The Spatial Distribution of Copepod Functional Traits in a Highly Anthropized Mediterranean Coastal Marine Region
Abstract
1. Introduction
2. Materials and Methods
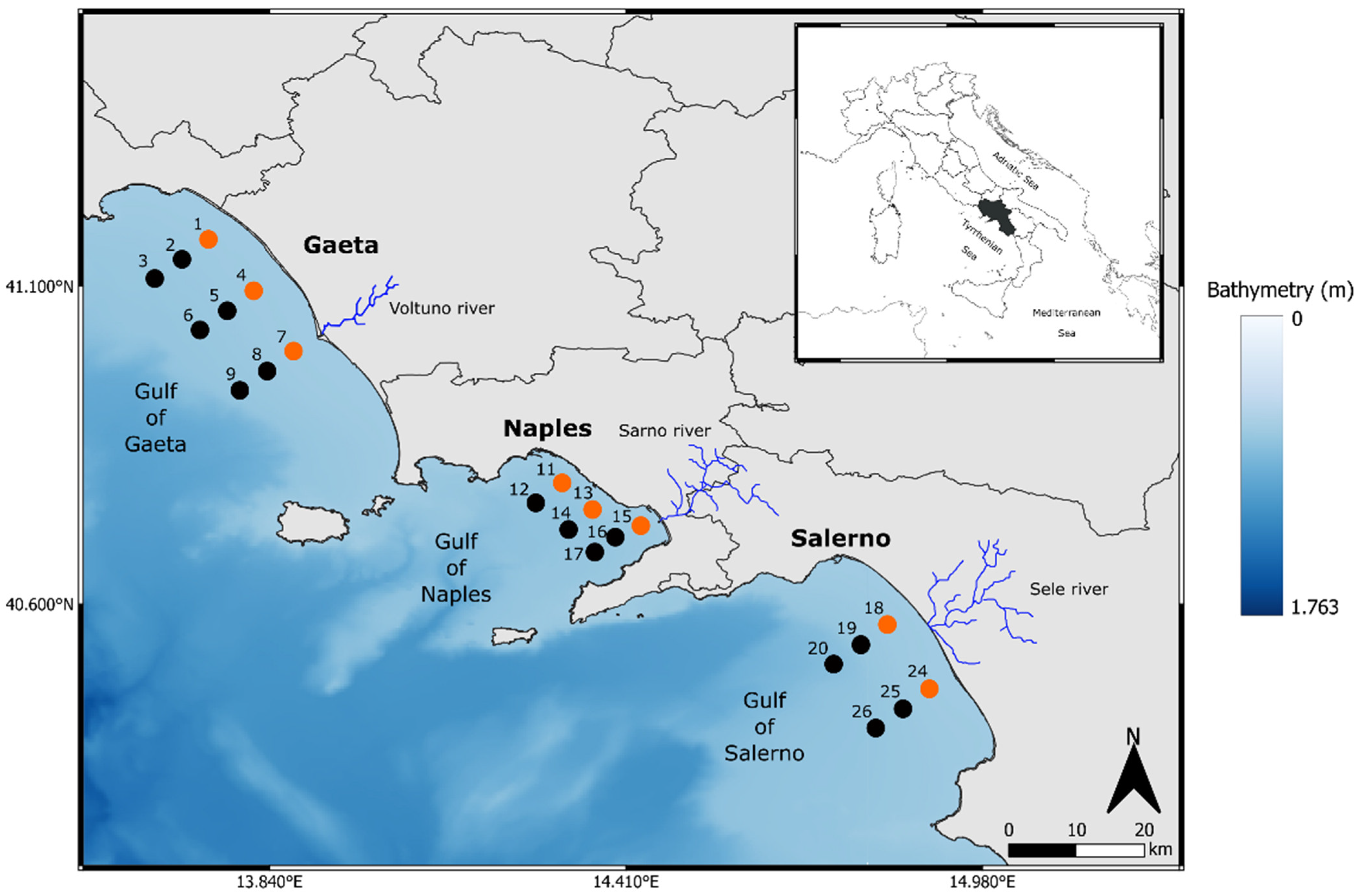
2.1. Sampling Area and Environmental Data
2.2. Mesozooplankton Sampling and Analysis
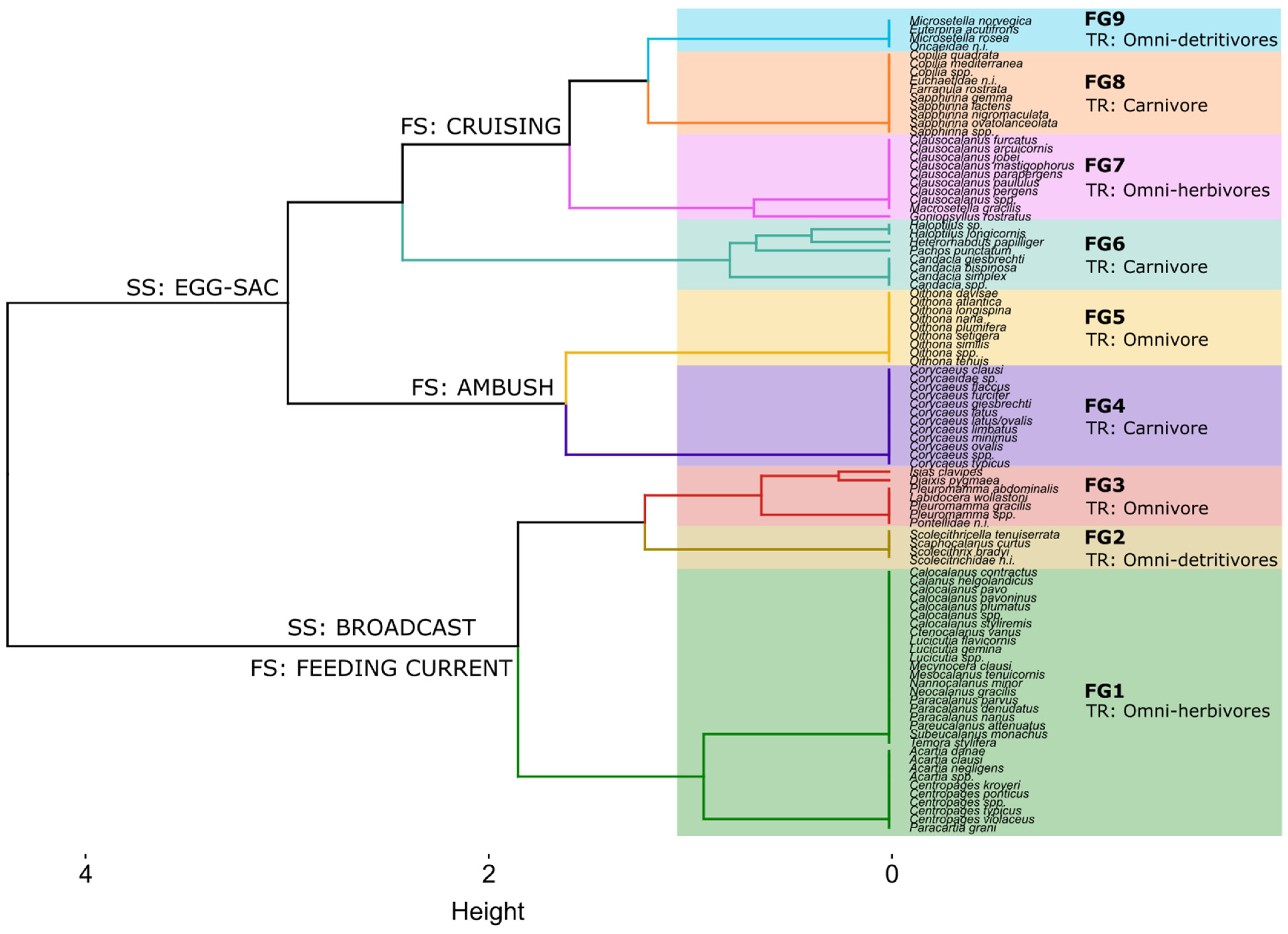
2.3. Copepod Functional Traits
2.4. Data Analysis
3. Results
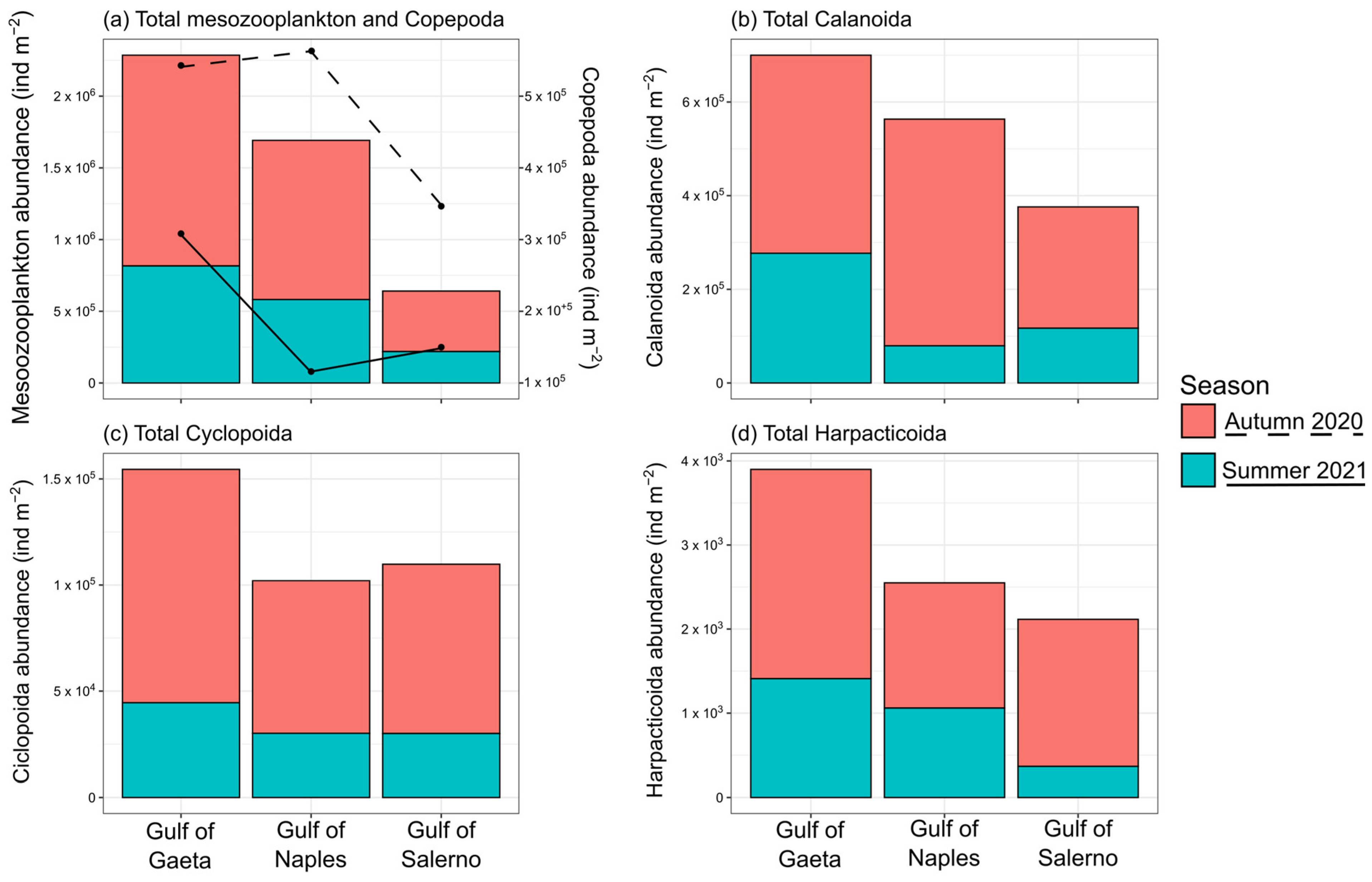
3.1. Environmental Variables
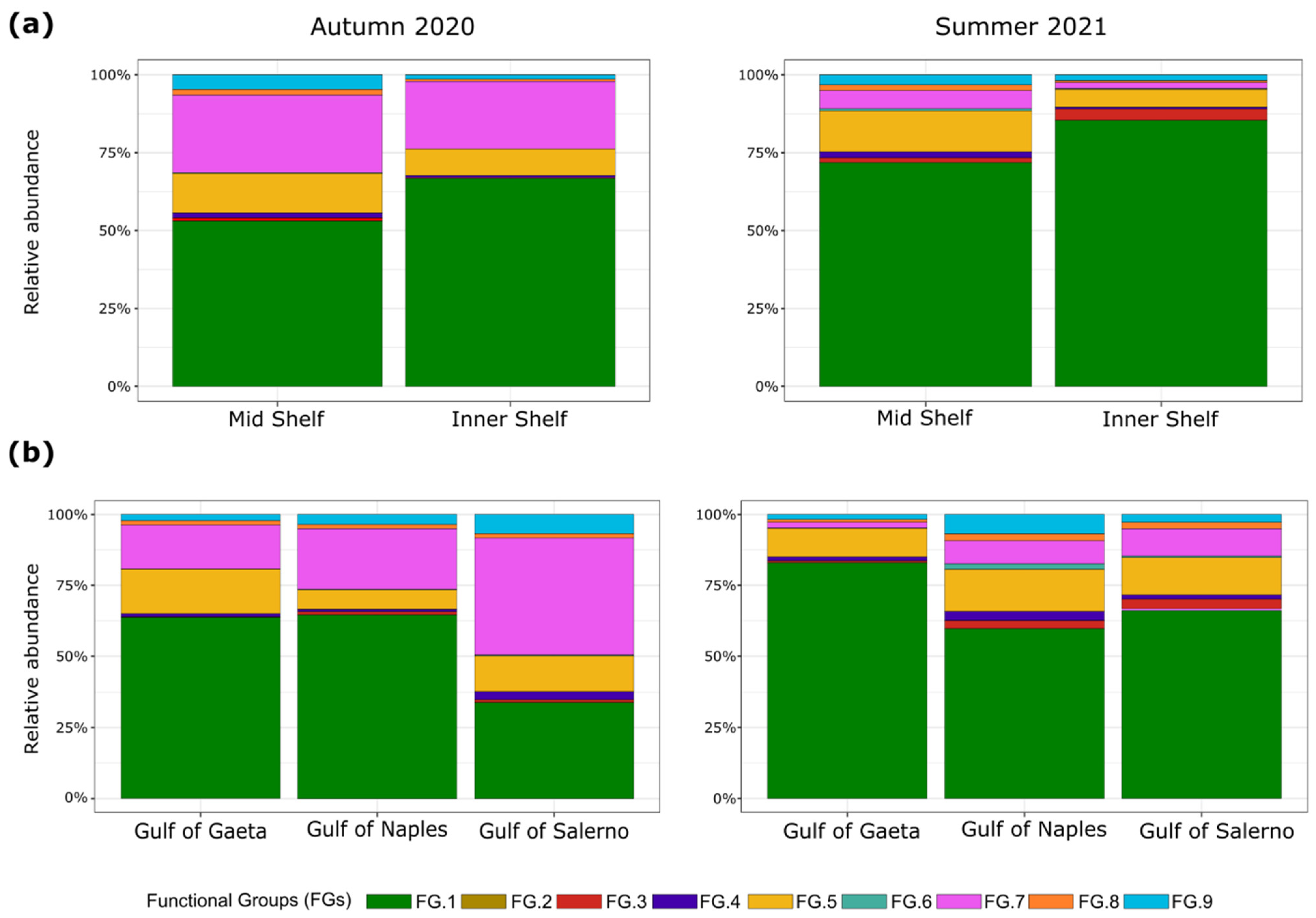
3.2. Copepod Functional Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buitenhuis, E.; Le Quéré, C.; Aumont, O.; Beaugrand, G.; Bunker, A.; Hirst, A.; Ikeda, T.; O’Brien, T.; Piontkovski, S.; Straile, D. Biogeochemical Fluxes through Mesozooplankton. Glob. Biogeochem. Cycles 2006, 20, GB2003. [Google Scholar] [CrossRef]
- Siokou-Frangou, I.; Shiganova, T.; Christou, E.D.; Kamburska, L.; Gubanova, A.; Konsulov, A.; Musaeva, E.; Skryabin, V.; Khoroshilov, V. Mesozooplankton Communities in the Aegean and Black Seas: A Comparative Study. Mar. Biol. 2004, 144, 1111–1126. [Google Scholar] [CrossRef]
- Mazzocchi, M.G.; Siokou, I.; Tirelli, V.; Bandelj, V.; Fernandez de Puelles, M.L.; Örek, Y.A.; de Olazabal, A.; Gubanova, A.; Kress, N.; Protopapa, M.; et al. Regional and Seasonal Characteristics of Epipelagic Mesozooplankton in the Mediterranean Sea Based on an Artificial Neural Network Analysis. J. Mar. Syst. 2014, 135, 64–80. [Google Scholar] [CrossRef]
- Zamora-Terol, S.; Novotny, A.; Winder, M. Molecular Evidence of Host-Parasite Interactions between Zooplankton and Syndiniales. Aquat. Ecol. 2021, 55, 125–134. [Google Scholar] [CrossRef]
- Steinberg, D.K.; Landry, M.R. Zooplankton and the Ocean Carbon Cycle. Annu. Rev. Mar. Sci. 2017, 9, 413–444. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.T. Zooplankton Fecal Pellets, Marine Snow, Phytodetritus and the Ocean’s Biological Pump. Prog. Oceanogr. 2015, 130, 205–248. [Google Scholar] [CrossRef]
- Kiørboe, T. What Makes Pelagic Copepods so Successful? J. Plankton Res. 2011, 33, 677–685. [Google Scholar] [CrossRef]
- Berggreen, U.; Hansen, B.; Kiørboe, T. Food Size Spectra, Ingestion and Growth of the copepodAcartia Tonsa during Development: Implications for Determination of Copepod Production. Mar. Biol. 1988, 99, 341–352. [Google Scholar] [CrossRef]
- Frost, B.W. Effects of Size and Concentration of Food Particles on the Feeding Behavior of the Marine Planktonic Copepod Calanus Pacificus1. Limnol. Oceanogr. 1972, 17, 805–815. [Google Scholar] [CrossRef]
