Occurrence of Persistent Organochlorine Pollutants in Sediments from Lake Piediluco, Italy
Abstract
:1. Introduction
2. Materials and Methods
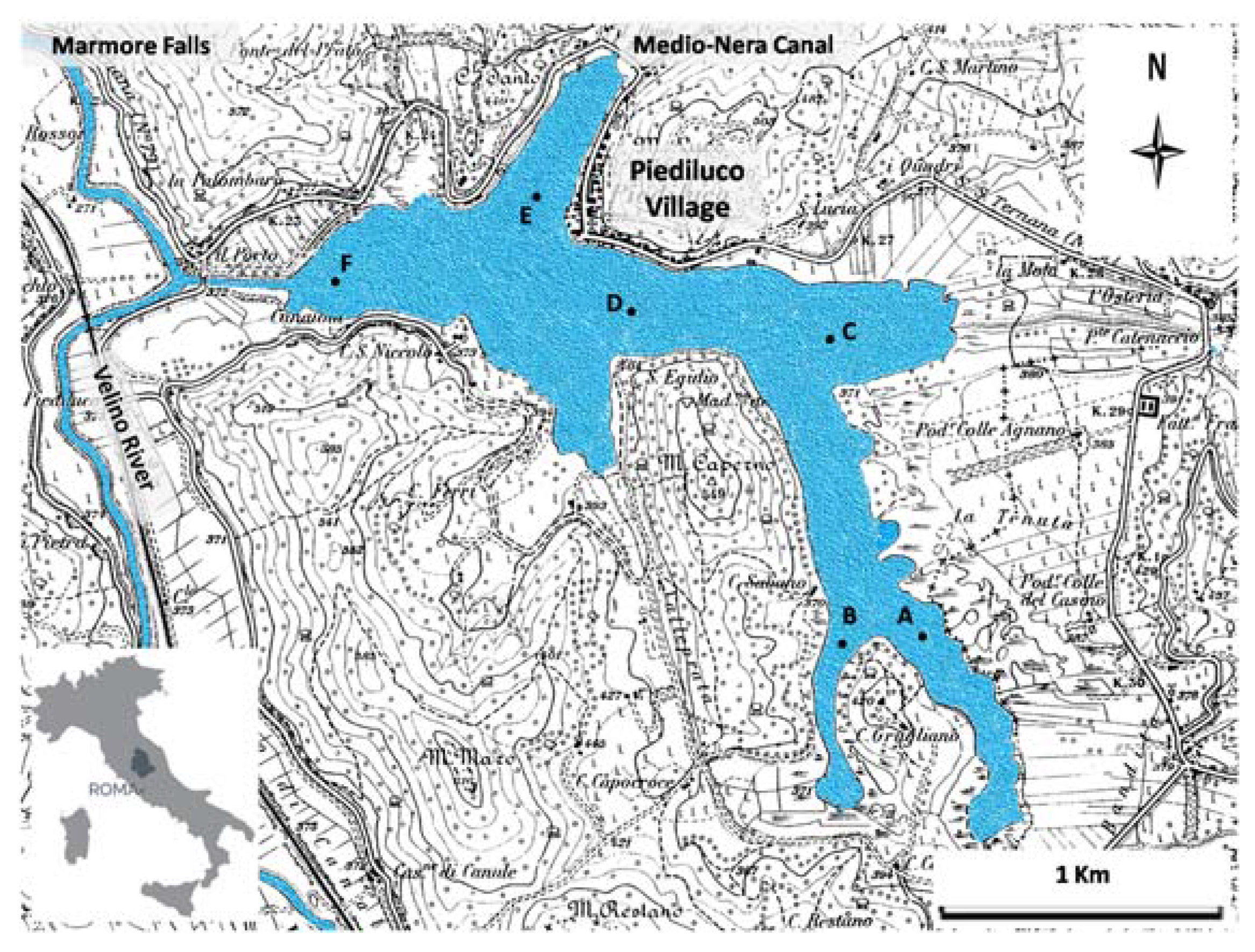
2.1. Study Area
2.2. Sample Collection and Pre-Treatment
2.3. Chemicals and Reagents
2.4. Extraction, Clean Up and Fractionation
2.5. Gas Chromatographic-Low Resolution Tandem Mass Spectrometry (GC-LRMS/MS) and Multi Reaction Monitoring (MRM) Analysis
3. Results and Discussion
3.1. Organochlorine Pesticides
3.2. Polychlorobiphenyls
3.3. Dioxins and Furans
3.4. Statistical Analysis
3.5. Ecological Risk Assessment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brils, J. Sediment monitoring and the European Water Framework Directive. Ann. Ist. Super. Sanità 2008, 44, 218–223. [Google Scholar]
- Mac Donald, D.D.; Ingersoll, C.G. Introduction. In An Ecosystem-Based Framework for Assessing and Managing Contaminated Sediments; Fisheries Foundation: Washington, DC, USA, 2002; Volume 1, pp. 1–3. [Google Scholar]
- Barceló, D. Analysis of soil, sediment and sludge. Trend Anal. Chem. 2004, 23, 677–679. [Google Scholar] [CrossRef]
- Mochungong, P.; Zhu, J. DDTs, PCBs and PBDEs contamination in Africa, Latin America and South-southeast Asia—A review. AIMS Environ. Sci. 2015, 2, 374–399. [Google Scholar] [CrossRef]
- Mac Donald, R.W.; Shaw, D.P.; Gray, C. Contaminants in Lake Sediments and Fish. Available online: http://publications.gc.ca/collections/collection_2015/ec/En47-119-1999-4-eng.pdf (accessed on 20 December 2022).
