Effect of Pharmaceutical Compounds (Diclofenac, Ibuprofen, and Erythromycin) on the Heterotrophic Behaviors of Biomass of a Membrane Bioreactor to Treat Urban Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Pilot Plant of Membrane Bioreactor
2.2. Operating Conditions
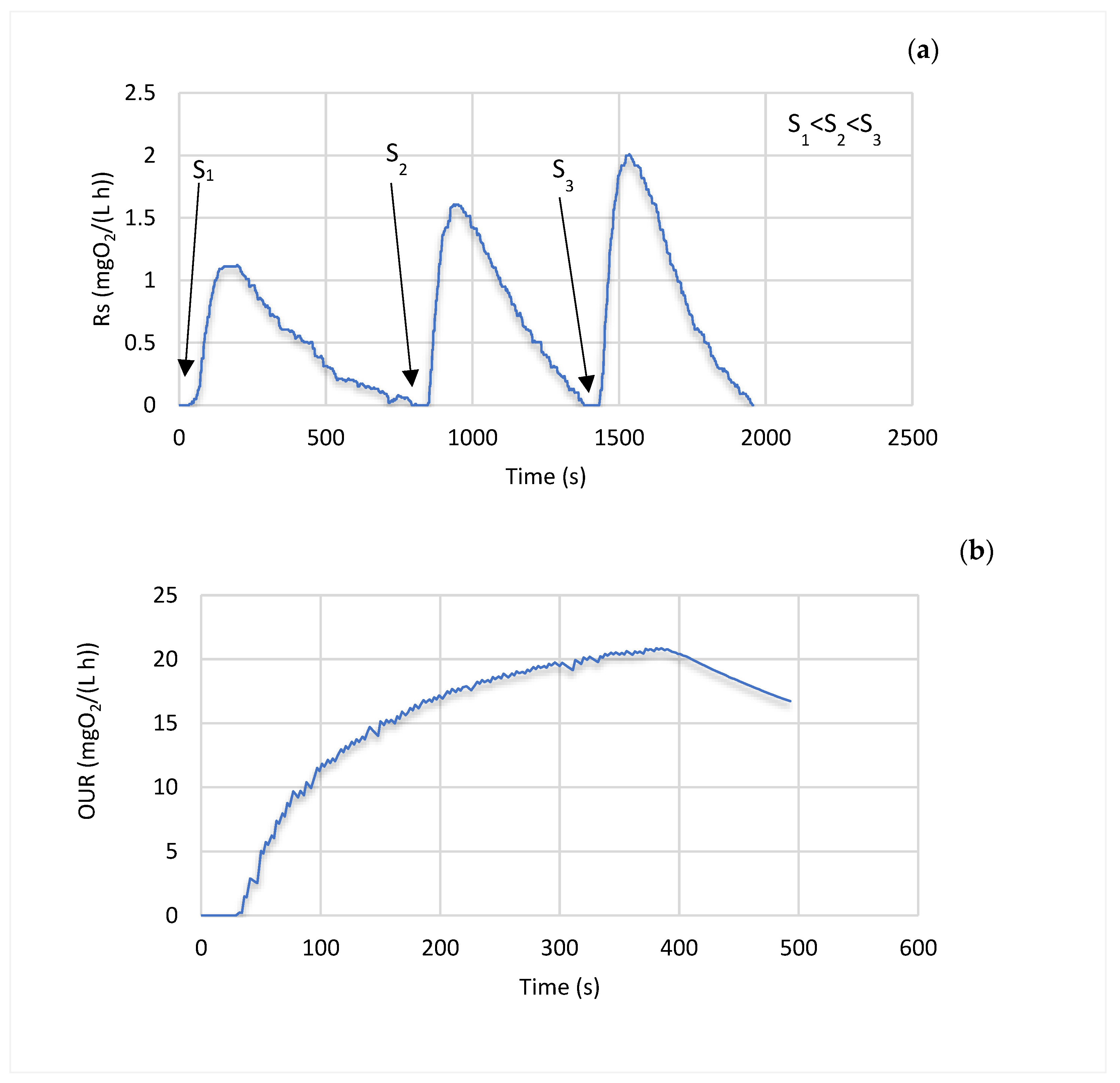
2.3. Dosing Study
2.4. Experimental Procedure
2.5. Statistical Analysis
3. Results and Discussion