- Jonsson, P.; Tiselius, P. Feeding Behaviour, Prey Detection and Capture Efficiency of the Copepod Acartia Tonsa Feeding on Planktonic Ciliates. Mar. Ecol. Prog. Ser. 1990, 60, 35–44. [Google Scholar] [CrossRef]
- DeMott, W.R. Feeding Selectivities and Relative Ingestion Rates of Daphnia and Bosmina1. Limnol. Oceanogr. 1982, 27, 518–527. [Google Scholar] [CrossRef]
- Teegarden, G. Copepod Grazing Selection and Particle Discrimination on the Basis of PSP Toxin Content. Grad. Sch. Oceanogr. Fac. Publ. 1999, 181, 163–176. [Google Scholar] [CrossRef]
- Barton, A.D.; Pershing, A.J.; Litchman, E.; Record, N.R.; Edwards, K.F.; Finkel, Z.V.; Kiørboe, T.; Ward, B.A. The Biogeography of Marine Plankton Traits. Ecol. Lett. 2013, 16, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the Concept of Trait Be Functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Díaz, S.; Cabido, M. Vive La Différence: Plant Functional Diversity Matters to Ecosystem Processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Litchman, E.; Ohman, M.D.; Kiørboe, T. Trait-Based Approaches to Zooplankton Communities. J. Plankton Res. 2013, 35, 473–484. [Google Scholar] [CrossRef]
- Becker, É.C.; Mazzocchi, M.G.; de Macedo-Soares, L.C.P.; Costa Brandão, M.; Santarosa Freire, A. Latitudinal Gradient of Copepod Functional Diversity in the South Atlantic Ocean. Prog. Oceanogr. 2021, 199, 102710. [Google Scholar] [CrossRef]
- Benedetti, F.; Gasparini, S.; Ayata, S.-D. Identifying Copepod Functional Groups from Species Functional Traits. J. Plankton Res. 2016, 38, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Pomerleau, C.; Sastri, A.R.; Beisner, B.E. Evaluation of Functional Trait Diversity for Marine Zooplankton Communities in the Northeast Subarctic Pacific Ocean. J. Plankton Res. 2015, 37, 712–726. [Google Scholar] [CrossRef]
- Helenius, L.K.; Leskinen, E.; Lehtonen, H.; Nurminen, L. Spatial Patterns of Littoral Zooplankton Assemblages along a Salinity Gradient in a Brackish Sea: A Functional Diversity Perspective. Estuar. Coast. Shelf Sci. 2017, 198, 400–412. [Google Scholar] [CrossRef]
- Benedetti, F.; Ayata, S.-D.; Irisson, J.-O.; Adloff, F.; Guilhaumon, F. Climate Change May Have Minor Impact on Zooplankton Functional Diversity in the Mediterranean Sea. Divers. Distrib. 2019, 25, 568–581. [Google Scholar] [CrossRef]
- Alcaraz, M.; Calbet, A. Zooplankton Ecology. In Marine Ecology; Encyclopedia of Life Support Systems (EOLSS): Paris, France, 2003; Volume I, pp. 295–318. [Google Scholar]