- Harkey, G.A.; Landrum, P.F.; Klaine, S.J. Comparison of whole-sediment, elutriate and pore water exposures for use in assessing sediment-associated organic contaminants in bioassays. Environ. Toxicol. Chem. 1994, 13, 1315–1329. [Google Scholar] [CrossRef]
- Förstner, U. Traceability of sediment analysis. Trend Anal. Chem. 2004, 23, 217–236. [Google Scholar] [CrossRef] [Green Version]
- Sandin, L.; Solimini, A.G. Fresh water ecosystem structure–function relationships: From theory to application. Freshw. Biol. 2009, 54, 2017–2024. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, E.; Zhang, E.; Luo, W.; Chen, L.; Wang, C.; Lin, Q. Historical records and sources of polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides (OCPs) in sediment from a representative plateau lake, China. Chemosphere 2017, 173, 78–88. [Google Scholar] [CrossRef]
- Mackay, D.; Fraser, A. Bioaccumulation of Persistent Organic Chemicals: Mechanisms and Models. Environ. Pollut. 2000, 110, 375–391. [Google Scholar] [CrossRef]
- Ingersoll, C.G.; Mac Donald, D.D. Interpretation of the Results of Sediment Quality Investigations. In Guidance Manual to Support the Assessment of Contaminated Sediments in Freshwater, Estuarine, and Marine Ecosystems in British Columbia; Environmental Protection Division, British Columbia Ministry of Water, Land and Air Protection: Victoria, BC, Australia, 2003; Volume III. [Google Scholar]
- Binelli, A.; Provini, A. Risk for human health of some POPs due to fish from Lake Iseo. Ecotoxicol. Environ. Saf. 2004, 58, 139–145. [Google Scholar] [CrossRef]
- Roche, H.; Vollaire, Y.; Persic, A.; Buet, A.; Oliveira-Ribeiro, C.; Coulet, E.; Banas, D.; Ramade, F. Organochlorines in the Vaccarès Lagoon trophic web (Biosphere Reserve of Camargue, France). Environ. Pollut. 2009, 157, 2493–2506. [Google Scholar] [CrossRef]
- United Nations Environment Programme. UNEP 2009 Annual Report. Available online: http://hdl.handle.net/20.500.11822/7824 (accessed on 24 February 2023).
- UNEP. ‘Chemicals Proposed for Listing under the Convention’, Stockholm Convention. 2018. Available online: http://chm.pops.int/TheConvention/ThePOPs/ChemicalsProposedforListing/tabid/2510/Default.aspx (accessed on 18 March 2023).
- Lorenzoni, M.; Carosi, A.; Pedicillo, G.; Trusso, A. A comparative study on the feeding competition of the European perch Perca fluviatilis L. and the ruffe Gymnocephalus cernuus (L.) in Lake Piediluco (Umbria, Italy). BFPP/Bull. Fr. Pêche Piscic. 2007, 387, 35–57. [Google Scholar] [CrossRef] [Green Version]
- LaPorta, G.; Angeli, V.; Bicchi, A.; Carosi, A.; Pedicillo, G.; Viali, P.; Lorenzoni, M. Variations in the fish community in lake Piediluco (Italy) caused by changes in the lakes trophic status and the introduction of alien species. J. Appl. Ichthyol. 2010, 26, 53–59. [Google Scholar] [CrossRef]
- DiVeroli, A.; Goretti, E.; Marcucci, C.; Fabrizi, A.; Scopetta, L.; Di Giovanni, M.V. Incidence of larvae mouth part deformities in Chironomus plumosus and Procladius sp. From Piediluco Lake, Italy. Bol. Mus. Mun. Funchal 2008, 13, 13–20. [Google Scholar]
- Mastrantuono, L.; Pilotto, F.; Rossopinti, A.; Bazzanti, M.; Solimini, A.G. Response of littoral macroinvertebrates to morphological disturbances in Mediterranean lakes: The case of Lake Piediluco (central Italy). Fundam. Appl. Limnol. 2015, 186, 297–310. [Google Scholar] [CrossRef]
- DiVeroli, A.; Selvaggi, R.; Pellegrino, R.M.; Goretti, E. Sediment toxicity and deformities of chironomia larvae in Lake Piediluco (Central Italy). Chemosphere 2010, 79, 33–39. [Google Scholar] [CrossRef]
- Mearelli, M. Eutrofizzazione. Riv. Idrobiol. 1988, 27, 651–661. [Google Scholar]
- Cioffi, F.; Gallerano, F. Response of lake Piediluco to the change of hydrodynamic conditions and nutrient load reductions. Ecol. Model. 2000, 135, 199–229. [Google Scholar] [CrossRef]
- Umbria Regional Agency for Environmental Protection (ARPA Umbria). Monografia15/Caratterizzazione Ambientale del Lago di Piediluco. 2005. Available online: https://www.arpa.umbria.it/au/pta/monografie/MONOGRAFIA%2015.pdf (accessed on 30 January 2023).
