Abstract
Nickel (Ni) is an essential micronutrient for plants, responsible for metabolizing urea nitrogen (urea-N) by urease and mitigating abiotic and oxidative stresses through the glyoxalase (Gly) and glutathione (GSH) cycles. However, excess Ni is toxic to flora at >100 mg kg−1, except for hyperaccumulators that tolerate >1000 mg kg−1 Ni. This review discusses the benefits of Ni nutrient management for soil fertility, improving food security, and minimizing adverse environmental impacts from urea overapplication. Many farming soils are Ni deficient, suggesting that applying 0.05–5 kg ha−1 of Ni improves yield and urea-N use efficiency. Applied foliar and soil Ni fertilizers decrease biotic stresses primarily by control of fungal diseases. The bioavailability of Ni is the limiting factor for urease synthesis in plants, animal guts, and the soil microbiome. Improved urease activity in plants and subsequently through feed in livestock guts reduces the release of nitrous oxide and nitrite pollutants. Fertilizer Ni applied to crops is dispersed in vegetative tissue since Ni is highly mobile in plants and is not accumulated in fruit or leafy tissues to cause health concerns for consumers. New methods for micronutrient delivery, including rhizophagy, recycled struvite, and nanoparticle fertilizers, can improve Ni bioavailability in farming systems.
1. Introduction
Metallic trace elements in soils can be classified as either essential and beneficial nutrients or toxins based on the biological response they induce in living organisms. While some metallic trace elements, such as lead, have no compensatory beneficial effects in living organisms [1], others, such as manganese (Mn) and zinc (Zn), are essential nutrients that become toxic at elevated levels of bioavailability [2]. Nickel (Ni) is an essential nutrient that plays a key role in the metabolism of most living organisms, yet in the field of environmental science, it is primarily observed as a hazardous contaminant. This review outlines the environmental and ecological significance of Ni nutrient management in farming systems. Elemental Ni forms −1 to +4 oxidation states [3]; however, it is broadly present as a divalent cation Ni2+ in soils worldwide [4]. Being the 24th most abundant element in the earth’s crust, Ni reported soil content ranges from 0.2 to 450 mg kg−1, and its estimated global average is 70–85 mg kg−1 [5,6,7,8,9,10,11,12]. Analysis of Ni in over three thousand samples representative of U.S. agricultural soils [13] found an average 17 mg Ni kg−1 dry weight, ranging from 7.4 mg kg−1 Ni in ultisols to 76 mg kg−1 Ni in vertisols [14]. Nickel in soils originates primarily from mineral weathering, where iron- and phosphate-bearing minerals such as serpentinites [6,15] tend to have a higher Ni content than most sedimentary minerals. Leaching is an additional factor affecting soil nickel content, whereby soils with elevated water tables or ones undergoing extensive leaching tend to have lower nickel content [16]. Soluble nickel transport occurs through sorption and desorption reaction series, dominated primarily by soil pH, followed by sorption site abundance and degree of soil solution wetting. Sorption of Ni2+ cations shows higher affinity to iron oxide sites, where desorption increases proportionally to lower soil pH and, to a lesser extent, by changes in redox (Eh) conditions as a function of soil moisture content and microbial activities [17,18].
Secondary Ni input in soils occurs through deposition from Ni-carrying particles of fire ash, volcanic activity, and aeolic dust [19]. Anthropogenic nickel addition to soil occurs primarily through atmospheric depositions of particulate matter that are byproducts from fuel combustion or waste incineration, fertilizers (primarily P fertilizers), and irrigation with contaminated waters. These processes often result in localized elevated soil Ni concentrations one to three orders higher than the local average. Anthropogenic aeolian deposition of Ni to the farming system is associated with proximity to copper-Ni (Cu-Ni) smelting plants [20,21], coal-burning plants [22], dusting of crops [23], or proximity to waste incineration sites [24]. Advancements in methods for predicting aeolian heavy metal deposition differentiate the effect of localized inputs from global particle deposition based on typical ratios of major elements such as magnesium to calcium (Mg/Ca) within potential particle source regions together with modeled air mass trajectories [25]. Waste sludges and bottom ash are used as soil amendments to improve soil structure and can pose a risk for Ni accumulation in crops, as reviewed by Dellantonio [26,27]. A recent study demonstrated the added value of mixing the two for improving soil fertility without increasing the risk of heavy metal accumulation in plants [28]. The application of refurbished waters for crop production is increasing in drought-prone regions and requires consideration for Ni content, as it was associated with elevating farming soil Ni [29].