- Barnett, A.J.; Finlay, K.; Beisner, B.E. Functional Diversity of Crustacean Zooplankton Communities: Towards a Trait-Based Classification. Freshw. Biol. 2007, 52, 796–813. [Google Scholar] [CrossRef]
- Vogt, R.J.; Peres-Neto, P.R.; Beisner, B.E. Using Functional Traits to Investigate the Determinants of Crustacean Zooplankton Community Structure. Oikos 2013, 122, 1700–1709. [Google Scholar] [CrossRef]
- Veríssimo, H.; Patrício, J.; Gonçalves, É.; Moura, G.C.; Barbosa, J.E.L.; Gonçalves, A.M.M. Functional Diversity of Zooplankton Communities in Two Tropical Estuaries (NE Brazil) with Different Degrees of Human-Induced Disturbance. Mar. Environ. Res. 2017, 129, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Hulot, F.D.; Lacroix, G.; Lescher-Moutoué, F.; Loreau, M. Functional Diversity Governs Ecosystem Response to Nutrient Enrichment. Nature 2000, 405, 340–344. [Google Scholar] [CrossRef]
- Vassallo, P.; Bellardini, D.; Castellano, M.; Dapueto, G.; Povero, P. Structure and Functionality of the Mesozooplankton Community in a Coastal Marine Environment: Portofino Marine Protected Area (Liguria). Diversity 2022, 14, 19. [Google Scholar] [CrossRef]
- Benedetti, F.; Vogt, M.; Righetti, D.; Guilhaumon, F.; Ayata, S.-D. Do Functional Groups of Planktonic Copepods Differ in Their Ecological Niches? J. Biogeogr. 2018, 45, 604–616. [Google Scholar] [CrossRef]
- D’Ortenzio, F.; Ribera d’Alcalà, M. On the Trophic Regimes of the Mediterranean Sea: A Satellite Analysis. Biogeosciences 2009, 6, 139–148. [Google Scholar] [CrossRef]
- Selig, E.R.; Hole, D.G.; Allison, E.H.; Arkema, K.K.; McKinnon, M.C.; Chu, J.; De Sherbinin, A.; Fisher, B.; Glew, L.; Holland, M.B.; et al. Mapping Global Human Dependence on Marine Ecosystems. Conserv. Lett. 2019, 12, e12617. [Google Scholar] [CrossRef]
- He, Q.; Silliman, B.R. Climate Change, Human Impacts, and Coastal Ecosystems in the Anthropocene. Curr. Biol. CB 2019, 29, R1021–R1035. [Google Scholar] [CrossRef]
- Millennium Ecosystem Assessment (Program). Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005; ISBN 978-1-59726-040-4. [Google Scholar]
- Crain, C.M.; Halpern, B.S.; Beck, M.W.; Kappel, C.V. Understanding and Managing Human Threats to the Coastal Marine Environment. Ann. N. Y. Acad. Sci. 2009, 1162, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Appolloni, L.; Sandulli, R.; Vetrano, G.; Russo, G.F. A New Approach to Assess Marine Opportunity Costs and Monetary Values-in-Use for Spatial Planning and Conservation; the Case Study of Gulf of Naples, Mediterranean Sea, Italy. Ocean Coast. Manag. 2018, 152, 135–144. [Google Scholar] [CrossRef]