- EuropeanCommunity. Directive2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Communities 2000, 22, L327. [Google Scholar]
- Mearelli, M.; Tiberi, O. Caratteristichefisiograficheedatifisico-chimici. Monografia–Contributi alla conoscenza del Lago di Piediluco. Riv. Idrobiol. 1988, 27, 607–633. [Google Scholar]
- Italian Electric Energy Agency (ENEL). Monitoraggio del Lago di Piediluco; Rapporto finale; Italian Electric Energy Agency: Rome, Italy, 1997. [Google Scholar]
- Umbria Regional Agency for Environmental Protection (ARPA Umbria). Rilievo Batimetrico del Lago di Piediluco; Rapporto finale; ARPA Umbria: Perugia, Italy, 2001. [Google Scholar]
- De Felip, E.; Miniero, R. Analytical procedures adepte to determine microcontaminants in lagoon sediments. In Rapporti ISTISAN 99/28; Istituto Superiore di Sanità: Roma, Italy, 1999; ISSN 1123-3117. [Google Scholar]
- Sebastiani, B.; Falcinelli, S. Contamination of Plants from Amazonia by Environmental Pollution. Environments 2018, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Küchler, T.; Brzezinski, H. Application GC-MS/MS for the analysis of PCDD/Fs in sewage effluents. Chemosphere 2000, 40, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Bettinetti, R.; Galassi, S.; Guilizzoni, P.; Quadroni, S. Sediment analysis to support the recent glacial origin of DDTpollution in Lake Iseo (Northern Italy). Chemosphere 2011, 85, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Marziali, L.; Guzzella, L.; Salerno, F.; Marchetto, A.; Valsecchi, L.; Tasselli, S.; Roscioli, C.; Schiavon, A. Twenty-year sediment contamination trends in some tributaries of Lake Maggiore (Northern Italy): Relation with anthropogenic factors. Environ. Sci. Pollut. Res. 2021, 28, 38193–38208. [Google Scholar] [CrossRef] [PubMed]
- Kalajzic, T.; Bianchi, M.; Muntau, H.; Kettrup, A. Polychlorinatedbiphenyls (PCBs) and organochlorine pesticides (OCPs) in the sediments of an Italian drinking water reservoir. Chemosphere 1998, 36, 1615–1625. [Google Scholar] [CrossRef]
- Hong, S.H.; Yim, U.H.; Shim, W.J.; Oh, J.E.; Lee, I.S. Horizontal and vertical distribution of PCBs and chlorinated pesticides in sediments from Masan Bay, Korea. Mar. Pollut. Bull. 2003, 46, 244–253. [Google Scholar] [CrossRef]
- Said, T.O.; Okbah, M.A.; Mohamed, L.A.; Othman, I.M. Detection of persistent OCPs and PCBs in the near-shore coastal waters of Alexandria, Egypt. Environ. Monit. Assess. 2015, 187, 353. [Google Scholar] [CrossRef]
- Perez-Maldonado, I.N.; Trejo, A.; Ruepert, C.; Jovel Rdel, C.; Mendez, M.P.; Ferrari, M.; Saballos-Sobalvarro, E.; Alexander, C.; Yáñez-Estrada, L.; Lopez, D.; et al. Assessment of DDT levels in selected environmental media and biological samples from Mexico and Central America. Chemosphere 2010, 78, 1244–1249. [Google Scholar] [CrossRef]
- Martínez-Salinas, R.I.; Díaz-Barriga, F.; Batres-Esquivel, L.E.; Pérez-Maldonado, I.N. Assessment of the levels of DDT and its metabolites in soil and dust samples from Chiapas, Mexico. Bull. Environ. Contam. Toxicol. 2011, 86, 33–37. [Google Scholar] [CrossRef]
- Qiu, X.H.; Zhu, T. Using the o,p’-DDT/p,p’-DDT ratio to identify DDT sources in China. Chemosphere 2010, 81, 1033–1038. [Google Scholar] [CrossRef]
- Boul, H.L.; Garnham, M.L.; Hucker, D.; Baird, D.; Aislabie, J. The influence of agricultural practices on the levels of DDT and its residues in soil. Environ. Sci. Technol. 1994, 28, 1397–1402. [Google Scholar] [CrossRef]