Agricultural soil Ni inputs are primarily from impurities in applied P fertilizers. In regions where limits are set on P fertilizers’ Ni content, they range from 2000 mg kg−1 for rock phosphates to 50 mg kg−1 for organic fertilizers that often carry only 1–6% P as P2O5 [30,31]. The effective kg ha−1 Ni application rates of such fertilizers derive from the fertilizer source available P percent and will fall short of the 0.6 kg ha−1 Ni soil fertility recommendation for most plants [6,32]. While some urban and industrial soils exhibit elevated Ni content, a growing body of evidence suggests that many agricultural soils are Ni deficient [33,34,35], except for the relatively rare cases where Ni is deposited to agricultural soils through contamination primarily by aeolian deposition or as a side effect of irrigation and soil amendments [23,36,37]. As Ni content in fertilizer is becoming more regulated like other heavy metals such as cadmium and lead, the deficit in soil Ni is expected to increase without addressing Ni nutrient management [30,38].
Elevated soil Ni is known to cause human health and environmental risks [39,40]; however, adequate nutrient management of Ni can improve crop yield and supplement livestock Ni nutritional requirements [41]. A recent review by Spears [42] demonstrated that Ni can improve the growth of livestock by enhancing the nutrient conversion efficiency of livestock gut microbiomes. Application of ≤5 mg kg−1 Ni feed improved urease activity in the gut of ruminant livestock [cattle (B. taurus), sheep (Ovis aries), goat (Capra hircus)] and to some extent in nonruminants, primarily if receiving supplementary urea in feed [chick (Gallus gallus), pigs (Sus domesticus L.)]. The likely mechanisms for improved animal weight gains are retention of protein and ammonia in the animal gut (≤7.5% gain in calves) by enhancing gut microbiome enzymatic activity [43]. Improving livestock urea conversion is environmentally significant since excreted urea-N in feces substantially contributes to livestock nitrous oxide greenhouse gas (GHG) emissions [44]. Under sufficient soil Ni conditions, ingesting Ni from food is the main source of human Ni intake, ranging from 2.3 to 7.4 µg kg−1 d−1, which does not pose a human health risk. However, elevated soil nickel and its subsequently elevated accumulation in crops and ingested foods may cause human health problems, primarily in younger children who tend to ingest higher Ni doses per kg mass or people with Ni allergy [45,46]. Research on elevated Ni content in foods reported higher concentrations in seeds and leafy vegetables, and the absorption rates of Ni ingested from food in humans were estimated at <5% [47,48,49]. While most countries currently do not impose regulations regarding limits for food Ni content, a total daily ingestion maximum value recommendation of 13 µg kg−1 d−1 Ni was recently established based on an estimated benchmark lower dose value of 1.3 mg kg d−1 Ni [50].
Research into Ni application for crop production is looking into the potential benefits of Ni in improving crop production, crop yield, and N conversion, as outlined in Figure 1. Plants absorb Ni through either passive transport or active transport, with the majority of Ni uptake being through plant roots [51]. Factors such as pH, oxides sorption sites, competing cations in soil solution, and organic matter all play a role in its availability, and this is correlated with the free Ni cation activity in soil solution [19,52,53,54,55,56,57]. Ni can also be readily taken up by the plant leaves when foliar fertilization is applied, although this is not the primary intake pathway that plants have evolved [51,56]. Plant Ni is transported primarily from the roots to the shoots and leaves by the xylem and translocated via the phloem to the buds, fruits, and seeds [57,58,59,60,61,62,63]. There is a discrepancy between the abundance of soil Ni and its bioavailability to plants, resulting from higher intake rates of soil divalent cations of similar hydration geometry with higher abundances, whereby iron (Fe) >> zinc (Zn) > Cu > Ni [6,64]. To mitigate the effect of competing soil cations on Ni intake and for practical reasons related to micronutrient application, Ni is often applied in a foliar form integrated into the ongoing nutrient management plan [65,66,67]. Ni also acts as a fungicide to mitigate biotic stresses in crop plants, providing an added benefit to soil and foliar Ni application [68]. This review will outline the current state of research regarding Ni in agricultural soils and agronomic and environmental aspects related to crop production. Evaluating the relationship between soil Ni bioavailability and soil fertility can highlight means to improve sustainable farming through nutrient management.