- Tornero, V.; Ribera d’Alcalà, M. Contamination by Hazardous Substances in the Gulf of Naples and Nearby Coastal Areas: A Review of Sources, Environmental Levels and Potential Impacts in the MSFD Perspective. Sci. Total Environ. 2014, 466–467, 820–840. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Dennis, R.L.; Whitall, D.R. Atmospheric Deposition of Nitrogen: Implications for Nutrient over-Enrichment of Coastal Waters. Estuaries 2002, 25, 677–693. [Google Scholar] [CrossRef]
- Ribera d’Alcalà, M.; Conversano, F.; Corato, F.; Licandro, P.; Mangoni, O.; Marino, D.; Mazzocchi, M.G.; Modigh, M.; Montresor, M.; Nardella, M.; et al. Seasonal Patterns in Plankton Communities in a Pluriannual Time Series at a Coastal Mediterranean Site (Gulf of Naples): An Attempt to Discern Recurrences and Trends. Sci. Mar. 2004, 68, 65–83. [Google Scholar] [CrossRef]
- Zingone, A.; D’Alelio, D.; Mazzocchi, M.G.; Montresor, M.; Sarno, D.; Team, L.-M. Time Series and beyond: Multifaceted Plankton Research at a Marine Mediterranean LTER Site. Nat. Conserv. 2019, 34, 273–310. [Google Scholar] [CrossRef]
- Kokoszka, F.; Saviano, S.; Botte, V.; Iudicone, D.; Zambianchi, E.; Cianelli, D. Gulf of Naples Advanced Model (GNAM): A Multiannual Comparison with Coastal HF Radar Data and Hydrological Measurements in a Coastal Tyrrhenian Basin. J. Mar. Sci. Eng. 2022, 10, 1044. [Google Scholar] [CrossRef]
- Margiotta, F.; Balestra, C.; Buondonno, A.; Casotti, R.; D’Ambra, I.; Di Capua, I.; Gallia, R.; Mazzocchi, M.G.; Merquiol, L.; Pepi, M.; et al. Do Plankton Reflect the Environmental Quality Status? The Case of a Post-Industrial Mediterranean Bay. Mar. Environ. Res. 2020, 160, 104980. [Google Scholar] [CrossRef]
- Montresor, M.; Prisco, C.D.; Sarno, D.; Margiotta, F.; Zingone, A. Diversity and Germination Patterns of Diatom Resting Stages at a Coastal Mediterranean Site. Mar. Ecol. Prog. Ser. 2013, 484, 79–95. [Google Scholar] [CrossRef]
- Zingone, A.; Tortora, C.; D′Alelio, D.; Margiotta, F.; Sarno, D. Assembly Rules Vary Seasonally in Stable Phytoplankton Associations of the Gulf of Naples (Mediterranean Sea). Mar. Ecol. 2023, 44, e12730. [Google Scholar] [CrossRef]
- Mazzocchi, M.G.; Di Capua, I.; Kokoszka, F.; Margiotta, F.; Ribera d’Alcalà, M.; Sarno, D.; Zingone, A.; Licandro, P. Coastal Mesozooplankton Respond to Decadal Environmental Changes via Community Restructuring. Mar. Ecol. 2023, 44, e12746. [Google Scholar] [CrossRef]
