, and ribosome recycling factor. Int. J. Biochem. Cell Biol. 2012, 44, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.N.; Wortham, B.W.; Lines, J.L.; Fetherston, J.D.; Perry, R.D.; Oliveira, M.A. Polyamines are essential for the formation of plague biofilm. J. Bacteriol. 2006, 188, 2355–2363. [Google Scholar] [CrossRef] [PubMed]
- Karatan, E.; Duncan, T.R.; Watnick, P.I. NspS, a predicted polyamine sensor, mediates activation of Vibrio cholerae biofilm formation by norspermidine. J. Bacteriol. 2005, 187, 7434–7443. [Google Scholar] [CrossRef] [PubMed]
- Wortham, B.W.; Patel, C.N.; Oliveira, M.A. Review article Polyamines in bacteria: Pleiotropic effects yet specific mechanisms. Adv. Exp. Med. Biol. 2007, 603, 106–115. [Google Scholar] [PubMed]
- Pan, Y.H.; Liao, C.C.; Kuo, C.C.; Duan, K.J.; Liang, P.H.; Yuan, H.S.; Hu, S.T.; Chak, K.F. The critical roles of polyamines in regulating ColE7 production and restricting ColE7 uptake of the colicin-producing Escherichia coli. J. Biol. Chem. 2006, 281, 13083–13091. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, I.M.; Silva, M.; Schuch, R.; Walker, W.A.; Siber, A.M.; Maurelli, A.T.; McCormick, B.A. Cadaverine prevents the escape of Shigella flexneri from the phagolysosome: A connection between bacterial dissemination and neutrophil transepithelial signaling. J. Infect. Dis. 2001, 184, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Swiatlo, E. A multifaceted role for polyamines in bacterial pathogens. Mol. Microbiol. 2008, 68, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.G.; Vazquez-Juarez, R.C.; Tutt, C.B.; Garcia-Gallegos, J.G. Pathoadaptive mutation that mediates adherence of shiga toxin-producing Escherichia coli O111. Infect. Immun. 2005, 73, 4766–4776. [Google Scholar] [CrossRef] [PubMed]
- Ware, D.; Jiang, Y.; Lin, W.; Swiatlo, E. Involvement of potD in Streptococcus pneumoniae polyamine transport and pathogenesis. Infect. Immun. 2006, 74, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Nanduri, B.; Swiatlo, E.; Ma, Y.; Pendarvis, K. Polyamine biosynthesis and transport mechanisms are crucial for fitness and pathogenesis of Streptococcus pneumoniae. Microbiology 2011, 157 Pt 2, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Swiatlo, E. Immunization with polyamine transport protein PotD protects mice against systemic infection with Streptococcus pneumoniae. Infect. Immun. 2006, 74, 5888–5892. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Briles, D.E.; King, J.; Hale, Y.; Swiatlo, E. Mucosal immunization with polyamine transport protein D (PotD) protects mice against nasopharyngeal colonization with Streptococcus pneumoniae. Exp. Biol. Med. 2009, 234, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Tettelin, H.; Nelson, K.E.; Paulsen, I.T.; Eisen, J.A.; Read, T.D.; Peterson, S.; Heidelberg, J.; DeBoy, R.T.; Haft, D.H.; Dodson, R.J.; et al. Complete Genome Sequence of a Virulent Isolate of Streptococcus pneumoniae. Science 2001, 293, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Kees Leenhouts, G.V.J.K. A lactococcal pWV01-based integration toolbox for bacteria. Methods Cell Sci. 1998, 20, 35–50. [Google Scholar] [CrossRef]