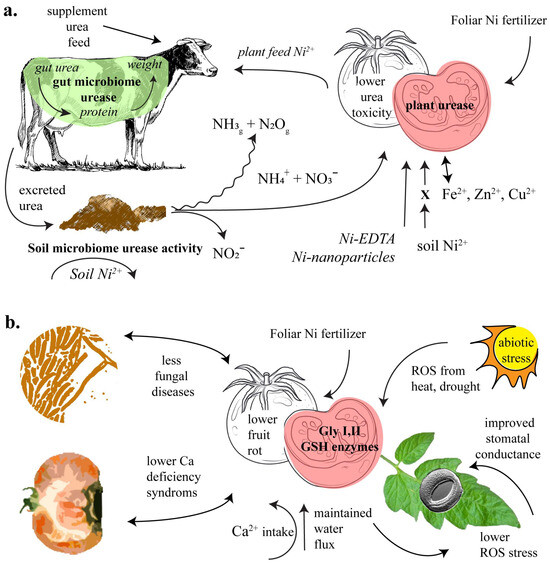
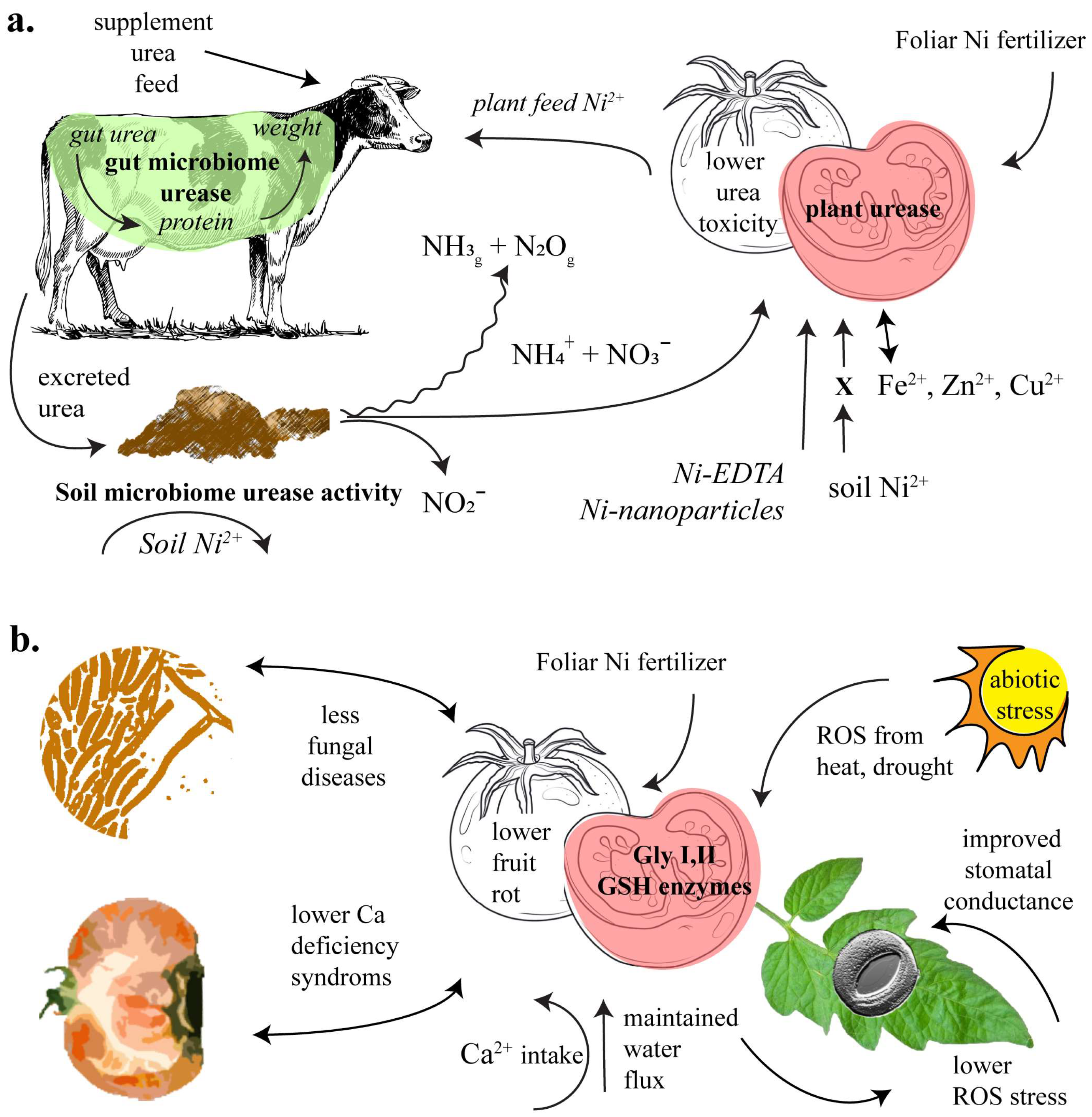
Figure 1.
Depiction of Ni’s role in the farming soil system, governed by the activity of urease (a), and plant glyoxalase and glutathione (GSH) cycle enzymes (b).