- de Ruggiero, P.; Esposito, G.; Napolitano, E.; Iacono, R.; Pierini, S.; Zambianchi, E. Modelling the Marine Circulation of the Campania Coastal System (Tyrrhenian Sea) for the Year 2016: Analysis of the Dynamics. J. Mar. Syst. 2020, 210, 103388. [Google Scholar] [CrossRef]
- Ragosta, M.; Mazzocchi, M.G.; Macchiato, M. Differentiation of Copepod Assemblages in Coastal Waters of the Tyrrhenian Sea. Oceanol. Acta 1995, 18, 479–491. [Google Scholar]
- Careddu, G.; Costantini, M.L.; Calizza, E.; Carlino, P.; Bentivoglio, F.; Orlandi, L.; Rossi, L. Effects of Terrestrial Input on Macrobenthic Food Webs of Coastal Sea Are Detected by Stable Isotope Analysis in Gaeta Gulf. Estuar. Coast. Shelf Sci. 2015, 154, 158–168. [Google Scholar] [CrossRef]
- Rossi, L.; Calizza, E.; Careddu, G.; Rossi, D.; Orlandi, L.; Jona-Lasinio, G.; Aguzzi, L.; Costantini, M.L. Space-Time Monitoring of Coastal Pollution in the Gulf of Gaeta, Italy, Using δ15N Values of Ulva Lactuca, Landscape Hydromorphology, and Bayesian Kriging Modelling. Mar. Pollut. Bull. 2018, 126, 479–487. [Google Scholar] [CrossRef]
- Triassi, M.; Nardone, A.; Giovinetti, M.C.; De Rosa, E.; Canzanella, S.; Sarnacchiaro, P.; Montuori, P. Ecological Risk and Estimates of Organophosphate Pesticides Loads into the Central Mediterranean Sea from Volturno River, the River of the “Land of Fires” Area, Southern Italy. Sci. Total Environ. 2019, 678, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, C.; Lega, M.; Fusco, G.; Bishop, P.; Endreny, T. Characterization of Terrestrial Discharges into Coastal Waters with Thermal Imagery from a Hierarchical Monitoring Program. Water 2017, 9, 500. [Google Scholar] [CrossRef]
- Iermano, I.; Liguori, G.; Iudicone, D.; Buongiorno Nardelli, B.; Colella, S.; Zingone, A.; Saggiomo, V.; Ribera d’Alcalà, M. Filament Formation and Evolution in Buoyant Coastal Waters: Observation and Modelling. Prog. Oceanogr. 2012, 106, 118–137. [Google Scholar] [CrossRef]
- Cianelli, D.; Uttieri, M.; Buonocore, B.; Falco, P.; Zambardino, G.; Zambianchi, E. Dynamics of a Very Special Mediterranean Coastal Area: The Gulf of Naples. Mediterr. Ecosyst. Dyn. Conserv. 2012, 7, 129–150. [Google Scholar]
- Baldantoni, D.; Bellino, A.; Lofrano, G.; Libralato, G.; Pucci, L.; Carotenuto, M. Biomonitoring of Nutrient and Toxic Element Concentrations in the Sarno River through Aquatic Plants. Ecotoxicol. Environ. Saf. 2018, 148, 520–527. [Google Scholar] [CrossRef]
- Kokoszka, F.; Conversano, F.; Iudicone, D.; Ferron, B.; Bouruet-Aubertot, P. A Turbulence Survey in the Gulf of Naples, Mediterranean Sea, during the Seasonal Destratification. J. Mar. Sci. Eng. 2023, 11, 499. [Google Scholar] [CrossRef]