- Qiu, X.; Zhu, T.; Yao, B.; Hu, S. Contribution of Dicofol to the current DDT pollution in China. Environ. Sci. Technol. 2005, 39, 4385–4390. [Google Scholar] [CrossRef]
- Manaca, M.N.; Grimalt, J.O.; Garí, M.; Sacarlal, J.; Sunyer, J.; Gonzalez, R.; Dobaño, C.; Menendez, C.; Alonso, P.L. Assessment of exposure to DDT and metabolites after indoor residual spraying through the analysis of thatch material from rural African dwellings. Environ. Sci. Pollut. Res. Int. 2012, 19, 756–762. [Google Scholar] [CrossRef] [Green Version]
- Tarcau, D.; Cucu-Man, S.; Boruvkova, J.; Klanova, J.; Covaci, A. Organochlorine pesticides in soil, moss and tree-bark fromNorth-Eastern Romania. Sci. Total Environ. 2013, 456, 317–324. [Google Scholar] [CrossRef]
- Yuan, G.L.; Sun, Y.; Qin, J.-X.; Li, J.; Wang, G.-H. Chiral signature of α-HCH and o,p′-DDT in the soil and grass of the Central Tibetan Plateau, China. Sci. Total Environ. 2014, 500, 147–154. [Google Scholar] [CrossRef]
- Da, C.; Liu, G.; Tang, Q.; Li, J. Distribution, sources, and ecological risks of organochlorine pesticides in surface sediments from the Yellow River Estuary, China. Environ. Sci. Process. Impacts 2013, 15, 2288–2296. [Google Scholar] [CrossRef]
- Beckvar, N.; Lotufo, G.R. DDT and Other Organohalogen Pesticides in Aquatic Organisms. In Environmental Contaminants in Biota: Interpreting Tissue Concentrations, 2nd ed.; Beyer, W.N., James, P.M., Eds.; US-EPA: Boca Raton, FL, USA, 2011; pp. 48–91. [Google Scholar]
- Tavares, T.M.; Beretta, M.; Costa, M.C. Ratio of DDT/DDE in the all saints bay, Brazil and its use in environmental management. Chemosphere 1999, 38, 1445–1452. [Google Scholar] [CrossRef]
- Iwata, H.; Tanabe, S.; Ueda, K.; Tatsukawa, R. Persistent organochlorine residues in air, water, sediments, and soils from the lake Baikal region, Russia. Environ. Sci. Technol. 1995, 29, 792–801. [Google Scholar] [CrossRef]
- Ristola, T.; Pellinen, J.; Van Hoof, P.L.; Leppänen, M.E.; Kukkonen, J. Characterization of lake Ladoga sediments. II.Toxic chemicals. Chemosphere 1996, 32, 1179–1192. [Google Scholar] [CrossRef]
- Environment Canada. A Reconnaissance of Contaminants in the Frazer River Drainage Basin Using Dated Sediment Cores from Six Lakes, DOE FRAP 1998-19; Environment Canada: Fredericton, NB, Canada, 1998. [Google Scholar]
- Muir, D.C.G.; Alex Omelchenko, A.; Grift, N.P.; Savoie, D.; Khart, W.L.; Wilkinson, P.; Brunskill, G.J. Spatial Trends and Historical Deposition of Polychlorinated Biphenyls in Canadian Mid latitude and Arctic Lake Sediments. Environ. Sci. Technol. 1996, 30, 3609–3617. [Google Scholar] [CrossRef]
- Provini, A.; Galassi, S.; Guzzella, L.; Valli, C. PCB profiles in sediments of lakes Maggiore, Comoand Garda(Italy). Mar. Freshw. Res. 1995, 46, 129–136. [Google Scholar] [CrossRef]
- Vives, I.; Canuti, E.; Castro-Jiménez, J.; Christoph, E.H.; Eisenreich, S.J.; Hanke, G.; Huber, T.; Mariani, G.; Mueller, A.; Skejo, H.; et al. Occurrence of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs), polychlorinatedbiphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in Lake Maggiore (Italy and Switzerland). J. Environ. Monit. 2007, 9, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Rockne, K.J.; Sturchio, N.; Song, W.; Ford, J.C.; Wei, H. PCBs in sediments of the GreatLakes—Distribution and trends, homolog and chlorine patterns, and in situ degradation. Environ. Pollut. 2009, 157, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Zitko, V. Chlorobiphenyls in sediments. Environ. Pollut. 2007, 145, 401. [Google Scholar] [CrossRef] [PubMed]