2. The Role of Ni in Soil Fertility and the Plant-Rhizosphere Environment
The importance of Ni for crop production was recognized as early as one hundred years ago [69], with speculation about the potential benefits of Ni, together with other micronutrients, in mitigating nutrient deficiency syndromes such as bitter pit, which cause costly yield losses in apples (Malus domestica). However, only in recent decades was Ni added to the plant essential nutrient list [70], with an identified physiological role in urea and ureides cycles or redox reactions that mitigate abiotic stresses [35]. Lower soil Ni bioavailability is associated with several crop nutrient deficiency syndromes (Table 1), including blossom end rot [71] in tomato and mouse-ear in pecan (Carya illinoinensis) and hazelnut (genus Corylus) [34,72]. Soil Ni plays a significant role in plant and soil microbiome metabolism through 11 known Ni-dependent enzymes [73], of which urease and glyoxalase I and II (Gly-I, II) are plant Ni metalloenzymes (Figure 2). Ni is also speculated to influence the regulation of glutathione (GSH) synthesis [74,75,76], which is key to mitigating reactive oxygen species (ROS) stress in plants as well as most living organisms [77,78]. On the other hand, an excess of Ni in soil, often resulting from anthropomorphic activity, becomes a source of abiotic stress by generating ROS [79] and suppressing metalloenzyme activity through competitive inhibition or allosteric regulation (protein/protein, protein/metal), as reviewed by Alfano et al. [80].

Table 1.
Nutrient deficiency syndromes in plants associated or speculated to be associated with Ni.
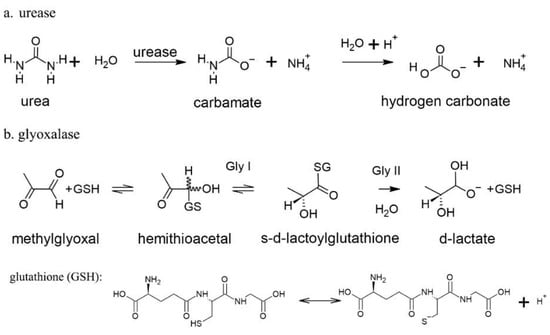
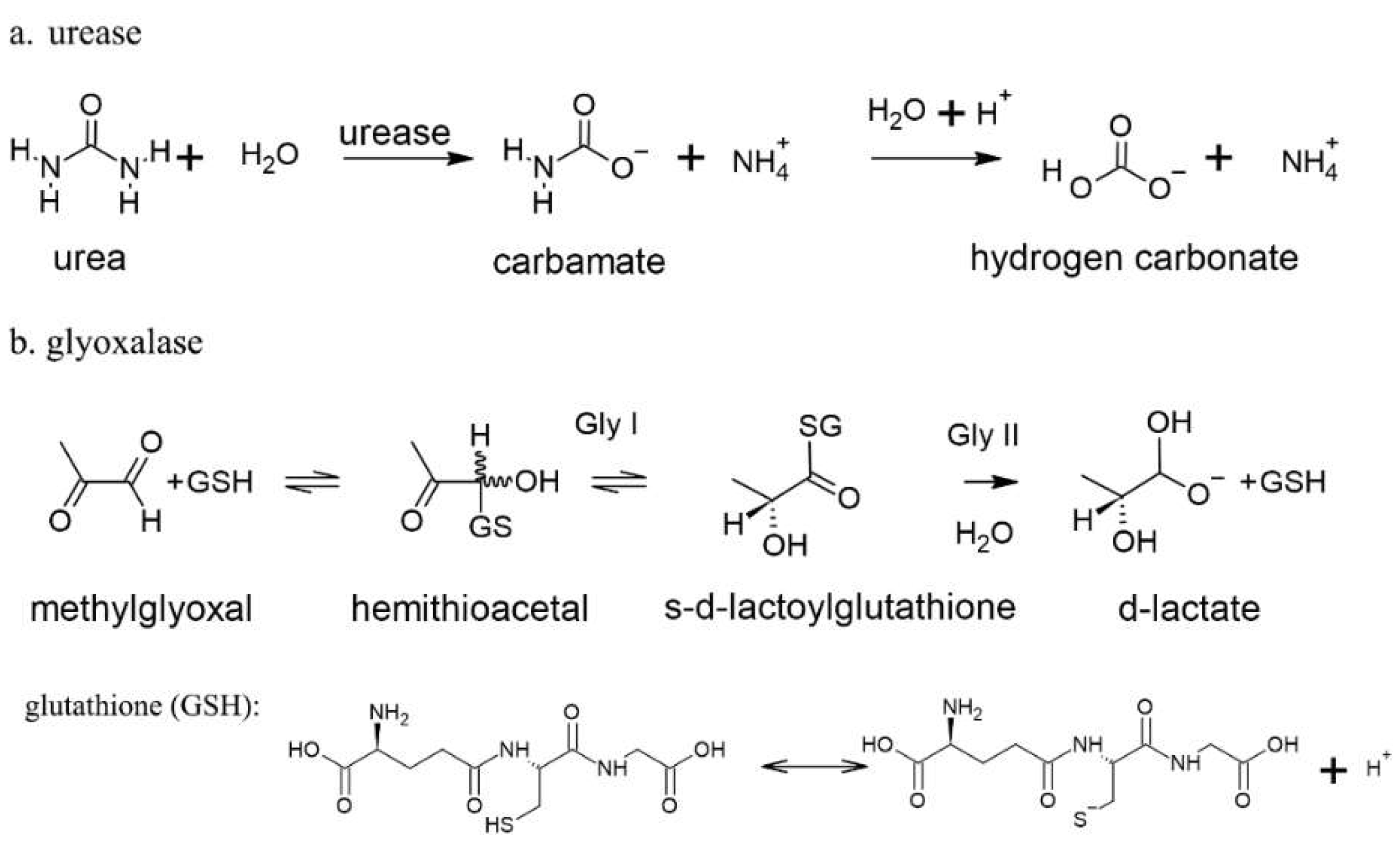
Figure 2.