- Kokoszka, F.; Le Roux, B.; Iudicone, D.; Conversano, F.; Ribera d’Alcalá, M. Long-Term Variability of the Coastal Ocean Stratification in the Gulf of Naples: Two Decades of Monitoring the Marine Ecosystem at the LTER–MC Site, between Land and Open Mediterranean Sea. Mar. Ecol. 2023, 44, e12725. [Google Scholar] [CrossRef]
- Mazzocchi, M.G. Mesozooplankton Abundance and Species Composition in the Gulf of Naples and the Gulf of Salerno in March 1987. Part 2 [dataset]. Stazione Zool. Anton Dohrn 2008. [Google Scholar] [CrossRef]
- Menghan, W.; Stefano, A.; Annamaria, L.; Claudia, C.; Antonio, C.; Wanjun, L.; Marco, S.; Angela, D.; Benedetto, D.V. Compositional Analysis and Pollution Impact Assessment: A Case Study in the Gulfs of Naples and Salerno. Estuar. Coast. Shelf Sci. 2015, 160, 22–32. [Google Scholar] [CrossRef]
- De Rosa, E.; Montuori, P.; Triassi, M.; Masucci, A.; Nardone, A. Occurrence and Distribution of Persistent Organic Pollutants (POPs) from Sele River, Southern Italy: Analysis of Polychlorinated Biphenyls and Organochlorine Pesticides in a Water–Sediment System. Toxics 2022, 10, 662. [Google Scholar] [CrossRef] [PubMed]
- de Ruggiero, P.; Napolitano, E.; Iacono, R.; Pierini, S.; Spezie, G. A Baroclinic Coastal Trapped Wave Event in the Gulf of Naples (Tyrrhenian Sea). Ocean Dyn. 2018, 68, 1683–1694. [Google Scholar] [CrossRef]
- Ciannelli, L.; Cannavacciuolo, A.; Konstandinidis, P.; Mirasole, A.; Wong-Ala, J.A.T.K.; Guerra, M.T.; D’Ambra, I.; Riginella, E.; Cianelli, D. Ichthyoplankton Assemblages and Physical Characteristics of Two Submarine Canyons in the South Central Tyrrhenian Sea. Fish. Oceanogr. 2022, 31, 480–496. [Google Scholar] [CrossRef]
- Rose, M. Faune de France, N°26: Copépodes Pélagiques. Available online: https://www.abebooks.it/1933-Faune-France-N%C2%B026-Cop%C3%A9podes-P%C3%A9lagiques/31096854703/bd (accessed on 22 March 2024).
- Trégouboff, G.; Rose, M. MANUEL DE PLANCTONOLOGIE MEDITERRANEENNE. Available online: https://doris.ffessm.fr/Bibliographie/MANUEL-DE-PLANCTONOLOGIE-MEDITERRANEENNE (accessed on 22 March 2024).
- Castellani, C.; Edwards, M. Marine Plankton: A Practical Guide to Ecology, Methodology, and Taxonomy; Oxford University Press: Oxford, UK, 2017; ISBN 978-0-19-183569-8. [Google Scholar]
- Razouls; Desreumaux; Kouwenberg; de Bovée Marine Planktonic Copepods: Home Page. Available online: https://copepodes.obs-banyuls.fr/en/ (accessed on 22 March 2024).