- Baldassarri, L.T.; D’Agostino, O.; DeFelip, E.; Di Domenico, A.; Fulgenzi, A.R.; Iacovella, N.; LaRocca, C.; Rodriguez, F.; Volpi, F. The ratio of 2,2′,3,4′,5′,6-Hexachlorobiphenyl (149) to 2,2′,4,4′,5,5′-Hexachlorobiphenyl (153) as a possible degradational and analytical indicator. Microchem. J. 1997, 22, 1–11. [Google Scholar] [CrossRef]
- Elliot, J.E.; Martin, P.A. Chlorinated hydrocarbon contaminats in Grebesans Seaducks wintering on the coast of British Columbia, Canada: 1988–1993. Environ. Monit. Assess. 1998, 53, 337–362. [Google Scholar] [CrossRef]
- Naffrechoux, E.; Cottin, N.; Pignol, C.; Arnaud, F.; Jenny, J.P.; Perga, M.E. Historical Profiles of PCB in Dated Sediment Cores Suggest Recent Lake Contamination through the “Halo Effect”. Environ. Sci. Technol. 2015, 49, 1303–1310. [Google Scholar] [CrossRef]
- Vartiainen, T.; Mannio, J.; Korhonen, M.; Kinnunen, K.; Strandman, T. Levels of PCDD, PCDF and PCB in Dated Lake Sediments in Subarctic Finland. Chemosphere 1997, 34, 1341–1350. [Google Scholar] [CrossRef]
- Umlauf, G.; Canuti, E.; Castro-Jimenez, J.; Christoph, E.H.; Eisenreich, S.J.; Ghiani, M.; Hanke, G.; Mariani, G.; Mueller, A.; Tourliti, V.; et al. Spatial distribution of PCDD/F in surface sediments of lake Maggiore (Italy). Organohalog. Compd. 2006, 68, 1141–1145. [Google Scholar]
- Dopico, M.; Gomez, A. Review of the current state and main sources of dioxins around the world. J. Air Waste Manag. Assoc. 2015, 65, 1033–1049. [Google Scholar] [CrossRef]
- Wagrowski, D.M.; Hite, R.A. Insights into the global distribution of polychlorinated dibenzo-p-dioxins and dibenzofurans. Environ. Sci. Technol. 2000, 34, 2952–2958. [Google Scholar] [CrossRef]
- Grochowalski, A.; Lassen, C.; Holtzer, M.; Sadowski, M.; Hudyma, T. Determination of PCDDs, PCDFs, PCBs and HCB Emissions from the Metallurgical Sector in Poland. Environ. Sci. Pollut. Res. 2007, 14, 326–332. [Google Scholar] [CrossRef]
- Meloni, D.; Carpine, F. Cinquanta Anni (1953–2002) di Osservazioni Meteo a Terni; di Terni, P., Ed.; Umbria: Perugia, Italy, 2004; p. 79. [Google Scholar]
- MacDonald, D.D.; Dipinto, L.M.; Field, J.; Ingersoll, C.G.; Long, E.R.; Scwartz, R.C. Development and evaluation of consensus-based sediment effectc on centrations for polychlorinated biphenyls. Environ. Toxicol. Chem. 2000, 19, 1403–1413. [Google Scholar] [CrossRef]
- MacDonald, D.D.; Ingersoll, C.G.; Berger, T.A. Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch. Environ. Contam. Toxicol. 2000, 39, 20–31. [Google Scholar] [CrossRef]
- Ingersoll, C.G.; MacDonald, D.D.; Wang, N.; Crane, J.L.; Field, L.J.; Haverland, P.S.; Kemble, N.E.; Lindskoog, R.A.; Severn, C.G.; Smorong, D.E. Predictions of sediment toxicity using consensus-based freshwater sediment quality guidelines. Arch. Environ. Contam. Toxicol. 2001, 41, 8–21. [Google Scholar] [CrossRef]