Mechanistic description of two known plant Ni metalloenzymes: urease (a) and glyoxalase I and II (b) with glutathione (GSH) dissociation reactions.
Recent studies that investigated better soil nutrient management recommendations for Ni evaluated standard methods for soil analysis, such as Mehlich-3 [83], that use ethylenediaminetetraacetic acid (EDTA) extraction or diethylene triamine penta-acetic acid (DTPA) soil test [84] for estimating crop soil Ni availability values [33,55,85]. The DTPA method was used to evaluate the soil Ni application rate of 5 mg kg−1 for soybean (Glycine max). This study related higher Ni extraction rates with an increase in soybean yield, and a critical level of 0.2 mg kg−1 DTPA extractable Ni was established for light-texture soils. In another study, a meta-analysis evaluating the Ni Mehlich-3 method that uses EDTA extraction values for ryegrass (Lolium multifolorum) soils in the states of Illinois and Wisconsin in the U.S. demonstrated Ni deficiency values for the majority of soils tested [33].
3. Transport and Accumulation of Ni in Plants
The most dominant factor in soil Ni availability is the parent rock classification, where mafic or ultramafic sources produce higher soil Ni content [55,86]. Studies that evaluated Ni availability in crop soils also related an increase in soil Ni intake by plants to lower soil pH [87], higher clay content, soil rhizosphere biological activity [88], and, to a lesser extent, organic matter content [32,35]. The kinetics of soil solution Ni exchange and its effect on plant Ni intake were evaluated using an isotopic exchange kinetics approach with a 63Ni radioisotope [55]. This evaluation looked into the rates of Ni dissolution and intake by plants with Ni assimilation capabilities ranging from normal to hyperaccumulators [89]. When entering plants, Ni is mobile in both the xylem and phloem through bonding with peptides [90] and other low-molecular-weight organic ligand carriers. These ligands vary between plant leaves, shoots, or roots as a result of overall chemical composition and pH differences [91]. Such ligands will typically form a 4–5 coordination number geometry with Ni [92,93] that varies in bond strength. Evaluation of bonding energy through X-ray absorption near-edge structure spectroscopy in Ni hyperaccumulator plants found the following increased bonding energies: succinate ≤ glutamate < malate ≤ citrate < oxalate [93]. The extrusion of plant ligands from roots into the rhizosphere was hypothesized to increase the intake of soil Ni through chelation assistance absorption [94], suggesting that chelated ligand-Ni is transferred across membranes using active transporters in a process that is not fully understood. The high mobility of Ni means it can seamlessly translocate from older to younger leaves [63,95,96,97,98,99].
About 50% of Ni that is taken up by the plant is stored in the root system, while the distribution of Ni throughout the plant (leaf organelles, leaves, and stems) can vary by plant species [100,101,102]. Hyperaccumulators are defined as plants that can uptake Ni at concentrations >1000 mg kg−1 on a dry weight basis [89,103]. Hyperaccumulators may have developed this relationship with Ni to defend against herbivores and pathogens [104,105]. Much is still unknown about the Ni intake mechanisms and related metabolic processes regarding Ni hyperaccumulators. It is believed poorly selective zinc transporter-like protein (ZRT-IRT-like) transporters may catalyze the absorption of Ni into the roots, and a ligand in the root cytosol may chelate Ni and prevent cytotoxicity [106,107,108,109]. Due to the overall lower Ni content in most plants, it does not tend to create ROS directly; however, it can indirectly cause oxidative stress through the disruption of metabolism [106].