- Kiørboe, T. How Zooplankton Feed: Mechanisms, Traits and Trade-Offs. Biol. Rev. 2011, 86, 311–339. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.; Wydler, J.; Vogt, M. Copepod Functional Traits and Groups Show Divergent Biogeographies in the Global Ocean. J. Biogeogr. 2023, 50, 8–22. [Google Scholar] [CrossRef]
- Brun, P.; Payne, M.R.; Kiørboe, T. A Trait Database for Marine Copepods. Earth Syst. Sci. Data 2017, 9, 99–113. [Google Scholar] [CrossRef]
- Li, Y.; Ge, R.; Chen, H.; Zhuang, Y.; Liu, G.; Zheng, Z. Functional Diversity and Groups of Crustacean Zooplankton in the Southern Yellow Sea. Ecol. Indic. 2022, 136, 108699. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2020. [Google Scholar]
- Kiørboe, T.; Sabatini, M. Reproductive and Life Cycle Strategies in Egg-Carrying Cyclopoid and Free-Spawning Calanoid Copepods. J. Plankton Res. 1994, 16, 1353–1366. [Google Scholar] [CrossRef]
- Kiørboe, T.; Hirst, A.G. Shifts in Mass Scaling of Respiration, Feeding, and Growth Rates across Life-Form Transitions in Marine Pelagic Organisms. Am. Nat. 2014, 183, E118–E130. [Google Scholar] [CrossRef] [PubMed]
- van Someren Gréve, H.; Almeda, R.; Kiørboe, T. Motile Behavior and Predation Risk in Planktonic Copepods. Limnol. Oceanogr. 2017, 62, 1810–1824. [Google Scholar] [CrossRef]
- Borme, D.; Tirelli, V.; Palomera, I. Feeding Habits of European Pilchard Late Larvae in a Nursery Area in the Adriatic Sea. J. Sea Res. 2013, 78, 8–17. [Google Scholar] [CrossRef]
- Costalago, D.; Garrido, S.; Palomera, I. Comparison of the Feeding Apparatus and Diet of European Sardines Sardina Pilchardus of Atlantic and Mediterranean Waters: Ecological Implications. J. Fish Biol. 2015, 86, 1348–1362. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.T. The Importance of Small Planktonic Copepods and Their Roles in Pelagic Marine Food Webs. Zool. Stud. 2004, 43, 255–266. [Google Scholar]
- Tönnesson, K.; Tiselius, P. Diet of the Chaetognaths Sagitta Setosa and S. Elegans in Relation to Prey Abundance and Vertical Distribution. Mar. Ecol. Prog. Ser. 2005, 289, 177–190. [Google Scholar] [CrossRef]
- Mazzocchi, M.G.; Dubroca, L.; García-Comas, C.; Capua, I.D.; Ribera d’Alcalà, M. Stability and Resilience in Coastal Copepod Assemblages: The Case of the Mediterranean Long-Term Ecological Research at Station MC (LTER-MC). Prog. Oceanogr. 2012, 97–100, 135–151. [Google Scholar] [CrossRef]
- Williamson, C.E.; Sanders, R.W.; Moeller, R.E.; Stutzman, P.L. Utilization of Subsurface Food Resources for Zooplankton Reproduction: Implications for Diel Vertical Migration Theory. Limnol. Oceanogr. 1996, 41, 224–233. [Google Scholar] [CrossRef]
- Doan-Nhu, H.; Nguyen, T.-V.; Do-Huu, H.; Montoya, J.P.; Nguyen-Ngoc, L. Copepods Key Traits in Diverse Habitats of Tropical Waters. J. Plankton Res. 2022, 44, 158–174. [Google Scholar] [CrossRef]
- Siokou-Frangou, I.; Papathanassiou, E. Differentiation of Zooplankton Populations in a Polluted Area. Mar. Ecol. Prog. Ser. 1991, 76, 41–51. [Google Scholar] [CrossRef]
- Uriarte, I.; Villate, F. Differences in the Abundance and Distribution of Copepods in Two Estuaries of the Basque Coast (Bay of Biscay) in Relation to Pollution. J. Plankton Res. 2005, 27, 863–874. [Google Scholar] [CrossRef][Green Version]
- Peralba, À.; Mazzocchi, M.G. Vertical and Seasonal Distribution of Eight Clausocalanus Species (Copepoda: Calanoida) in Oligotrophic Waters. ICES J. Mar. Sci. 2004, 61, 645–653. [Google Scholar] [CrossRef]
- Cornils, A.; Wend-Heckmann, B.; Held, C. Global Phylogeography of Oithona Similis s.l. (Crustacea, Copepoda, Oithonidae)—A Cosmopolitan Plankton Species or a Complex of Cryptic Lineages? Mol. Phylogenet. Evol. 2017, 107, 473–485. [Google Scholar] [CrossRef]
- Saiz, E.; Griffell, K.; Calbet, A.; Isari, S. Feeding Rates and Prey: Predator Size Ratios of the Nauplii and Adult Females of the Marine Cyclopoid Copepod Oithona Davisae. Limnol. Oceanogr. 2014, 59, 2077–2088. [Google Scholar] [CrossRef]
- Cheng, W.; Akiba, T.; Omura, T.; Tanaka, Y. On the Foraging and Feeding Ability of Oithona Davisae (Crustacea, Copepoda). Hydrobiologia 2014, 741, 167–176. [Google Scholar] [CrossRef]
- Almeda, R.; van Someren Gréve, H.; Kiørboe, T. Prey Perception Mechanism Determines Maximum Clearance Rates of Planktonic Copepods. Limnol. Oceanogr. 2018, 63, 2695–2707. [Google Scholar] [CrossRef]
- Castellani, C.; Robinson, C.; Smith, T.; Lampitt, R.S. Temperature Affects Respiration Rate of Oithona Similis. Mar. Ecol. Prog. Ser. 2005, 285, 129–135. [Google Scholar] [CrossRef]
- Almeda, R.; Alcaraz, M.; Calbet, A.; Saiz, E. Metabolic Rates and Carbon Budget of Early Developmental Stages of the Marine Cyclopoid Copepod Oithona Davisae. Limnol. Oceanogr. 2011, 56, 403–414. [Google Scholar] [CrossRef]
- Cianelli, D.; D’Alelio, D.; Uttieri, M.; Sarno, D.; Zingone, A.; Zambianchi, E.; Ribera d’Alcalà, M. Disentangling Physical and Biological Drivers of Phytoplankton Dynamics in a Coastal System. Sci. Rep. 2017, 7, 15868. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.T. Zooplankton Feeding Ecology: Contents of Fecal Pellets of the Cyclopoid Copepods Oncaea Venusta, Corycaeus Amazonicus, Oithona Plumifera, and O. Simplex from the Northern Gulf of Mexico. Mar. Ecol. 1986, 7, 289–302. [Google Scholar] [CrossRef]