- Bricker, A.L.; Camilli, A. Transformation of a type 4 encapsulated strain of Streptococcus pneumoniae. FEMS Microbiol. Lett. 1999, 172, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Granok, A.B.; Parsonage, D.; Ross, R.P.; Caparon, M.G. The RofA Binding Site in Streptococcus pyogenes is utilized in multiple transcriptional pathways. J. Bacteriol. 2000, 182, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G.; Acar, H.; Nandipati, A.; Barlow, M. Growth rates made easy. Mol. Biol. Evol. 2014, 31, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, A.; Hoyland, C.N.; Vollmer, D.; Bisle, S.; Cleverley, R.M.; Johnsborg, O.; Havarstein, L.S.; Lewis, R.J.; Vollmer, W. Attachment of capsular polysaccharide to the cell wall in Streptococcus pneumoniae. Microb. Drug Resist. 2012, 18, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Rychli, K.; Guinane, C.M.; Daly, K.; Hill, C.; Cotter, P.D. Generation of nonpolar deletion mutants in Listeria monocytogenes using the “SOEing” method. In Listeria Monocytogenes: Methods and Protocols; Jordan, K., Fox, E.M., Wagner, M., Eds.; Springer: New York, NY, USA, 2014; pp. 187–200. [Google Scholar]
- Rai, A.N.; Thornton, J.A.; Stokes, J.; Sunesara, I.; Swiatlo, E.; Nanduri, B. Polyamine transporter in Streptococcus pneumoniae is essential for evading early innate immune responses in pneumococcal pneumonia. Sci. Rep. 2016, 6, 26964. [Google Scholar] [CrossRef] [PubMed]
- Andon, N.L.; Hollingworth, S.; Koller, A.; Greenland, A.J.; Yates, J.R.; Haynes, P.A. Proteomic characterization of wheat amyloplasts using identification of proteins by tandem mass spectrometry. Proteomics 2002, 2, 1156–1168. [Google Scholar] [CrossRef]
- Qian, W.J.; Jacobs, J.M.; Camp, D.G., 2nd; Monroe, M.E.; Moore, R.J.; Gritsenko, M.A.; Calvano, S.E.; Lowry, S.F.; Xiao, W.; Moldawer, L.L.; et al. Comparative proteome analyses of human plasma following in vivo lipopolysaccharide administration using multidimensional separations coupled with tandem mass spectrometry. Proteomics 2005, 5, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2008, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Kashiwagi, K.; Kawai, G.; Ishihama, A.; Igarashi, K. Polyamine enhancement of the synthesis of adenylate cyclase at the translational level and the consequential stimulation of the synthesis of the RNA polymerase sigma 28 subunit. J. Biol. Chem. 2001, 276, 16289–16295. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
- Vizcaino, J.A.; Csordas, A.; del-Toro, N.; Dianes, J.A.; Griss, J.; Lavidas, I.; Mayer, G.; Perez-Riverol, Y.; Reisinger, F.; Ternent, T.; et al. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 2016, 44, D447–D456. [Google Scholar] [CrossRef] [PubMed]
- Battig, P.; Hathaway, L.J.; Hofer, S.; Muhlemann, K. Serotype-specific invasiveness and colonization prevalence in Streptococcus pneumoniae correlate with the lag phase during in vitro growth. Microbes Infect. 2006, 8, 2612–2617. [Google Scholar] [CrossRef] [PubMed]
- Melin, M.; Trzcinski, K.; Meri, S.; Kayhty, H.; Vakevainen, M. The capsular serotype of Streptococcus pneumoniae is more important than the genetic background for resistance to complement. Infect. Immun. 2010, 78, 5262–5270. [Google Scholar] [CrossRef] [PubMed]
- Bentley, S.D.; Aanensen, D.M.; Mavroidi, A.; Saunders, D.; Rabbinowitsch, E.; Collins, M.; Donohoe, K.; Harris, D.; Murphy, L.; Quail, M.A.; et al. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2006, 2, e31. [Google Scholar] [CrossRef] [PubMed]