What may be the missing link in describing plant Ni transport is the role of endophytic bacteria and fungi. Endophytes are microbes that are typically bacteria or fungi that live in the tissue of plants asymptomatically and help with nutrition uptake, stress tolerance, development, and pathogen defense [110]. Multiple endophytes have already been shown to facilitate increased Ni tolerance and uptake in plants and prevent stymied growth from Ni toxicity in hyperaccumulator plants [111,112,113,114,115,116,117,118,119]. These microbes may play a vital part in the mechanisms of biogeochemical processes relative to Ni. A proposed mechanism of nutrient extraction from these endophytic microbes is the rhizophagy cycle, a two-stage mechanism where nutrient-carrying endophytes enter plant roots, are stripped of their nutrients, and then extruded back into the rhizosphere [120]. Plant endophytes carry a significant amount of micronutrients sorbed to their cell walls and can manipulate rhizosphere pH to improve the bioavailability of Ni needed for their enzyme functions. Following the first stage of rhizophagy, where the endophyte enters the plant root nodes, nutrients are extracted from the microbes to plant tissues by induced oxidative conditions in the root cell plasma membranes [121,122,123]. Hyperaccumulators exposed to Ni also have higher ROS levels, thus suggesting ROS detoxification capabilities rather than preventing Ni-induced ROS formation from happening [105]. Increased levels of ROS could be tied to the rhizophagy cycle and point to increased relationships with multiple endophytic microbes, which could provide an explanation of why Ni hyperaccumulators are able to cope better with Ni toxicity than non-hyperaccumulators. Further studies into the endophyte rhizophagy cycle regarding its relationship with Ni are needed to adopt better practices for Ni nutrient management and utilize the potential benefits of endophytic biofertilizers.
4. Nickel and Biotic Stress in Plants
Nickel can mitigate biotic stresses in plants and has often been used as a fungicide. The use of Ni as a fungicide has an extensive history, dating back to 1908, when the fungicidal effects of Ni compounds were established [124]. In 1961, a U.S. patent (No. 2,971,880) was issued to Rohm and Haas Co. for the application of Ni as a fungicide [125], and by 1963, over 149 scientific references observed Ni activities against various fungi [68]. Ni appears to work as a fungicide by disrupting carbohydrate metabolism and DNA repair with the creation of reactive oxygen species (ROS) and membrane damage. This occurs due to Ni replacing the original metal in the metalloenzymes, which eliminates the redox function of the metal cofactor, as reviewed in detail by Mecomber and Gerwien et al. [126,127]. It appears that the fungicidal properties may be related to its role in the activation of urease [128,129]. The antifungal properties seem to be from an enzymatic-independent mechanism that involves fungal membrane permeabilization rather than the normal catalytic activity of urease [129]. This is not predicated on ureolytic activity [130], which may indicate that Ni’s fungicidal role could be the stabilization of urease and less turnover of the enzyme [128]. With some plant varieties being damaged by very low Ni amounts [128,131] and due to the possibility of plant toxicity, Ni is not currently applied regularly as a fungicide [128]. Human health concerns and potential carcinogenic/toxic effects, mostly from Ni dusting [132,133,134], may have also limited its application as a fungicide. In contrast, recent studies that evaluated the fungicide effect also demonstrated a significant effect on fungal disease control at relatively low application rates (Table 2).

Table 2.
Effect of foliar Ni application on crop fungal diseases.
Using Ni as part of foliar or soil nutrient management plan can also assist with growing resistivity to Cu-based fungicides by many microbial pathogens. Inorganic Cu is an expansively used fungicide that has diminished efficacy resulting from an increase in resistivity traits, as reviewed by Lamichhane et al. [139]. Expansion of Ni nutrient management adoption demonstrated that Ni application was found to have a lesser phytotoxic effect than previously expected and a positive fungicide response in crops [72,124]. These findings, together with the need for a readily available solution for Cu-resistant plant disease, merit the reconsideration of Ni as a supplemental fungicide treatment in areas where resistivity to Cu products is high [140].
5. Advancement in Ni Delivery to Plants and Future Research
While there is a large body of research on chelated micronutrients and foliar fertilizer applications to improve the bioavailability of Ni for crop plants [35], there is a need for more research on methods for delivering micronutrients using high-surface-area and nanoparticle fertilizers. These newer approaches were reported to be most applicable in farming systems where foliar spraying is not regularly applied due to agrotechnical limitations or where they offer a lower-cost alternative to chelated products [141]. Slow-release fertilizers with high surface areas, such as struvite (MgNH4PO4·6H2O) [142,143], a mineral product of phosphate and ammonium recovery from wastewater [144], demonstrated the ability to deliver micronutrients to crops [145]. Recovered P minerals, such as struvite, have a high affinity for Ni sorption during the precipitation process [146,147,148,149]. Expanding the use of struvite and other regenerated fertilizers with high surface areas to deliver Ni and other micronutrients can advance the goal of adding value to nutrient recovery and thus making it more economically viable.