- Sun, R.; Wang, Y.; Wang, C.; Xiang, P.; Chen, X.; Xing, B. Research Advance in the Taxonomy and Ecology of Oncaeidae Giesbrecht, 1893. Front. Mar. Sci. 2022, 9, 919877. [Google Scholar] [CrossRef]
- Böttger-Schnack, R.; Schnack, D.; Weikert, H. Biological Observations on Small Cyclopoid Copepods in the Red Sea. J. Plankton Res. 1989, 11, 1089–1101. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Watanabe, Y.; Ishida, H.; Harimoto, T.; Furusawa, K.; Suzuki, S.; Ishizaka, J.; Ikeda, T.; Takahashi, M.M. Community and Trophic Structures of Pelagic Copepods down to Greater Depths in the Western Subarctic Pacific (WEST-COSMIC). Deep Sea Res. Part Oceanogr. Res. Pap. 2002, 49, 1007–1025. [Google Scholar] [CrossRef]
- Amran, M.A.; Daming, W.S. Estimation of Coastal Waters Turbidity Using Sentinel-2 Imagery. Geod. Cartogr. 2023, 49, 180–185. [Google Scholar] [CrossRef]
- Lee, A.J.; Folkard, A.R. Factors Affecting Turbidity in the Southern North Sea. ICES J. Mar. Sci. 1969, 32, 291–302. [Google Scholar] [CrossRef]
- Paffenhöfer, G.-A. On the Ecology of Marine Cyclopoid Copepods (Crustacea, Copepoda). J. Plankton Res. 1993, 15, 37–55. [Google Scholar] [CrossRef]
- Annabi-Trabelsi, N.; Guermazi, W.; Leignel, V.; Al-Enezi, Y.; Karam, Q.; Ali, M.; Ayadi, H.; Belmonte, G. Effects of Eutrophication on Plankton Abundance and Composition in the Gulf of Gabès (Mediterranean Sea, Tunisia). Water 2022, 14, 2230. [Google Scholar] [CrossRef]
- Besiktepe, S.; Kucuksezgin, F.; Besiktepe, S.T.; Eronat, C.; Gonul, T.; Kurt, T.T.; Sayın, E.; Gubanova, A. Variations in Copepod Composition and Diversity in Relation to Eutrophication and Hydrology in İzmir Bay, Aegean Sea. Mar. Pollut. Bull. 2023, 197, 115745. [Google Scholar] [CrossRef]
- Carman, K.R.; Thistle, D. Microbial Food Partitioning by Three Species of Benthic Copepods. Mar. Biol. 1985, 88, 143–148. [Google Scholar] [CrossRef]
- Kleppel, G.S.; Pieper, R.E. Phytoplankton Pigments in the Gut Concents of Planktonic Copepods from Coastal Waters off Southern California. Mar. Biol. 1984, 78, 193–198. [Google Scholar] [CrossRef]
- Landry, M.R.; Lehner-Fournier, J.M.; Fagerness, V.L. Predatory Feeding Behavior of the Marine Cyclopoid Copepod Corycaeus Anglicus. Mar. Biol. 1985, 85, 163–169. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Onbé, T. Evidence of Selective Feeding on Larvaceans by the Pelagic Copepod Candacia Bipinnata (Calanoida: Candaciidae). J. Plankton Res. 1989, 11, 869–872. [Google Scholar] [CrossRef]
- Battuello, M.; Sartor, R.M.; Brizio, P.; Nurra, N.; Pessani, D.; Abete, M.C.; Squadrone, S. The Influence of Feeding Strategies on Trace Element Bioaccumulation in Copepods (Calanoida). Ecol. Indic. 2017, 74, 311–320. [Google Scholar] [CrossRef]
- López-Urrutia, Á.; Harris, R.P.; Smith, T. Predation by Calanoid Copepods on the Appendicularian Oikopleura Dioica. Limnol. Oceanogr. 2004, 49, 303–307. [Google Scholar] [CrossRef]
- Everett, J.D.; Baird, M.E.; Buchanan, P.; Bulman, C.; Davies, C.; Downie, R.; Griffiths, C.; Heneghan, R.; Kloser, R.J.; Laiolo, L.; et al. Modeling What We Sample and Sampling What We Model: Challenges for Zooplankton Model Assessment. Front. Mar. Sci. 2017, 4, 77. [Google Scholar] [CrossRef]
- Brandão, M.C.; Benedetti, F.; Martini, S.; Soviadan, Y.D.; Irisson, J.-O.; Romagnan, J.-B.; Elineau, A.; Desnos, C.; Jalabert, L.; Freire, A.S.; et al. Macroscale Patterns of Oceanic Zooplankton Composition and Size Structure. Sci. Rep. 2021, 11, 15714. [Google Scholar] [CrossRef]
- Pierini, S.; Simioli, A. A Wind-Driven Circulation Model of the Tyrrhenian Sea Area. J. Mar. Syst. 1998, 18, 161–178. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellardini, D.; Vannini, J.; Russo, L.; Buondonno, A.; Saggiomo, M.; Vassallo, P.; Mazzocchi, M.G.; D’Alelio, D.; Licandro, P. The Spatial Distribution of Copepod Functional Traits in a Highly Anthropized Mediterranean Coastal Marine Region. Environments 2024, 11, 113. https://doi.org/10.3390/environments11060113
Bellardini D, Vannini J, Russo L, Buondonno A, Saggiomo M, Vassallo P, Mazzocchi MG, D’Alelio D, Licandro P. The Spatial Distribution of Copepod Functional Traits in a Highly Anthropized Mediterranean Coastal Marine Region. Environments. 2024; 11(6):113. https://doi.org/10.3390/environments11060113
Chicago/Turabian StyleBellardini, Daniele, Jessica Vannini, Luca Russo, Angela Buondonno, Maria Saggiomo, Paolo Vassallo, Maria Grazia Mazzocchi, Domenico D’Alelio, and Priscilla Licandro. 2024. "The Spatial Distribution of Copepod Functional Traits in a Highly Anthropized Mediterranean Coastal Marine Region" Environments 11, no. 6: 113. https://doi.org/10.3390/environments11060113
APA StyleBellardini, D., Vannini, J., Russo, L., Buondonno, A., Saggiomo, M., Vassallo, P., Mazzocchi, M. G., D’Alelio, D., & Licandro, P. (2024). The Spatial Distribution of Copepod Functional Traits in a Highly Anthropized Mediterranean Coastal Marine Region. Environments, 11(6), 113. https://doi.org/10.3390/environments11060113