- Yother, J. Capsules of Streptococcus pneumoniae and other bacteria: Paradigms for polysaccharide biosynthesis and regulation. Annu. Rev. Microbiol. 2011, 65, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; He, Y.; Wang, K.Y.; Wang, J.; Zeng, Y.K.; Chen, Y.X.; Chen, D.; Geng, Y.; OuYang, P. cpsJ gene of Streptococcus iniae is involved in capsular polysaccharide synthesis and virulence. Antonie Van Leeuwenhoek 2016, 109, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Diaz, J.; Rubio-Del-Campo, A.; Yebra, M.J. Regulatory insights into the production of UDP-N-acetylglucosamine by Lactobacillus casei. Bioengineered 2012, 3, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Reichmann, N.T.; Grundling, A. Location, synthesis and function of glycolipids and polyglycerolphosphate lipoteichoic acid in Gram-positive bacteria of the phylum Firmicutes. FEMS Microbiol. Lett. 2011, 319, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.; Gonzalez, A.; Stelter, M.; Perez-Dorado, I.; Kahn, R.; Morales, M.; Moscoso, M.; Campuzano, S.; Campillo, N.E.; Mobashery, S.; et al. Crystal structure of CbpF, a bifunctional choline-binding protein and autolysis regulator from Streptococcus pneumoniae. EMBO Rep. 2009, 10, 246–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarre, W.W.; Ton-That, H.; Faull, K.F.; Schneewind, O. Multiple enzymatic activities of the murein hydrolase from staphylococcal phage. Identification of a d-alanyl-glycine endopeptidase activity. J. Biol. Chem. 1999, 274, 15847–15856. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, I.; Peters, K.; Stahlmann, C.; Hakenbeck, R.; Denapaite, D. Penicillin-binding protein 2X of Streptococcus pneumoniae: The mutation Ala707Asp within the C-terminal PASTA2 domain leads to destabilization. Microb. Drug Resist. 2014, 20, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jones, V.C.; Scherman, M.S.; Mahapatra, S.; Crick, D.; Bhamidi, S.; Xin, Y.; McNeil, M.R.; Ma, Y. Expression, essentiality, and a microtiter plate assay for mycobacterial GlmU, the bifunctional glucosamine-1-phosphate acetyltransferase and N-acetylglucosamine-1-phosphate uridyltransferase. Int. J. Biochem. Cell Biol. 2008, 40, 2560–2571. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, T.; Ibrahim, S. Choline kinase, a novel drug target for the inhibition of Streptococcus pneumoniae. Antibiotics 2017, 6, E20. [Google Scholar] [CrossRef] [PubMed]
- McAllister, L.J.; Tseng, H.J.; Ogunniyi, A.D.; Jennings, M.P.; McEwan, A.G.; Paton, J.C. Molecular analysis of the psa permease complex of Streptococcus pneumoniae. Mol. Microbiol. 2004, 53, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Yesilkaya, H.; Kadioglu, A.; Gingles, N.; Alexander, J.E.; Mitchell, T.J.; Andrew, P.W. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect. Immun. 2000, 68, 2819–2826. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. Iron-sulphur clusters and the problem with oxygen. Mol. Microbiol. 2006, 59, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Chekabab, S.M.; Harel, J.; Dozois, C.M. Interplay between genetic regulation of phosphate homeostasis and bacterial virulence. Virulence 2014, 5, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Chekabab, S.M.; Jubelin, G.; Dozois, C.M.; Harel, J. PhoB activates Escherichia coli O157:H7 virulence factors in response to inorganic phosphate limitation. PLoS ONE 2014, 9, e94285. [Google Scholar] [CrossRef] [PubMed]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Gruning, N.M.; Kruger, A.; Tauqeer Alam, M.; et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. Camb. Philos. Soc. 2015, 90, 927–963. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.B.; Lolkema, J.S. CcpA-dependent carbon catabolite repression in bacteria. Microbiol. Mol. Biol. Rev. 2003, 67, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.; Baliga, N.S.; Camilli, A. Catabolite control protein A (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae. J. Bacteriol. 2005, 187, 8340–8349. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Dam, P.; Chou, J.; Olman, V.; Xu, Y. DOOR: A database for prokaryotic operons. Nucleic Acids Res. 2009, 37, D459–D463. [Google Scholar] [CrossRef] [PubMed]