Nanotechnology, which includes engineered particulate minerals [150], metal–oxide frameworks [151], and nanoconjugate [152] nanoparticles, was reported in recent studies as beneficial for improving the bioavailability of nutrients and micronutrients in agricultural soils [153,154,155]. These synthetic components have distinctive chemical and physical properties depending on particle size (with regards to the size distribution and surface area), shape, solubility, structure, and assemblage [156,157,158,159]. Nanoparticles are more effective, efficient, reactive, and soluble than their bulk equivalents [158,159,160,161]. Nanoparticles also allow for a more controlled, targeted delivery (including to specific tissues), which can reduce the use of plant-protection products, optimize nutrient management and thus increase yields, and decrease nutrient losses from fertilizers [159]. Given the low dosage and the low amount of Ni needed by plants, this may be a multi-beneficial solution for agriculture. If developed and applied correctly, this nickel nanoparticle could be used as a fertilizer and fungicide combination, thus reducing the overall application of both by farmers. Thus far, nickel-related nanoparticles and nanoconjugates have been used successfully as fertilizes [162] and have been shown to be effective as fungicides against Fusarium oxysporum, Colletotrichum gloeosporioides, Dematophora necatrix [156], Fusarium wilt [137,156], Fusarium solani [163], and Pyricularia oryzae [164]. While nanoparticles present agronomical benefit potential, the risk of environmental toxicity to plants and humans suggests that further studies are needed to adopt field-scale application [159,165,166,167,168,169].
6. Discussion
Understanding the role Ni plays in the agronomic environment is evolving from being labeled a pollutant to recognition of its functions for improving nutrient management for crop production. While there is an overwhelming consensus in the scientific community regarding the risk that Ni contamination poses to soil health, the application rates of Ni for crop production ranging from 0.05 to 5 kg ha−1 are not environmentally significant and comply with current Ni regulations regarding soil amendments [30]. The potential environmental benefits of Ni nutrient management are primarily in improving nitrogen conversion and lessening the harm from abiotic stress related to climate change (drought, elevated heat) that improve crop yield to meet global food production goals [170]. The high sorption affinity of Ni in most soils diverts agronomic delivery practices towards foliar application in orchards, row crops, and field crops, integrating it with the ongoing foliar nutrient management plan. Undetected Ni deficiency in crop plants decreases plant urea-N use efficiency, resulting in excess N losses to nitrous oxide and nitrate. It is also apparent that Ni deficiency is likely in the feed of livestock, resulting in diminished urease activity in the livestock gut biome. The global urea price crisis of recent years [169], as well as the increasing regulatory push to limit N application in agricultural systems [170], are compelling justifications for adopting Ni nutrient management to enhance urea-N use efficiency.
The relationship between crop Ni availability and abiotic stress mitigation is less clear, yet the application of Ni has been reported to mitigate drought stress, heat stress, and Ca deficiency syndromes. Evaluation of Ni hyperaccumulators was used to identify Ni-regulated genes and determine the biogeochemistry of Ni transport in plants [93,171,172,173]. Foliar application of Ni is considered to be the most agronomically effective delivery method since it eliminates soil sorption and competition with cations of Fe, Zn, Cu, and similar divalent cations on roots’ active transport sites. A drawback to foliar Ni application is that it might not be applicable for farming systems where the occurrence of Ni deficiency is well established, such as in grazing pastures where it is not cost-effective. In addition, while commonly occurring levels of soil nickel have little effect on farm safety, inhaling Ni aerosols such as the ones formed by foliar sprayers are known to be carcinogenic and toxic to humans and animals [174,175]. A newer generation of Ni delivery methods is proposed by promoting plant rhizophagy, using recycled nutrients enriched with Ni, or nanoparticles of Ni, as an alternative to the more costly chelated Ni products such as Ni EDTA. Chelation with EDTA, such as the proposed Ni EDTA, is considered most effective in crops where liquid fertigation is needed [176]. Another disadvantage of chelated fertilizers, other than higher costs, is that they pose an environmental risk by increasing the bioavailability of undesirable heavy metal contaminants, as reviewed by Pinto et al. [177]. Recent work suggests the adoption of biodegradable chelates as a less environmentally harmful approach, which is likely to be applicable to Ni chelates [178].