- Ingersoll, C.G.; MacDonald, D.D.; Brumbaugh, W.G.; Johnson, B.T.; Kemble, N.E.; Kunz, J.L.; May, T.W.; Wang, N.; Smith, J.R.; Sparks, D.W.; et al. Toxicity assessment of sediments from the Grand Calumet River and Indiana Harbor Canal in north western Indiana. Arch. Environ. Contam. Toxicol. 2002, 43, 156–167. [Google Scholar] [CrossRef]
- Macfarlane, M.; MacDonald, D.D. Criteria for Managing Contaminated Sediment in British Columbia; Environmental Protection Division, British Columbia Ministry of Water, Landand Air Protection: Victoria, BC, Australia, 2002. [Google Scholar]
- Long, E.R.; Field, L.J.; MacDonald, D.D. Predicting toxicity in marine sediments with numerical sediment quality guidelines. Environ. Toxicol. Chem. 1998, 17, 714–727. [Google Scholar] [CrossRef]



| Parameter | Value |
|---|---|
| Perimeter (km) | 14.84 |
| Area (km2) | 1.67 |
| Volume (Mm3) | 18.56 |
| Maximum depth (m) | 21 |
| Mean depth (m) | 10.9 |
| Length (km) | 2.8 |
| Mean width (km) | 0.39 |
| Sinuosity index | 3.28 |
| Mean annual temperature (°C) | 15.33 |
| Site | Sampling Location | Depth (m) | Thickness Sediment (cm) | ||
|---|---|---|---|---|---|
| S | A | S | A | ||
| A | 42°31′18.1″ N 12°46′10.7″ E | 8.5 | 7.5 | 21 | 17 |
| B | 42°31′17.2″ N 12°45′55.1″ E | 6.0 | 5.5 | 21 | 26 |
| C | 42°31′53.8″ N 12°46′06.2″ E | 9.0 | 7.5 | 15 | 33 |
| D | 42°31′59.5″ N 12°45′29.1″ E | 18.5 | 19.0 | 27 | 36 |
| E | 42°32′21.1″ N 12°45′11.9″ E | 14.5 | 15.0 | 21 | 26 |
| F | 42°32′03.7″ N 12°44′30.8″ E | 5.0 | 5.0 | 11 | 15 |
| Site | A | B | C | D | E | F | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Season | S | A | S | A | S | A | S | A | S | A | S | A |
| α-HCH 2 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 |
| β-HCH 3 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 |
| γ-HCH 4 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 | <0.030 |
| HCB 5 | <0.007 | <0.007 | <0.007 | <0.007 | <0.007 | <0.007 | 0.021 | <0.007 | 0.007 | <0.007 | <0.007 | 0.010 |
| Heptachlor | <0.020 | <0.020 | <0.020 | <0.020 | <0.020 | <0.020 | <0.020 | <0.020 | <0.020 | <0.020 | <0.020 | <0.020 |
| p,p’-DDT 6 | 0.023 | <0.023 | 0.043 | 0.078 | <0.023 | <0.023 | 0.067 | <0.023 | 2.172 | 0.302 | <0.023 | 0.261 |
| o,p’-DDT 6 | 0.042 | 0.026 | 0.036 | 0.037 | 0.054 | 0.018 | 0.051 | 0.068 | 0.500 | 0.150 | 0.070 | 0.260 |
| p,p’-DDE 7 | 0.408 | 0.281 | 0.359 | 0.217 | 0.302 | 0.178 | 0.306 | 0.152 | 1.917 | 0.351 | 0.381 | 0.632 |
| o,p’-DDE 7 | <0.020 | <0.020 | <0.020 | <0.020 | 0.064 | 0.089 | 0.020 | <0.020 | 0.091 | 0.031 | 0.045 | 0.035 |
| p,p’-DDD 8 | 0.075 | 0.080 | 0.118 | 0.057 | 0.085 | 0.088 | 0.124 | 0.081 | 3.499 | 0.353 | 0.456 | 0.522 |
| o,p’-DDD 8 | <0.020 | <0.020 | <0.020 | <0.020 | <0.020 | <0.020 | <0.020 | <0.020 | <0.020 | <0.020 | <0.020 | <0.020 |
| Totals | 0.548 | 0.387 | 0.556 | 0.389 | 0.505 | 0.373 | 0.589 | 0.301 | 8.185 | 1.186 | 0.951 | 1.720 |
| Sample Sites | A | B | C | D | E | F | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seasons | S | A | S | A | S | A | S | A | S | A | S | A |
| DCB 2 | ||||||||||||
| 10 | 0.002 | <0.002 | 0.002 | 0.004 | 0.003 | 0.004 | 0.003 | 0.006 | 0.002 | 0.007 | 0.009 | 0.002 |
| 4 | 0.012 | 0.008 | 0.015 | 0.033 | 0.020 | 0.013 | 0.011 | 0.028 | 0.011 | 0.051 | 0.040 | 0.007 |
| 8 | 0.008 | 0.006 | 0.008 | 0.023 | 0.014 | 0.009 | 0.011 | 0.022 | 0.004 | 0.023 | 0.070 | 0.009 |
| 15 | 0.004 | <0.003 | 0.003 | 0.014 | 0.006 | 0.006 | 0.005 | 0.013 | <0.003 | 0.013 | 0.014 | 0.004 |
| TCB 3 | ||||||||||||