- Ware, D.; Watt, J.; Swiatlo, E. Utilization of putrescine by Streptococcus pneumoniae during growth in choline-limited medium. J. Microbiol. 2005, 43, 398–405. [Google Scholar] [PubMed]
- Koski, P.; Vaara, M. Polyamines as Constituents of the Outer Membranes of Escherichia coli and Salmonella typhimurium. J. Bacteriol. 1991, 173, 3695–3699. [Google Scholar] [CrossRef] [PubMed]
- Yethon, J.A.; Vinogradov, E.; Perry, M.B.; Whitfield, C. Mutation of the lipopolysaccharide core glycosyltransferase encoded by waaG destabilizes the outer membrane of Escherichia coli by interfering with core phosphorylation. J. Biotechnol. 2000, 182, 5620–5623. [Google Scholar] [CrossRef]
- Kamio, Y. Structural specificity of diamines covalently linked to peptidoglycan for cell growth of Veillonella alcalescens and Selenomonas ruminantium. J. Bacteriol. 1987, 169, 4837–4840. [Google Scholar] [CrossRef] [PubMed]
- Samartzidou, H.; Mehrazin, M.; Xu, Z.; Benedik, M.J.; Delcour, A.H. Cadaverine inhibition of porin plays a role in cell survival at acidic pH. J. Bacteriol. 2003, 185, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Paterson, G.K.; Blue, C.E.; Mitchell, T.J. Role of two-component systems in the virulence of Streptococcus pneumoniae. J. Med. Microbiol. 2006, 55 Pt 4, 355–363. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, L.S.; Swiatlo, E. Should pneumococcal vaccines eliminate nasopharyngeal colonization? MBio 2016, 7, e00545-16. [Google Scholar] [CrossRef] [PubMed]
- Maurelli, A.T.; Fernandez, R.E.; Bloch, C.A.; Rode, C.K.; Fasano, A. Black holes and bacterial pathogenicity: A large genomic deletion that enhances the virulence of Shigella spp. and enteroinvasive Escherichia coli. Proc. Natl. Acad. Sci. USA 1998, 95, 3943–3948. [Google Scholar] [CrossRef] [PubMed]
- Duggan, J.M.; You, D.; Cleaver, J.O.; Larson, D.T.; Garza, R.J.; Guzman Pruneda, F.A.; Tuvim, M.J.; Zhang, J.; Dickey, B.F.; Evans, S.E. Synergistic interactions of TLR2/6 and TLR9 induce a high level of resistance to lung infection in mice. J. Immunol. 2011, 186, 5916–5926. [Google Scholar] [CrossRef] [PubMed]
- Wortham, B.W.; Oliveira, M.A.; Fetherston, J.D.; Perry, R.D. Polyamines are required for the expression of key Hms proteins important for Yersinia pestis biofilm formation. Environ. Microbiol. 2010, 12, 2034–2047. [Google Scholar] [CrossRef] [PubMed]
- Feldman, C.; Anderson, R. Recent advances in our understanding of Streptococcus pneumoniae infection. F1000Prime Rep. 2014, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Hardy, G.G.; Magee, A.D.; Ventura, C.L.; Caimano, M.J.; Yother, J. Essential role for cellular phosphoglucomutase in virulence of type 3 Streptococcus pneumoniae. Infect. Immun. 2001, 69, 2309–2317. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.J. Interaction of gram-positive microorganisms with complement. Curr. Top. Microbiol. Immunol. 1985, 121, 159–187. [Google Scholar] [PubMed]
- Paterson, G.K.; Orihuela, C.J. Pneumococci: Immunology of the innate host response. Respirology 2010, 15, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, A.T.; Orihuela, C.J. Anatomical site-specific contributions of pneumococcal virulence determinants. Pneumonia 2016, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.N.; Austrian, R.; Sreenivasan, P.K.; Masure, H.R. Phase variation in pneumococcal opacity: Relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 1994, 62, 2582–2589. [Google Scholar] [PubMed]