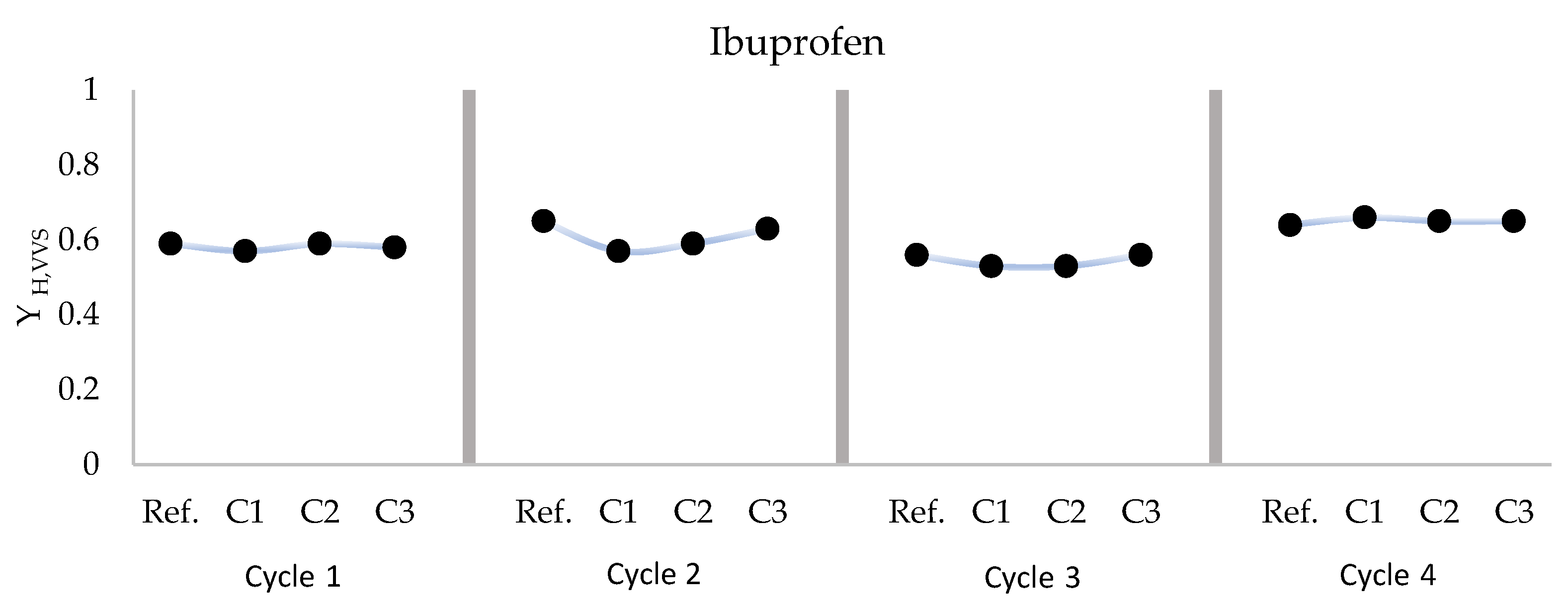
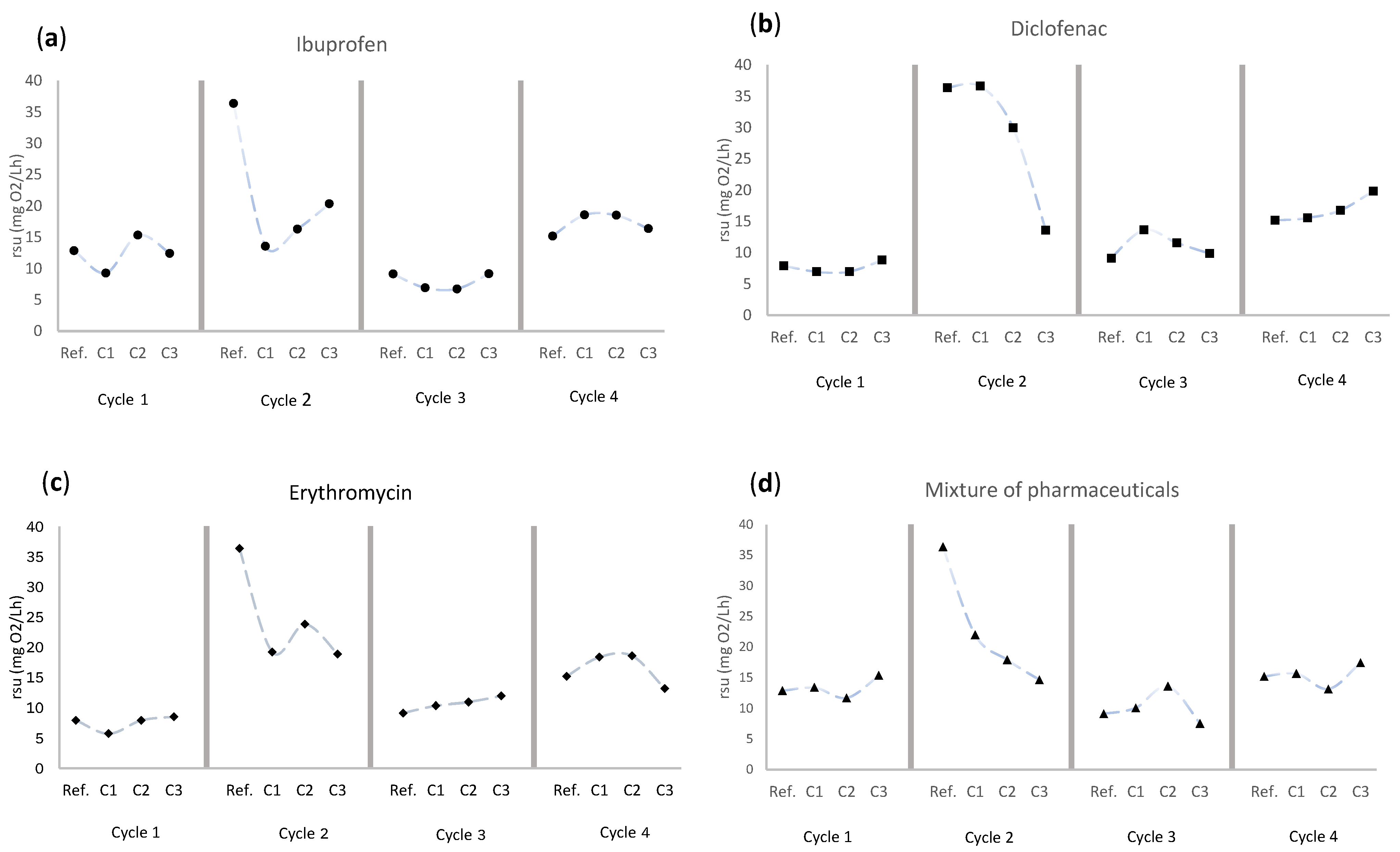
3.1. Ibuprofen
3.2. Diclofenac
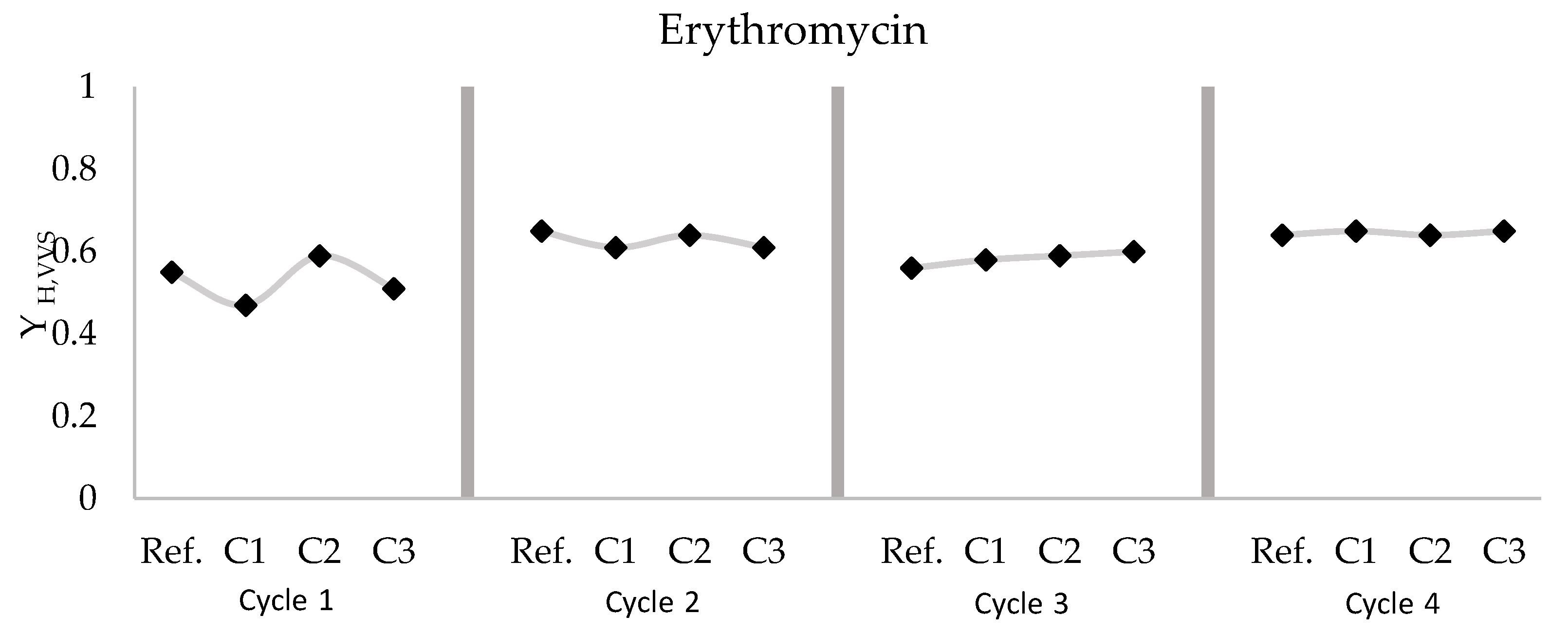
3.3. Erythromycin
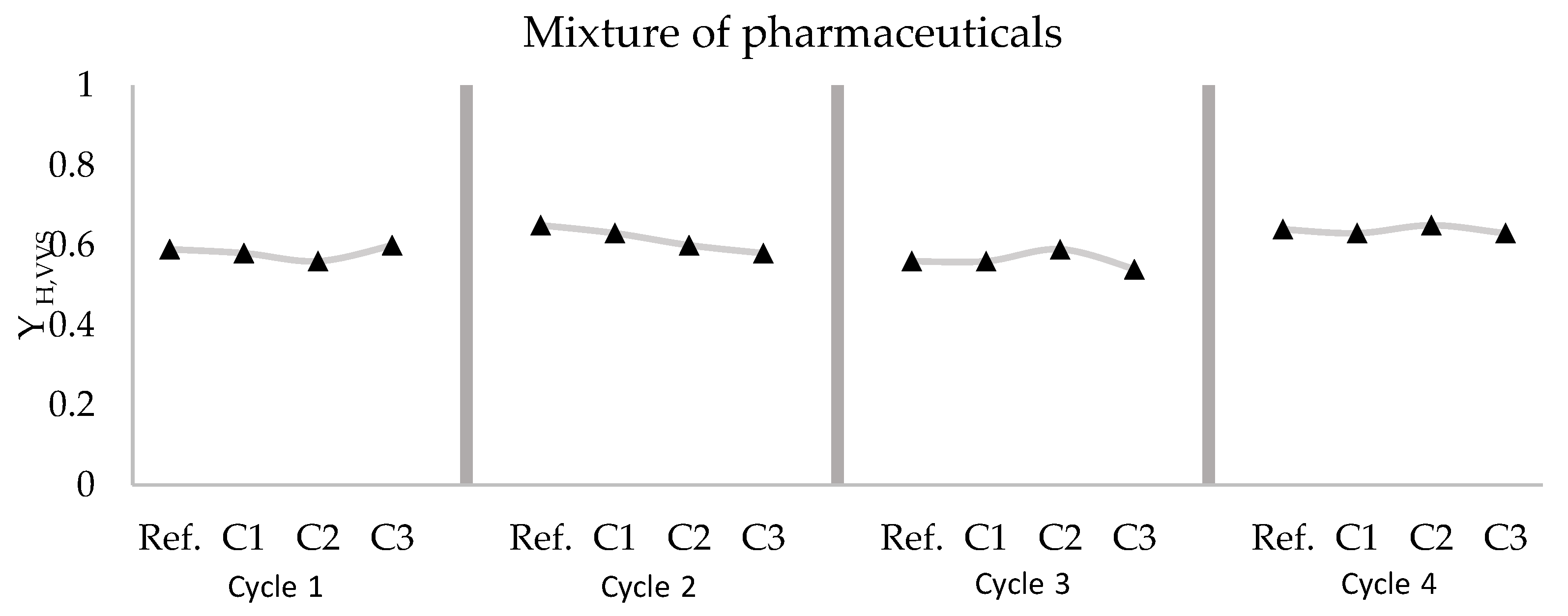
3.4. Mixture of Ibuprofen, Diclofenac, and Erythromycin
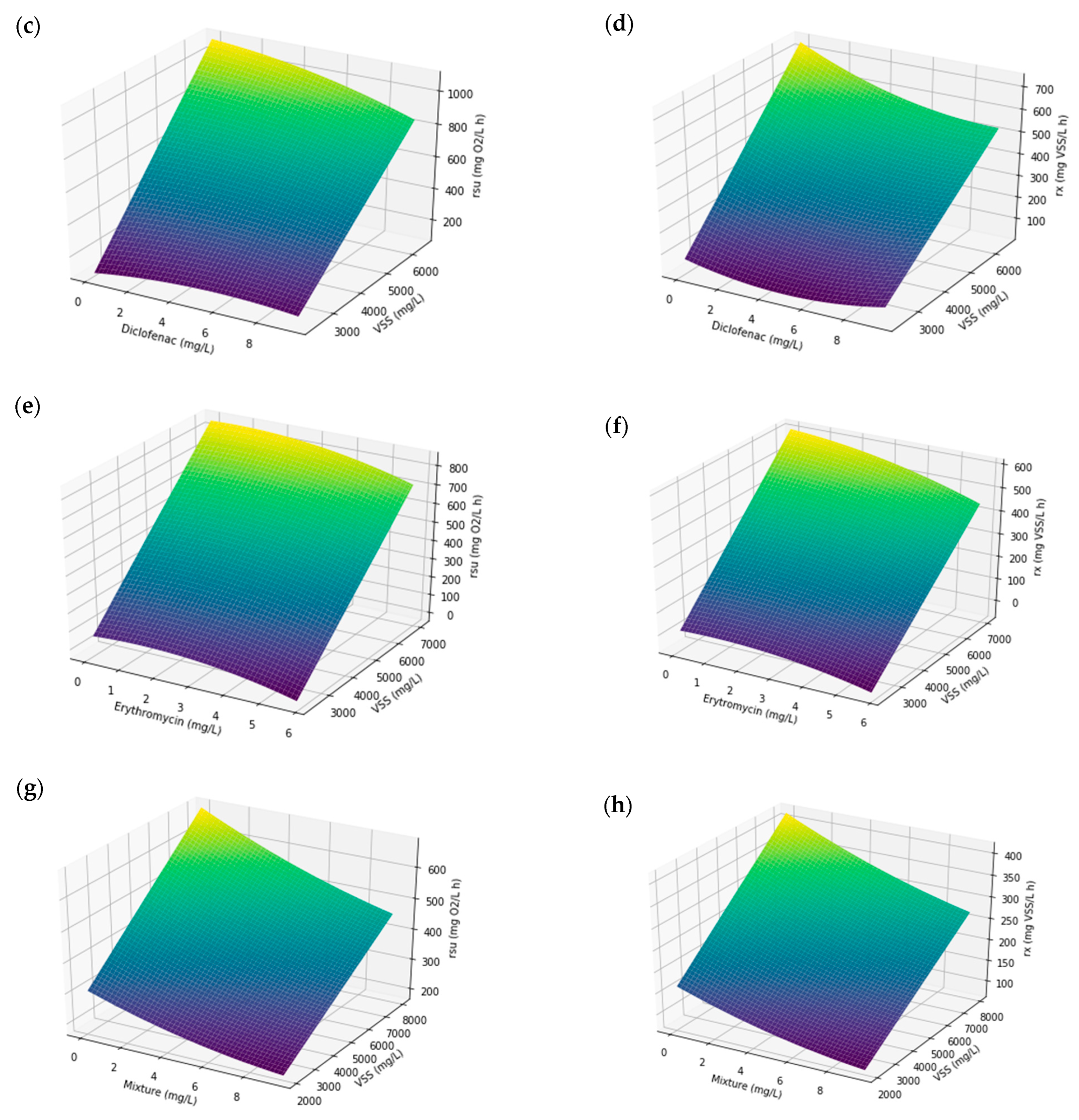
3.5. Combined Effect of Operational Variables
4. Conclusions
- At a HRT of 6 h, the heterotrophic biomass showed a higher microbial activity than a HRT of 12 h and the effect of the pharmaceutical on the biomass is higher. Regardless of the MLSS concentration and pharmaceutical type, the higher SRT causes the lower effect of dosing in the heterotrophic biomass. Furthermore, the erythromycin is the most affected pharmaceutical in the heterotrophic biomass since it is an antibiotic.