| 16 + 32 | 0.004 | <0.004 | 0.005 | 0.042 | 0.024 | <0.004 | <0.004 | <0.004 | 0.012 | 0.022 | 0.068 | 0.009 |
| 28 + 31 | 0.027 | 0.034 | 0.030 | 0.111 | 0.096 | 0.066 | 0.032 | 0.128 | 0.030 | 0.120 | 0.144 | 0.030 |
| 20 | 0.011 | 0.010 | 0.011 | 0.061 | 0.035 | 0.056 | <0.006 | <0.006 | 0.009 | 0.045 | 0.059 | 0.006 |
| 33 | 0.009 | <0.005 | 0.008 | 0.049 | <0.005 | <0.005 | 0.005 | <0.005 | <0.005 | 0.022 | 0.033 | 0.012 |
| 22 | 0.008 | <0.004 | 0.004 | <0.004 | <0.004 | 0.026 | 0.007 | 0.024 | 0.010 | <0.004 | 0.027 | 0.013 |
| TeCB 4 | ||||||||||||
| 52 | 0.033 | 0.038 | 0.017 | 0.131 | 0.067 | 0.060 | 0.049 | 0.142 | 0.043 | 0.126 | 0.248 | 0.051 |
| 49 | 0.012 | 0.013 | 0.009 | 0.036 | 0.022 | 0.018 | 0.018 | 0.057 | 0.023 | 0.054 | 0.094 | 0.021 |
| 47 + 48 | 0.006 | <0.005 | <0.005 | 0.029 | 0.015 | 0.012 | 0.010 | 0.025 | 0.015 | 0.026 | 0.051 | 0.005 |
| 44 | 0.015 | 0.012 | 0.008 | 0.051 | 0.029 | 0.029 | 0.023 | 0.075 | 0.021 | 0.066 | 0.120 | 0.027 |
| 42 | 0.002 | <0.002 | <0.002 | 0.008 | <0.002 | <0.002 | 0.006 | 0.027 | <0.002 | 0.012 | 0.029 | 0.004 |
| 41 | 0.003 | <0.002 | 0.002 | 0.012 | 0.010 | 0.010 | 0.007 | 0.044 | 0.009 | 0.025 | 0.050 | <0.002 |
| 64 | 0.011 | 0.014 | 0.006 | 0.031 | 0.023 | 0.022 | 0.018 | 0.072 | 0.017 | 0.042 | 0.079 | 0.014 |
| 74 | 0.015 | 0.012 | 0.009 | 0.047 | 0.038 | 0.022 | 0.038 | 0.112 | 0.022 | 0.046 | 0.136 | 0.018 |
| 70 | 0.032 | 0.031 | 0.016 | 0.096 | 0.072 | 0.074 | 0.054 | 0.177 | 0.049 | 0.107 | 0.318 | 0.056 |
| 80 | 0.021 | 0.015 | 0.013 | 0.042 | 0.054 | 0.029 | 0.037 | 0.107 | 0.038 | 0.062 | 0.244 | 0.024 |
| 60 | 0.011 | 0.006 | 0.011 | 0.043 | 0.023 | 0.036 | 0.023 | 0.043 | 0.011 | 0.039 | 0.119 | 0.012 |
| 77 | 0.006 | 0.012 | 0.005 | <0.005 | 0.014 | 0.019 | 0.017 | <0.005 | <0.005 | 0.014 | 0.047 | <0.005 |
| PeCB 5 | ||||||||||||
| 82 | 0.003 | <0.003 | <0.003 | 0.013 | 0.015 | <0.003 | <0.003 | <0.003 | <0.003 | <0.003 | 0.054 | <0.003 |
| 93 | 0.036 | 0.030 | 0.015 | 0.096 | 0.068 | 0.064 | 0.057 | 0.156 | 0.069 | 0.140 | 0.252 | 0.053 |
| 92 | 0.020 | 0.011 | 0.006 | 0.012 | 0.023 | 0.030 | 0.015 | 0.072 | 0.028 | 0.030 | 0.054 | 0.013 |
| 84 | 0.017 | 0.006 | <0.006 | 0.033 | 0.015 | 0.033 | 0.015 | 0.020 | 0.016 | 0.035 | 0.085 | 0.015 |
| 101 | 0.049 | 0.046 | 0.027 | 0.123 | 0.117 | 0.087 | 0.084 | 0.191 | 0.119 | 0.183 | 0.240 | 0.080 |
| 99 | 0.020 | 0.016 | 0.010 | 0.049 | 0.047 | 0.031 | 0.036 | 0.097 | 0.045 | 0.075 | 0.090 | 0.037 |
| 97 | 0.008 | 0.012 | <0.008 | 0.030 | 0.021 | 0.020 | 0.019 | 0.042 | 0.028 | 0.038 | 0.081 | 0.024 |
| 87 | 0.018 | 0.020 | 0.006 | 0.055 | 0.045 | 0.049 | 0.035 | 0.087 | 0.034 | 0.066 | 0.163 | 0.037 |
| 85 | 0.006 | <0.006 | 0.005 | 0.013 | 0.010 | 0.016 | 0.014 | 0.039 | 0.017 | 0.019 | 0.087 | 0.011 |
| 110 | 0.048 | 0.058 | 0.028 | 0.161 | 0.116 | 0.124 | 0.109 | 0.258 | 0.126 | 0.179 | 0.711 | 0.097 |
| 118 + 123 | 0.045 | 0.047 | 0.025 | 0.124 | 0.120 | 0.099 | 0.098 | 0.225 | 0.098 | 0.156 | 0.693 | 0.085 |
| 105 | 0.018 | 0.018 | 0.012 | 0.065 | 0.058 | 0.048 | 0.039 | 0.073 | 0.031 | 0.066 | 0.213 | 0.038 |
| 126 | 0.004 | <0.004 | <0.004 | 0.028 | 0.009 | 0.006 | <0.004 | 0.020 | 0.016 | <0.004 | 0.058 | <0.004 |
| HxCB 6 | ||||||||||||
| 151 | 0.023 | <0.010 | 0.010 | 0.152 | 0.075 | 0.038 | 0.049 | 0.137 | 0.129 | 0.103 | 0.370 | 0.043 |