- Shainheit, M.G.; Mule, M.; Camilli, A. The core promoter of the capsule operon of Streptococcus pneumoniae is necessary for colonization and invasive disease. Infect. Immun. 2014, 82, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.M.; Farshchi Andisi, V.; Gradstedt, H.; Neef, J.; Kuipers, O.P.; Neves, A.R.; Bijlsma, J.J. Pyruvate oxidase influences the sugar utilization pattern and capsule production in Streptococcus pneumoniae. PLoS ONE 2013, 8, e68277. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Yang, J.; Dong, Y.; Swiatlo, E.; Zhang, J.R.; Metzger, D.W.; Bai, G. Deletion of arcD in Streptococcus pneumoniae D39 impairs its capsule and attenuates virulence. Infect. Immun. 2013, 81, 3903–3911. [Google Scholar] [CrossRef] [PubMed]
- Echlin, H.; Frank, M.W.; Iverson, A.; Chang, T.C.; Johnson, M.D.; Rock, C.O.; Rosch, J.W. Pyruvate Oxidase as a Critical Link between Metabolism and Capsule Biosynthesis in Streptococcus pneumoniae. PLoS Pathog. 2016, 12, e1005951. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kashiwagi, K. Modulation of protein synthesis by polyamines. IUBMB Life 2015, 67, 160–169. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sequence * (5′→3′) | Experiment |
|---|---|---|
| cadAF1 | AGCAAATATAAACCCGAGTAAAAA | Mutagenesis |
| cadAR1 | CAGGTACCGCTTGTGACCTGGAACATC | Mutagenesis |
| cadAF2 | CAGAGCTCGTTTCGGTTTGCGATTTT | Mutagenesis |
| cadAR2 | GATCTTCCGTCCCTTGGAG | Mutagenesis |
| cadAF-XbaI | TTCCCCGGGCCGTGCGAAAATCATCGCC | Complementation |
| cadAR-SacI | ATTCGAGGAAGACAGAGGTGTACTATTC | Complementation |
| gyrBF | CCGTCCTGCTGTTGAGACC | qRT-PCR |
| gyrBR | GTGAAGACCACCTGAAACCTTG | qRT-PCR |
| potDF | AAACCTGAAAATGCTCTCCAAAATG | qRT-PCR |
| potDR | CCTTATCTTCCTTTGTTTCCTCTGG | qRT-PCR |
| cps4AF | TCAAGTCAAGTCAGAATACCGATTTG | qRT-PCR |
| cps4AR | TCAAAGACACTATTTAGGACAATGGC | qRT-PCR |
| speEF | TGCGGATGATTTCGTCTACAATG | qRT-PCR |
| speER | CCAGTTCAGGATAGAGGGTTAATAC | qRT-PCR |
| aguAF | GCTTAGTCCTGGTCGCAATC | qRT-PCR |
| aguAR | CTGGGGATCATTTTCGTCAT | qRT-PCR |
| lys9F | GGCTTGACTGCTCTTCTTGG | qRT-PCR |
| lys9R | AGTAAGAACCTGGCGCAGAA | qRT-PCR |
| nspCF | ATGTATTTGCGCCTGCTTTC | qRT-PCR |
| nspCR | TGGTGCACAAGGGTCATAGA | qRT-PCR |
| Description | Protein | ΔcadA/TIGR4 (Fold Change) | Function |
|---|---|---|---|
| N-carbamoylputrescine amidase | *SP_0922 | −10.0 | Putrescine biosynthesis |
| Carboxynorspermidine decarboxylase | NspC | −5.0 | Spermidine biosynthesis |
| Homoserine dehydrogenase | Hom | −1.4 | Lysine biosynthesis |
| 4-hydroxy-tetrahydrodipicolinate synthase | DapA | −10.0 | Lysine biosynthesis |
| 4-hydroxy-tetrahydrodipicolinate reductase | DapB | −2.5 | Lysine biosynthesis |
| N-acetyldiaminopimelate deacetylase | SP_2096 | −2.5 | Lysine biosynthesis |
| Saccharopine dehydrogenase | Lys9 | −25.0 | Lysine biosynthesis |
| Aspartate-semialdehyde dehydrogenase | Asd | −25.0 | Lysine biosynthesis |
| 2,3,4,5-tetrahydropyridine-2-carboxylate N-Succinyl transferase | DapH | −1.7 | Lysine biosynthesis |
| 50S ribosomal protein L21 | RplU | −5.0 | Regulation of protein elongation |
| Ribosome maturation factor | RimP | -5.0 | Regulation of protein maturation |
| Lysine-tRNA ligase | LysS | −3.3 | Amino acid metabolism |
| Iron-compound ABC Transporter | FhuD | −50.0 | Iron complex ABC transporter |
| Phosphate-binding protein PstS 2 | PstS 2 | 41.0 | Phosphate ion transport |