- The higher temperature at a HRT of 6 h had less of an effect on the behavior of heterotrophic biomass under the presence of pharmaceuticals.
- Different response surfaces of the system were obtained to predict the expected behavior of the biomass against possible spills of the pharmaceuticals studied. When the biomass is dosed with the pharmaceuticals individually, a greater kinetic response is produced than when it is doped with a combination of the three pharmaceuticals. This slower kinetic response in the mixture of diclofenac, ibuprofen, and erythromycin indicates that there is a synergistic effect between them.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, A. A review of wastewater irrigation: Environmental implications. Resour. Conserv. Recycl. 2021, 168, 105454. [Google Scholar] [CrossRef]
- Romeiko, X.X. Comprehensive water footprint assessment of conventional and four alternative resource recovery based wastewater service options. Resour. Conserv. Recycl. 2019, 151, 104458. [Google Scholar] [CrossRef]
- Zhao, J.; Dang, Z.; Muddassir, M.; Raza, S.; Zhong, A.; Wang, X.; Jin, J. A New Cd(II)-Based Coordination Polymer for Efficient Photocatalytic Removal of Organic Dyes. Molecules 2023, 28, 6848. [Google Scholar] [CrossRef]
- Pan, Y.; Rao, C.; Tan, X.; Ling, Y.; Singh, A.; Kumar, A.; Li, B.; Liu, J. Cobalt-seamed C-methylpyrogallol[4]arene nanocapsules-derived magnetic carbon cubes as advanced adsorbent toward drug contaminant removal. Chem. Eng. J. 2022, 433, 133857. [Google Scholar] [CrossRef]
- Palma, P.; Fialho, S.; Lima, A.; Novais, M.H.; Costa, M.J.; Montemurro, N.; Pérez, S.; de Alda, M.L. Pharmaceuticals in a Mediterranean Basin: The influence of temporal and hydrological patterns in environmental risk assessment. Sci. Total Environ. 2020, 709, 136205. [Google Scholar] [CrossRef]
- Monteoliva-García, A.; Martín-Pascual, J.; Muñío, M.; Poyatos, J. Effects of carrier addition on water quality and pharmaceutical removal capacity of a membrane bioreactor—Advanced oxidation process combined treatment. Sci. Total Environ. 2020, 708, 135104. [Google Scholar] [CrossRef]
- Sabri, N.A.; Schmitt, H.; Van Der Zaan, B.; Gerritsen, H.W.; Zuidema, T.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Prevalence of antibiotics and antibiotic resistance genes in a wastewater effluent-receiving river in the Netherlands. J. Environ. Chem. Eng. 2020, 8, 102245. [Google Scholar] [CrossRef]
- Okoye, C.O.; Nyaruaba, R.; Ita, R.E.; Okon, S.U.; Addey, C.I.; Ebido, C.C.; Opabunmi, A.O.; Okeke, E.S.; Chukwudozie, K.I. Antibiotic resistance in the aquatic environment: Analytical techniques and interactive impact of emerging contaminants. Environ. Toxicol. Pharmacol. 2022, 96, 103995. [Google Scholar] [CrossRef]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef]
- Schröder, P.; Helmreich, B.; Škrbić, B.; Carballa, M.; Papa, M.; Pastore, C.; Emre, Z.; Oehmen, A.; Langenhoff, A.; Molinos, M.; et al. Status of hormones and painkillers in wastewater effluents across several European states—Considerations for the EU watch list concerning estradiols and diclofenac. Environ. Sci. Pollut. Resear. 2016, 23, 12835–12866. [Google Scholar] [CrossRef]
- Ali, A.M.; Rønning, H.T.; Alarif, W.; Kallenborn, R.; Al-Lihaibi, S.S. Occurrence of pharmaceuticals and personal care products in effluent-dominated Saudi Arabian coastal waters of the Red Sea. Chemosphere 2017, 175, 505–513. [Google Scholar] [CrossRef]
- Liwarska-Bizukojc, E.; Galamon, M.; Bernat, P. Kinetics of Biological Removal of the Selected Micropollutants and Their Effect on Activated Sludge Biomass. Water Air Soil Pollut. 2018, 229, 356. [Google Scholar] [CrossRef] [PubMed]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Mujtaba, G.; Lee, K. Treatment of real wastewater using co-culture of immobilized Chlorella vulgaris and suspended activated sludge. Water Res. 2017, 120, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Q.; Lam, J.C.; Li, W.-W.; Yu, H.-Q.; Lam, P.K. Spatial distribution and removal performance of pharmaceuticals in municipal wastewater treatment plants in China. Sci. Total Environ. 2017, 586, 1162–1169. [Google Scholar] [CrossRef]
- Gonzalez-Gil, L.; Fernandez-Fontaina, E.; Singh, R.R.; Lema, J.M.; Carballa, M.; Aga, D.S. Feeding composition and sludge retention time both affect (co-)metabolic biotransformation of pharmaceutical compounds in activated sludge systems. J. Environ. Chem. Eng. 2021, 9, 105123. [Google Scholar] [CrossRef]
- Sundararaman, S.; Kumar, J.A.; Deivasigamani, P.; Devarajan, Y. Emerging pharma residue contaminants: Occurrence, monitoring, risk and fate assessment—A challenge to water resource management. Sci. Total Environ. 2022, 825, 153897. [Google Scholar] [CrossRef]
- Zhou, S.; Di Paolo, C.; Wu, X.; Shao, Y.; Seiler, T.-B.; Hollert, H. Optimization of screening-level risk assessment and priority selection of emerging pollutants—The case of pharmaceuticals in European surface waters. Environ. Int. 2019, 128, 1–10. [Google Scholar] [CrossRef]
- Yang, J.-F.; Ying, G.-G.; Zhao, J.-L.; Tao, R.; Su, H.-C.; Liu, Y.-S. Spatial and seasonal distribution of selected antibiotics in surface waters of the Pearl Rivers, China. J. Environ. Sci. Health Part B 2011, 46, 272–280. [Google Scholar] [CrossRef]