| 135 | 0.018 | 0.028 | 0.023 | 0.109 | 0.040 | 0.057 | 0.057 | 0.125 | 0.113 | 0.071 | 0.338 | 0.049 |
| 149 | 0.068 | 0.072 | 0.040 | 0.278 | 0.195 | 0.136 | 0.194 | 0.498 | 0.420 | 0.265 | 1.469 | 0.132 |
| 131 | <0.012 | <0.012 | 0.012 | <0.012 | <0.012 | <0.012 | 0.022 | 0.041 | 0.040 | 0.023 | 0.164 | 0.028 |
| 146 | 0.009 | <0.009 | <0.009 | 0.030 | 0.016 | 0.017 | 0.026 | 0.065 | 0.059 | 0.039 | 0.184 | 0.021 |
| 153 | 0.080 | 0.075 | 0.047 | 0.304 | 0.210 | 0.092 | 0.196 | 0.418 | 0.381 | 0.260 | 1.352 | 0.150 |
| 141 + 179 | 0.020 | <0.020 | 0.020 | 0.085 | 0.076 | 0.066 | 0.053 | 0.127 | 0.104 | 0.095 | 0.235 | 0.037 |
| 130 | <0.032 | <0.032 | <0.032 | <0.032 | <0.032 | <0.032 | <0.032 | <0.032 | <0.032 | <0.032 | 0.032 | <0.032 |
| 137 | <0.017 | <0.017 | <0.017 | <0.017 | <0.017 | <0.017 | 0.017 | 0.027 | 0.024 | 0.030 | 0.051 | 0.018 |
| 138 + 158 | 0.045 | 0.046 | 0.034 | 0.150 | 0.142 | 0.087 | 0.097 | 0.210 | 0.163 | 0.148 | 0.567 | 0.080 |
| 128 | 0.016 | <0.013 | 0.013 | 0.051 | 0.032 | 0.033 | 0.039 | 0.073 | 0.044 | 0.051 | 0.097 | 0.031 |
| HpCB 7 | ||||||||||||
| 174 | 0.012 | <0.011 | 0.011 | 0.076 | <0.011 | 0.041 | 0.035 | 0.070 | 0.085 | 0.023 | 0.245 | 0.023 |
| 180 | 0.037 | 0.034 | 0.025 | 0.158 | 0.102 | 0.023 | 0.074 | 0.160 | 0.150 | 0.087 | 0.146 | 0.044 |
| 170 | 0.014 | 0.016 | 0.014 | 0.074 | 0.049 | 0.020 | 0.034 | 0.074 | 0.080 | 0.029 | 0.062 | 0.020 |
| OCB 8 | ||||||||||||
| 199 | <0.005 | 0.006 | <0.005 | 0.018 | <0.005 | <0.005 | 0.008 | 0.012 | 0.013 | 0.005 | 0.056 | 0.006 |
| 196 + 203 | 0.007 | <0.004 | 0.004 | 0.040 | 0.015 | <0.004 | 0.012 | 0.034 | 0.027 | 0.016 | 0.055 | 0.012 |
| Total PCBs | 0.891 | 0.750 | 0.570 | 3.190 | 2.210 | 1.701 | 1.809 | 4.454 | 2.781 | 3.153 | 10.206 | 1.506 |
| Σ dl-PCBs 9 | 0.411 | 0.014 | 0.008 | 2.828 | 0.924 | 0.633 | 0.026 | 2.039 | 1.622 | 0.033 | 5.922 | 0.015 |
| Compound | SQC ERL-ERM ng/g d.w. | Sediment Samples | SQG TEL-PEL ng/g d.w. | Sediment Samples | ||||
|---|---|---|---|---|---|---|---|---|
| <ERL | ERL-ERM | >ERM | <TEL | TEL-PEL | >PEL | |||
| p,p’-DDT | 1–7 | 11 | 1 | - | N.G. | - | - | - |
| p,p’-DDE | 2–15 | 11 | 1 | - | 1.42–6.8 | 11 | 1 | - |
| p,p’-DDD | 2–20 | 11 | 1 | - | 3.54–8.51 | 11 | 1 | - |
| ΣDDTs | 3–350 | 11 | 1 | - | 7–4500 | 11 | 1 | - |
| PCBs | 50–400 | 12 | - | - | 34–277 | 12 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercanti, T.; El Hachmi, M.; Falcinelli, S.; Sebastiani, B. Occurrence of Persistent Organochlorine Pollutants in Sediments from Lake Piediluco, Italy. Environments 2023, 10, 120. https://doi.org/10.3390/environments10070120
Mercanti T, El Hachmi M, Falcinelli S, Sebastiani B. Occurrence of Persistent Organochlorine Pollutants in Sediments from Lake Piediluco, Italy. Environments. 2023; 10(7):120. https://doi.org/10.3390/environments10070120
Chicago/Turabian StyleMercanti, Tommaso, Mohamed El Hachmi, Stefano Falcinelli, and Bartolomeo Sebastiani. 2023. "Occurrence of Persistent Organochlorine Pollutants in Sediments from Lake Piediluco, Italy" Environments 10, no. 7: 120. https://doi.org/10.3390/environments10070120
APA StyleMercanti, T., El Hachmi, M., Falcinelli, S., & Sebastiani, B. (2023). Occurrence of Persistent Organochlorine Pollutants in Sediments from Lake Piediluco, Italy. Environments, 10(7), 120. https://doi.org/10.3390/environments10070120