| Phosphate import ATP-binding protein PstB 3 | PstB 3 | 36.0 | Phosphate ion transport |
| Phosphate transport system permease protein | PstC | 7.0 | Phosphate ion transport |
| Phosphate-specific transport system accessory protein PhoU homolog | PhoU | 43.0 | Phosphate ion transport |
| ABC transporter, ATP-binding/permease protein | SP_2073 | −3.3 | Oligopeptide ABC transporter |
| Oligopeptide binding protein | OppA | −25.0 | Oligopeptide ABC transporter |
| Oligopeptide transport ATP-binding protein | OppD | −1.4 | Oligopeptide ABC transporter |
| Oligopeptide transport ATP-binding protein | OppF | −1.7 | Oligopeptide ABC transporter |
| Oligopeptide transport system permease protein | OppB | −1.7 | Oligopeptide ABC transporter |
| Manganese ABC transporter-substrate-binding lipoprotein | PsaA | 2.4 | Oxidative stress |
| Manganese ABC transporter, ATP -binding protein | PsaB | 6.8 | Oxidative stress |
| Penicillin-binding protein 2x | Pbp2X | −2.5 | Peptidoglycan biosynthesis |
| Choline kinase | Pck | −2.0 | Cell wall biosynthesis |
| UDP-glucose 4-epimerase | GalE-1 | −1.3 | Carbohydrate metabolism |
| Tagatose 1,6-diphosphate aldolase | LacD | 1.4 | Carbohydrate metabolism |
| Galactose-6-phosphate isomerase subunit | LacB | 2.1 | Carbohydrate metabolism |
| Catabolite control protein A | CcpA | −2.5 | Carbohydrate metabolism |
| Bifunctional protein | GlmU | −1.7 | UDP- GlcNAc synthesis |
| N-acetylglucosamine-6-phosphate deacetylase | NagA | 1.4 | N-acetylglucosamine degradation |
| N-acetylglucosamine-6-phosphate deaminase | NagB | 2.1 | N-acetylglucosamine degradation |
| Transketolase, C-terminal subunit | TktC | 67.0 | Pentose phosphate pathway |
| Transketolase, N-terminal subunit | TktN | 46.0 | Pentose phosphate pathway |
| Ascorbate-specific PTS, EIIC component | SgaT2 | 31.0 | Ascorbate utilization |
| Ascorbate-specific PTS system, EIIB component | SgaB2 | 32.0 | Ascorbate utilization |
| Phosphocarrier protein HPr | PtsH | 21.0 | Phosphotransferase system (PTS) |
| Gene | Description | ΔcadA/TIGR4 (Fold change) | p-Value |
|---|---|---|---|
| potD | Spermidine/putrescine ABC transporter, spermidine/putrescine-binding protein | −2.0 | 1.93E−04 |
| speE | Spermidine synthase | −27.0 | 1.29E−06 |
| cps4A | Capsular polysaccharide biosynthesis protein 4A | −2.0 | 2.03E−07 |
| lys9 | Saccharopine dehydrogenase | −26.0 | 3.83E−12 |
| nspC | Carboxynorspermidine decarboxylase | −34.0 | 4.70E−10 |
| aguA | Agmatine deiminase | −30.0 | 2.87E−12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamya, M.F.; Ayoola, M.B.; Park, S.; Shack, L.A.; Swiatlo, E.; Nanduri, B. The Role of Cadaverine Synthesis on Pneumococcal Capsule and Protein Expression. Med. Sci. 2018, 6, 8. https://doi.org/10.3390/medsci6010008
Nakamya MF, Ayoola MB, Park S, Shack LA, Swiatlo E, Nanduri B. The Role of Cadaverine Synthesis on Pneumococcal Capsule and Protein Expression. Medical Sciences. 2018; 6(1):8. https://doi.org/10.3390/medsci6010008
Chicago/Turabian StyleNakamya, Mary F., Moses B. Ayoola, Seongbin Park, Leslie A. Shack, Edwin Swiatlo, and Bindu Nanduri. 2018. "The Role of Cadaverine Synthesis on Pneumococcal Capsule and Protein Expression" Medical Sciences 6, no. 1: 8. https://doi.org/10.3390/medsci6010008
APA StyleNakamya, M. F., Ayoola, M. B., Park, S., Shack, L. A., Swiatlo, E., & Nanduri, B. (2018). The Role of Cadaverine Synthesis on Pneumococcal Capsule and Protein Expression. Medical Sciences, 6(1), 8. https://doi.org/10.3390/medsci6010008