- Ji, K.; Kim, S.; Han, S.; Seo, J.; Lee, S.; Park, Y.; Choi, K.; Kho, Y.-L.; Kim, P.-G.; Park, J.; et al. Risk assessment of chlortetracycline, oxytetracycline, sulfamethazine, sulfathiazole, and erythromycin in aquatic environment: Are the current environmental concentrations safe? Ecotoxicology 2012, 21, 2031–2050. [Google Scholar] [CrossRef]
- Mheidli, N.; Malli, A.; Mansour, F.; Al-Hindi, M. Occurrence and risk assessment of pharmaceuticals in surface waters of the Middle East and North Africa: A review. Sci. Total Environ. 2022, 851, 158302. [Google Scholar] [CrossRef]
- Bu, Q.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Pharmaceuticals and personal care products in the aquatic environment in China: A review. J. Hazard. Mater. 2013, 262, 189–211. [Google Scholar] [CrossRef]
- Li, Z.; Maier, M.P.; Radke, M. Screening for pharmaceutical transformation products formed in river sediment by combining ultrahigh performance liquid chromatography/high resolution mass spectrometry with a rapid data-processing method. Anal. Chim. Acta 2014, 810, 61–70. [Google Scholar] [CrossRef]
- Quintana, J.B.; Weiss, S.; Reemtsma, T. Pathways and metabolites of microbial degradation of selected acidic pharmaceutical and their occurrence in municipal wastewater treated by a membrane bioreactor. Water Res. 2005, 39, 2654–2664. [Google Scholar] [CrossRef]
- Toński, M.; Dołżonek, J.; Stepnowski, P.; Białk-Bielińska, A. Hydrolytic stability of selected pharmaceuticals and their transformation products. Chemosphere 2019, 236, 124236. [Google Scholar] [CrossRef]
- Maculewicz, J.; Kowalska, D.; Świacka, K.; Toński, M.; Stepnowski, P.; Białk-Bielińska, A.; Dołżonek, J. Transformation products of pharmaceuticals in the environment: Their fate, (eco)toxicity and bioaccumulation potential. Sci. Total Environ. 2022, 802, 149916. [Google Scholar] [CrossRef]
- Liu, J.; Lu, G.; Ding, J.; Zhang, Z.; Wang, Y. Tissue distribution, bioconcentration, metabolism, and effects of erythromycin in crucian carp (Carassius auratus). Sci. Total Environ. 2014, 490, 914–920. [Google Scholar] [CrossRef]
- Sharma, K.; Thakur, I.S.; Kaushik, G. Occurrence and distribution of pharmaceutical compounds and their environmental impacts: A review. Bioresour. Technol. Rep. 2021, 16, 100841. [Google Scholar] [CrossRef]
- Pino, M.R.; Val, J.; Mainar, A.M.; Zuriaga, E.; Español, C.; Langa, E. Acute toxicological effects on the earthworm Eisenia fetida of 18 common pharmaceuticals in artificial soil. Sci. Total Environ. 2015, 518–519, 225–237. [Google Scholar] [CrossRef]
- Minguez, L.; Pedelucq, J.; Farcy, E.; Ballandonne, C.; Budzinski, H.; Halm-Lemeille, M.-P. Toxicities of 48 pharmaceuticals and their freshwater and marine environmental assessment in northwestern France. Environ. Sci. Pollut. Res. 2016, 23, 4992–5001. [Google Scholar] [CrossRef]
- El-Bassat, R.; Touliabah, H.; Harisa, G. Toxicity of four pharmaceuticals from different classes to isolated plankton species. Afr. J. Aquat. Sci. 2012, 37, 71–80. [Google Scholar] [CrossRef]
- Geiger, E.; Hornek-Gausterer, R.; Saçan, M.T. Single and mixture toxicity of pharmaceuticals and chlorophenols to freshwater algae Chlorella vulgaris. Ecotoxicol. Environ. Saf. 2016, 129, 189–198. [Google Scholar] [CrossRef]
- Ding, T.; Yang, M.; Zhang, J.; Yang, B.; Lin, K.; Li, J.; Gan, J. Toxicity, degradation and metabolic fate of ibuprofen on freshwater diatom Navicula sp. J. Hazard. Mater. 2017, 330, 127–134. [Google Scholar] [CrossRef]
- Gu, Y.; Huang, J.; Zeng, G.; Shi, L.; Shi, Y.; Yi, K. Fate of pharmaceuticals during membrane bioreactor treatment: Status and perspectives. Bioresour. Technol. 2018, 268, 733–748. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal organic frameworks as efficient adsorbents for drugs from wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Díaz, V.; Antiñolo, L.; Capilla, J.M.P.; Almécija, M.C.; Muñío, M.d.M.; Martín-Pascual, J. Nutrient Removal and Membrane Performance of an Algae Membrane Photobioreactor in Urban Wastewater Regeneration. Membranes 2022, 12, 982. [Google Scholar] [CrossRef]
- Judd, S. The status of industrial and municipal effluent treatment with membrane bioreactor technology. Chem. Eng. J. 2016, 305, 37–45. [Google Scholar] [CrossRef]
- Antiñolo Bermúdez, L.; Leyva Díaz, J.C.; Martín Pascual, J.; Muñío Martínez, M.d.M.; Poyatos Capilla, J.M. Study of the Potential for Agricultural Reuse of Urban Wastewater with Membrane Bioreactor Technology in the Circular Economy Framework. Agronomy 2022, 12, 1877. [Google Scholar] [CrossRef]
- Goh, P.; Wong, K.; Ismail, A. Membrane technology: A versatile tool for saline wastewater treatment and resource recovery. Desalination 2022, 521, 115377. [Google Scholar] [CrossRef]
- Samal, K.; Bandyopadhyay, R.; Dash, R.R. Biological Treatment of Contaminants of Emerging Concern in Wastewater: A Review. J. Hazard. Toxic Radioact. Waste 2022, 26, 04022002. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Si, Y.; Zhang, S.; Yu, J.; Ding, B. Charged membranes based on spider silk-inspired nanofibers for comprehensive and continuous purification of wastewater. Nanotechnology 2021, 32, 495704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, J.; Liu, L.; Zhang, P.; Si, Y.; Zhang, S.; Yu, J.; Ding, B. Electroconductive nanofibrous membranes with nanosheet-based microsphere-threaded heterostructures enabling oily wastewater remediation. J. Mater. Chem. A 2021, 9, 15310–15320. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Xia, M.; Cai, M.; Nie, Z.; Gao, J. Green multifunctional PVA composite hydrogel-membrane for the efficient purification of emulsified oil wastewater containing Pb2+ ions. Sci. Total Environ. 2023, 856, 159271. [Google Scholar] [CrossRef] [PubMed]
- Mainardis, M.; Buttazzoni, M.; Cottes, M.; Moretti, A.; Goi, D. Respirometry tests in wastewater treatment: Why and how? A critical review. Sci. Total Environ. 2021, 793, 148607. [Google Scholar] [CrossRef] [PubMed]
- Arias-Navarro, M.; Villen-Guzman, M.; Perez-Recuerda, R.; Rodriguez-Maroto, J.M. The use of respirometry as a tool for the diagnosis of waste water treatment plants. A real case study in Southern Spain. J. Water Process. Eng. 2019, 29, 100791. [Google Scholar] [CrossRef]
- Mainardis, M.; Buttazzoni, M.; De Bortoli, N.; Mion, M.; Goi, D. Evaluation of ozonation applicability to pulp and paper streams for a sustainable wastewater treatment. J. Clean. Prod. 2020, 258, 120781. [Google Scholar] [CrossRef]
- Ferrari, B.; Paxéus, N.; Lo Giudice, R.; Pollio, A.; Garric, J. Ecotoxicological impact of pharmaceuticals found in treated wastewaters: Study of carbamazepine, clofibric acid, and diclofenac. Ecotoxicol. Environ. Saf. 2003, 55, 359–370. [Google Scholar] [CrossRef]
- Wijaya, L.; Alyemeni, M.; Ahmad, P.; Alfarhan, A.; Barcelo, D.; El-Sheikh, M.A.; Pico, Y. Ecotoxicological Effects of Ibuprofen on Plant Growth of Vigna unguiculata L. Plants 2020, 9, 1473. [Google Scholar] [CrossRef]
- Ping, Q.; Zhang, Z.; Ma, L.; Yan, T.; Wang, L.; Li, Y. The prevalence and removal of antibiotic resistance genes in full-scale wastewater treatment plants: Bacterial host, influencing factors and correlation with nitrogen metabolic pathway. Sci. Total Environ. 2022, 827, 154154. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Leyva-Díaz, J.; Calderón, K.; Rodríguez, F.; González-López, J.; Hontoria, E.; Poyatos, J. Comparative kinetic study between moving bed biofilm reactor-membrane bioreactor and membrane bioreactor systems and their influence on organic matter and nutrients removal. Biochem. Eng. J. 2013, 77, 28–40. [Google Scholar] [CrossRef]
- Leyva-Díaz, J.; Calero-Díaz, G.; López-López, C.; Martín-Pascual, J.; Torres, J.; Poyatos, J. Kinetic study of the effect of bisphenol A on the rates of organic matter removal, decay and biomass generation in a membrane bioreactor. Biochem. Eng. J. 2017, 128, 45–53. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Pérez-Gálvez, R.; Guadix, A.; Guadix, E.M. Influence of the parameters of the Rancimat test on the determination of the oxidative stability index of cod liver oil. LWT Food Sci. Technol. 2012, 51, 303–308. [Google Scholar] [CrossRef]
- Tambosi, J.L.; de Sena, R.F.; Favier, M.; Gebhardt, W.; José, H.J.; Schröder, H.F.; Moreira, R.d.F.P.M. Removal of pharmaceutical compounds in membrane bioreactors (MBR) applying submerged membranes. Desalination 2010, 261, 148–156. [Google Scholar] [CrossRef]
- Taheran, M.; Brar, S.K.; Verma, M.; Surampalli, R.; Zhang, T.; Valero, J. Membrane processes for removal of pharmaceutically active compounds (PhACs) from water and wastewaters. Sci. Total Environ. 2016, 547, 60–77. [Google Scholar] [CrossRef] [PubMed]
- Maeng, S.K.; Choi, B.G.; Lee, K.T.; Song, K.G. Influences of solid retention time, nitrification and microbial activity on the attenuation of pharmaceuticals and estrogens in membrane bioreactors. Water Res. 2013, 47, 3151–3162. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Hara, H.; Watanabe, Y. Elimination of Selected Acidic Pharmaceuticals from Municipal Wastewater by an Activated Sludge System and Membrane Bioreactors. Environ. Sci. Technol. 2007, 41, 3708–3714. [Google Scholar] [CrossRef] [PubMed]
- Londoño, Y.A.; A Peñuela, G. Biological Removal of Different Concentrations of Ibuprofen and Methylparaben in a Sequencing Batch Reactor (SBR). Water, Air Soil Pollut. 2015, 226, 393. [Google Scholar] [CrossRef]
- Jia, Y.; Khanal, S.K.; Yin, L.; Sun, L.; Lu, H. Influence of ibuprofen and its biotransformation products on different biological sludge systems and ecosystem. Environ. Int. 2020, 146, 106265. [Google Scholar] [CrossRef]
- Gurung, K.; Ncibi, M.C.; Sillanpää, M. Removal and fate of emerging organic micropollutants (EOMs) in municipal wastewater by a pilot-scale membrane bioreactor (MBR) treatment under varying solid retention times. Sci. Total Environ. 2019, 667, 671–680. [Google Scholar] [CrossRef]
- Dehnavi, H.A.; Amin, M.M.; Fatehizadeh, A.; Attar, H.M.; Ebrahimpour, K.; Bina, B. Assessment of toxicity and kinetic effects of erythromycin on activated sludge consortium by fast respirometry method. Environ. Health Eng. Manag. 2021, 8, 205–214. [Google Scholar] [CrossRef]
- Amin, M.M.; Zilles, J.L.; Greiner, J.; Charbonneau, S.; Raskin, L.; Morgenroth, E. Influence of the Antibiotic Erythromycin on Anaerobic Treatment of a Pharmaceutical Wastewater. Environ. Sci. Technol. 2006, 40, 3971–3977. [Google Scholar] [CrossRef] [PubMed]
- Rios-Miguel, A.B.; Jetten, M.S.M.; Welte, C.U. Effect of concentration and hydraulic reaction time on the removal of pharmaceutical compounds in a membrane bioreactor inoculated with activated sludge. Microb. Biotechnol. 2021, 14, 1707–1721. [Google Scholar] [CrossRef] [PubMed]
- Wunder, D.B.; Tan, D.T.; LaPara, T.M.; Hozalski, R.M. The effects of antibiotic cocktails at environmentally relevant concentrations on the community composition and acetate biodegradation kinetics of bacterial biofilms. Chemosphere 2013, 90, 2261–2266. [Google Scholar] [CrossRef] [PubMed]



| Cycle | HRT (h) | MLSS (mg L−1) | Average Temperature (°C) | SRT (Day) |
|---|---|---|---|---|
| 1 | 6 | 4256 ± 1023 | 21.4 ± 1.0 | 22.3 |
| 2 | 6 | 7477 ± 869 | 19.1 ± 2.6 | 10.7 |
| 3 | 12 | 6151 ± 386 | 20.0 ± 1.5 | 38.5 |
| 4 | 12 | 2888 ± 371 | 18.0 ± 1.1 | 36.5 |
| Pharmaceutical | Dosing 1 (mg L−1) | Dosing 2 (mg L−1) | Dosing 3 (mg L−1) |
|---|---|---|---|
| Diclofenac | 0.95 | 2.37 | 9.48 |
| Erythromycin | 0.58 | 1.44 | 5.76 |
| Ibuprofen | 0.06 | 0.13 | 0.56 |
| Mixture | Dosing 1 of the 3 compounds | Dosing 2 of the 3 compounds | Dosing 3 of the 3 compounds |
| Average YH,VVS | KM | μm | bH, d−1 | rsu (mg O2/Lh) (Higher) | MLSS (mg L−1) | ||
|---|---|---|---|---|---|---|---|
| Cycle 1 | Reference | 0.59 ± 0.02 | 3.97 | 0.009 | 0.063 | 12.84 | 4267 |
| C1 Ibuprofen | 0.57 ± 0.06 | 2.06 | 0.004 | 0.053 | 9.27 | 4500 | |
| C2 Ibuprofen | 0.59 ± 0.00 | 8.21 | 0.016 | 0.068 | 15.34 | 4967 | |
| C3 Ibuprofen | 0.58 ± 0.02 | 11.78 | 0.018 | 0.066 | 12.41 | 4967 | |
| Cycle 2 | Reference | 0.65 ± 0.00 | 2.73 | 0.011 | 0.067 | 36.37 | 7333 |
| C1 Ibuprofen | 0.57 ± 0.04 | 1.61 | 0.003 | 0.101 | 13.56 | 6833 | |
| C2 Ibuprofen | 0.59 ± 0.01 | 28.28 | 0.036 | 0.078 | 16.29 | 7567 | |
| C3 Ibuprofen | 0.63 ± 0.01 | 2.09 | 0.006 | 0.095 | 20.34 | 7367 | |
| Cycle 3 | Reference | 0.56 ± 0.04 | 21.42 | 0.019 | 0.039 | 9.14 | 6200 |
| C1 Ibuprofen | 0.53 ± 0.01 | 4.19 | 0.003 | 0.033 | 6.91 | 6867 | |
| C2 Ibuprofen | 0.53 ± 0.01 | 2.74 | 0.002 | 0.031 | 6.74 | 6867 | |
| C3 Ibuprofen | 0.56 ± 0.03 | 59.73 | 0.058 | 0.033 | 9.17 | 6133 | |
| Cycle 4 | Reference | 0.64 ± 0.00 | 2.65 | 0.013 | 0.028 | 15.17 | 2933 |
| C1 Ibuprofen | 0.66 ± 0.01 | 4.57 | 0.021 | 0.105 | 18.57 | 2300 | |
| C2 Ibuprofen | 0.65 ± 0.00 | 16.25 | 0.073 | 0.032 | 18.50 | 2300 | |
| C3 Ibuprofen | 0.65 ± 0.01 | 6.97 | 0.019 | 0.024 | 16.37 | 3000 |
| Average YH,VVS | KM | μm | bH, d−1 | rsu (mg O2/Lh) (Higher) | MLSS (mg L−1) | ||
|---|---|---|---|---|---|---|---|
| Cycle 1 | Reference | 0.55 ± 0.03 | 2.64 | 0.005 | 0.029 | 7.92 | 3133 |
| C1 Diclofenac | 0.55 ± 0.05 | 4.46 | 0.006 | 0.022 | 6.96 | 3133 | |
| C2 Diclofenac | 0.50 ± 0.04 | 1.62 | 0.003 | 0.027 | 6.98 | 3367 | |
| C3 Diclofenac | 0.57 ± 0.02 | 1.59 | 0.004 | 0.023 | 8.83 | 3367 | |
| Cycle 2 | Reference | 0.65 ± 0.00 | 2.73 | 0.011 | 0.067 | 36.37 | 7333 |
| C1 Diclofenac | 0.65 ± 0.01 | 4.04 | 0.016 | 0.047 | 36.62 | 7333 | |
| C2 Diclofenac | 0.64 ± 0.01 | 1.35 | 0.009 | 0.076 | 29.95 | 5800 | |
| C3 Diclofenac | 0.62 ± 0.00 | 16.09 | 0.021 | 0.046 | 13.60 | 5967 | |
| Cycle 3 | Reference | 0.56 ± 0.04 | 21.42 | 0.019 | 0.039 | 9.14 | 6200 |
| C1 Diclofenac | 0.61 ± 0.03 | 3.9 | 0.004 | 0.026 | 13.66 | 6000 | |
| C2 Diclofenac | 0.62 ± 0.00 | 2.90 | 0.005 | 0.027 | 11.58 | 6000 | |
| C3 Diclofenac | 0.58 ± 0.03 | 2.00 | 0.003 | 0.047 | 9.87 | 5667 | |
| Cycle 4 | Reference | 0.64 ± 0.00 | 2.65 | 0.013 | 0.028 | 15.17 | 2933 |
| C1 Diclofenac | 0.65 ± 0.00 | ND | ND | 0.037 | 15.57 | 2617 | |
| C2 Diclofenac | 0.65 ± 0.00 | 2.74 | 0.015 | 0.033 | 16.79 | 2617 | |
| C3 Diclofenac | 0.66 ± 0.00 | 2.50 | 0.017 | 0.035 | 19.84 | 2617 |
| Average YH,VVS | KM | μm | bH (d−1) | rsu (mg O2 L−1 h−1) (Higher) | MLSS (mg L−1) | ||
|---|---|---|---|---|---|---|---|
| Cycle 1 | Reference | 0.55 ± 0.03 | 2.64 | 0.005 | 0.029 | 7.92 | 3133 |
| C1 Erythromycin | 0.47 ± 0.03 | 5.50 | 0.004 | 0.033 | 5.71 | 3800 | |
| C2 Erythromycin | 0.59 ± 0.01 | 3.26 | 0.005 | 0.042 | 7.92 | 3800 | |
| C3 Erythromycin | 0.51 ± 0.04 | 2.05 | 0.003 | 0.031 | 8.52 | 3833 | |
| Cycle 2 | Reference | 0.65 ± 0.00 | 2.73 | 0.011 | 0.067 | 36.37 | 7333 |
| C1 Erythromycin | 0.61 ± 0.03 | 3.33 | 0.006 | 0.053 | 19.18 | 8033 | |
| C2 Erythromycin | 0.64 ± 0.01 | 1.91 | 0.006 | 0.026 | 23.80 | 8233 | |
| C3 Erythromycin | 0.61 ± 0.02 | 4.40 | 0.008 | 0.022 | 18.82 | 8233 | |
| Cycle 3 | Reference | 0.56 ± 0.04 | 21.42 | 0.019 | 0.039 | 9.14 | 6200 |
| C1 Erythromycin | 0.58 ± 0.02 | 22.98 | 0.026 | 0.034 | 10.34 | 6133 | |
| C2 Erythromycin | 0.59 ± 0.02 | 5.46 | 0.008 | 0.035 | 10.92 | 6000 | |
| C3 Erythromycin | 0.60 ± 0.01 | 2.22 | 0.004 | 0.030 | 11.91 | 6000 | |
| Cycle 4 | Reference | 0.64 ± 0.00 | 2.65 | 0.013 | 0.028 | 15.17 | 2933 |
| C1 Erythromycin | 0.65 ± 0.01 | 19.45 | 0.068 | 0.024 | 18.34 | 3000 | |
| C2 Erythromycin | 0.64 ± 0.00 | 3.73 | 0.016 | 0.017 | 18.54 | 3183 | |
| C3 Erythromycin | 0.65 ± 0.01 | 1.71 | 0.009 | 0.016 | 13.12 | 3183 |
| Average YH,VVS | KM | μm | bH, d−1 | rsu (mg O2/Lh) (Higher) | MLSS (mg L−1) | ||
|---|---|---|---|---|---|---|---|
| Cycle 1 | Reference | 0.59 ± 0.02 | 3.97 | 0.009 | 0.063 | 12.84 | 4267 |
| C1 Mixture | 0.58 ± 0.01 | 5.97 | 0.009 | 0.064 | 13.37 | 4267 | |
| C2 Mixture | 0.56 ± 0.02 | 9.98 | 0.007 | 0.035 | 11.64 | 6100 | |
| C3 Mixture | 0.60 ± 0.01 | ND | ND | 0.030 | 15.35 | 6100 | |
| Cycle 2 | Reference | 0.65 ± 0.00 | 2.73 | 0.011 | 0.067 | 36.37 | 7333 |
| C1 Mixture | 0.63 ± 0.00 | 3.65 | 0.007 | 0.023 | 21.97 | 8800 | |
| C2 Mixture | 0.60 ± 0.01 | 2.07 | 0.004 | 0.013 | 17.89 | 7933 | |
| C3 Mixture | 0.58 ± 0.01 | 8.86 | 0.009 | 0.013 | 14.62 | 7767 | |
| Cycle 3 | Reference | 0.56 ± 0.04 | 21.42 | 0.019 | 0.039 | 9.14 | 6200 |
| C1 Mixture | 0.56 ± 0.02 | 7.94 | 0.009 | 0.040 | 10.09 | 5733 | |
| C2 Mixture | 0.59 ± 0.04 | 20.89 | 0.027 | 0.034 | 13.58 | 5733 | |
| C3 Mixture | 0.54 ± 0.06 | 52.44 | 0.031 | 0.023 | 7.53 | 6633 | |
| Cycle 4 | Reference | 0.64 ± 0.00 | 2.65 | 0.013 | 0.028 | 15.17 | 2933 |
| C1 Mixture | 0.63 ± 0.00 | 2.37 | 0.012 | 0.033 | 15.66 | 3000 | |
| C2 Mixture | 0.65 ± 0.00 | 1.73 | 0.010 | 0.013 | 13.11 | 3367 | |
| C3 Mixture | 0.63 ± 0.00 | 13.99 | 0.054 | 0.024 | 17.41 | 3432 |
| a | b | c | d | e | f | g | h | |
|---|---|---|---|---|---|---|---|---|
| Ibuprofen | 322.142 | 73.6801 | 0.204046 | −704.479 | 0.0222387 | 273.9939 | −0.130788 | 172.215 |
| Diclofenac | 1343.2 | 144.411 | 0.50484 | 14.9641 | 0.0435368 | 3.62735 | 0.00723289 | 1.79009 |
| Erythromycin | 974.425 | 109.527 | 0.331094 | 7.85758 | 0.0276471 | 1.76038 | 0.00155738 | 5.36507 |
| Mixture of pharmaceuticals | 182.835 | 58.7241 | 0.177247 | 28.9007 | 0.0188858 | 1.77263 | 0.00168898 | 5.36507 |
| a | b | c | d | e | f | g | h | |
|---|---|---|---|---|---|---|---|---|
| Ibuprofen | 236.385 | 51.3016 | 0.123095 | −393.148 | 0.0143061 | 12.2028 | −0.0846876 | 109.116 |
| Diclofenac | 949.434 | 106.258 | 0.356699 | 26.2874 | 0.0324628 | 2.64388 | 0.0053223 | 2.34626 |
| Erythromycin | 768.074 | 82.166 | 0.254193 | 0.0943917 | 0.0215321 | 2.28943 | 0.0033023 | 3.35068 |
| Mixture of pharmaceuticals | 206.625 | 46.3584 | 0.126267 | 21.27 | 0.0138022 | 1.29993 | 0.000956014 | 0.57605 |
| R2 | HRT Optimal (h) | Optimal (Maximum) Value | |||
|---|---|---|---|---|---|
| rsu (mg O2 L−1 h−1) | VSS (mg L−1) | [Pharmaceutical] (mg L−1) | |||
| Ibuprofen | 0.9922 | 6 | 690.44 | 6433 | 0.56 |
| Diclofenac | 0.9597 | 6 | 1090.46 | 6433 | Not significant |
| Erythromycin | 0.8739 | 6 | 847.32 | 7033 | 0.68 |
| Mixture of pharmaceuticals | 0.9491 | 6 | 669.195 | 7933 | 0.238 |
| R2 | HRT Optimal (h) | Optimal (Maximum) Value | |||
|---|---|---|---|---|---|
| rx (mg VSS L−1 h−1) | VSS (mg L−1) | [Pharmaceutical] (mg L−1) | |||
| Ibuprofen | 0.9819 | 6 | 402.52 | 6433 | 0.56 |
| Diclofenac | 0.9332 | 6 | 729.673 | 6433 | 0.0032 |
| Erythromycin | 0.9200 | 6 | 603.40 | 7033 | Not significant |
| Mixture of pharmaceuticals | 0.9545 | 6 | 413.593 | 7033 | Not significant |
| Ibuprofen | Diclofenac | Erythromycin | Mixture of Pharmaceuticals | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | rsu p-Value | rx p-Value | rsu p-Value | rx p-Value | rsu p-Value | rx p-Value | rsu p-Value | rx p-Value |
| HRT | 0.0006 * | 0.0266 * | 0.0081 * | 0.0138 * | 0.2809 | 0.1310 | 0.0173 * | 0.0255 * |
| MLSS | 0.0001 * | 0.0041 * | 0.0016 * | 0.0248 * | 0.0046 * | 0.0024 * | 0.5519 | 0.3989 |
| [Pharmaceutical] | 0.8611 | 0.7099 | 0.8709 | 0.5363 | 0.3490 | 0.1272 | 0.0172 * | 0.0133 * |
| HRT · MLSS | 0.0000 * | 0.0000 * | 0.0001 * | 0.0002 * | 0.0021 * | 0.0005 * | 0.0005 * | 0.0002 * |
| HRT · [pharmaceutical] | 0.0072 * | 0.1263 | 0.0782 | 0.1405 | 0.7240 | 0.4120 | 0.1341 | 0.0904 |
| MLSS · [pharmaceutical] | 0.0001 * | 0.0011* | 0.0916 | 0.1560 | 0.8492 | 0.4777 | 0.2860 | 0.3330 |
| [pharmaceutical]2 | 0.4488 | 0.6195 | 0.5953 | 0.4503 | 0.6824 | 0.6395 | 0.2637 | 0.2364 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antiñolo Bermúdez, L.; Díaz Mendoza, V.; Poyatos Capilla, J.M.; Muñío Martínez, M.d.M.; Martín Pascual, J. Effect of Pharmaceutical Compounds (Diclofenac, Ibuprofen, and Erythromycin) on the Heterotrophic Behaviors of Biomass of a Membrane Bioreactor to Treat Urban Wastewater. Environments 2023, 10, 198. https://doi.org/10.3390/environments10120198
Antiñolo Bermúdez L, Díaz Mendoza V, Poyatos Capilla JM, Muñío Martínez MdM, Martín Pascual J. Effect of Pharmaceutical Compounds (Diclofenac, Ibuprofen, and Erythromycin) on the Heterotrophic Behaviors of Biomass of a Membrane Bioreactor to Treat Urban Wastewater. Environments. 2023; 10(12):198. https://doi.org/10.3390/environments10120198
Chicago/Turabian StyleAntiñolo Bermúdez, Laura, Verónica Díaz Mendoza, José Manuel Poyatos Capilla, María del Mar Muñío Martínez, and Jaime Martín Pascual. 2023. "Effect of Pharmaceutical Compounds (Diclofenac, Ibuprofen, and Erythromycin) on the Heterotrophic Behaviors of Biomass of a Membrane Bioreactor to Treat Urban Wastewater" Environments 10, no. 12: 198. https://doi.org/10.3390/environments10120198
APA StyleAntiñolo Bermúdez, L., Díaz Mendoza, V., Poyatos Capilla, J. M., Muñío Martínez, M. d. M., & Martín Pascual, J. (2023). Effect of Pharmaceutical Compounds (Diclofenac, Ibuprofen, and Erythromycin) on the Heterotrophic Behaviors of Biomass of a Membrane Bioreactor to Treat Urban Wastewater. Environments, 10(12), 198. https://doi.org/10.3390/environments10120198