The environmental significance of Ni nutrient management in agricultural soils is primarily the improvement in urea-N conversion by crop plants and, to a lesser extent, by livestock. Urea-N comprises 25% of all N applied in the United States [179], 40% in the European Union [180], 50% in China [181], and ~70% globally [182]. Thus, studies have demonstrated that increasing plant urea-N use efficiency can significantly decrease N application rate requirements. Current practice in many localities is to overapply urea-N by up to 50%, accounting for anticipated N losses to nitrous oxide, ammonia, and nitrite, and improving plant urease activity can significantly reduce these losses [176,183,184]. Additionally, the proposed relationship between Ni and mitigation of abiotic stress, the subsequent improved stomal water flux, and improved Ca intake by plants also affect the environmental impact of farming systems. Deficiencies of Ca are associated with decreased crop yield, and in the case of plants susceptible to BER (e.g., tomato and bell pepper), the occurrence of even the mildest symptoms will result in unmarketable fruits. Events of BER in vegetables are poorly predicted and frequently result in up to 80% fruit loss [185]. As a result, excessive irrigation and Ca application are commonly used to prevent Ca deficiency, resulting in water losses that limit food production in many regions globally [186]. Ni nutrient management is a potentially low-cost and readily available method to mitigate abiotic stresses contributing to Ca deficiencies without applying excessive irrigation.
7. Conclusions
Nutrient management of Ni in farming systems was reviewed to compare its potential direct adverse environmental effects with its agricultural and subsequently indirect environmental benefits. Reported recommended application rates for agricultural Ni of <5 kg ha−1 were not reported to be environmentally significant. Moreover, Ni fertilizer applications have demonstrated a beneficial effect from increasing urease and other Ni enzyme activities that enhance food security and decrease the overall environmental impact of agricultural activities. Enhanced plant urease activity prevented urea stress in plants, improved plant urea-N conversion, and decreased nitrous oxide GHG emissions. Advancements in research on the plant Ni metalloenzymes Gly I, II, as well as the GSH system, demonstrated that improving Ni intake in crop plants assists in developing drought resistance and heat-resistant crops. Mitigating abiotic stress in plants through Ni nutrient management lessens fruit yield losses caused by deficiency syndromes such as BER. Application of Ni at agricultural nutrient management levels also mitigated biotic stresses in a wide range of crop plants, primarily by suppressing fungal diseases. Ni is highly mobile in plants but tends to have lower bioavailability in soils, which is often resolved by using foliar fertilizer applications. Newer alternatives such as biofertilizers, the rhizophagy approach, high-surface-area products, and nanoparticle-based fertilizers have demonstrated the potential to improve Ni intake in plants and farming systems where foliar application is not applicable.
Author Contributions
Conceptualization, A.R., R.D., S.L. and J.H.; methodology, A.R. and J.H.; experiments, data collection, and analysis, A.R. and J.H.; writing—original draft preparation, A.R., R.D., S.L. and J.H.; writing—review and editing, A.R., R.D., S.L. and J.H.; funding acquisition, J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the USDA NE-SARE grant LNE22-449R.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pain, D.J.; Mateo, R.; Green, R.E. Effects of lead from ammunition on birds and other wildlife: A review and update. Ambio 2019, 48, 935–953. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Cotton, F.A.; Matusz, M.; Poli, R.; Feng, X. Dinuclear formamidinato complexes of nickel and palladium. J. Am. Chem. Soc. 1988, 110, 1144–1154. [Google Scholar] [CrossRef]
- Harasim, P. Nickel resources and sources. In Nickel in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2018; pp. 87–104. [Google Scholar]
- Alloway, B. Heavy Metals in Soils, 2nd ed.; Blackie Academic and Professional: London, UK, 1995. [Google Scholar]
- Gasparatos, D.; Barbayiannis, N. The Origin of Nickel in Soils. In Nickel in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2018; pp. 105–128. [Google Scholar]
- Kabata-Pendias, A.; Szteke, B. Trace Elements in Abiotic and Biotic Environments; Taylor & Francis: Boca Raton, FL, USA, 2015. [Google Scholar]
- Albanese, S.; Sadeghi, M.; Lima, A.; Cicchella, D.; Dinelli, E.; Valera, P.; Falconi, M.; Demetriades, A.; De Vivo, B.; Team, G.P. GEMAS: Cobalt, Cr, Cu and Ni distribution in agricultural and grazing land soil of Europe. J. Geochem. Explor. 2015, 154, 81–93. [Google Scholar] [CrossRef]
- Reimann, C.; de Caritat, P. Establishing geochemical background variation and threshold values for 59 elements in Australian surface soil. Sci. Total Environ. 2017, 578, 633–648. [Google Scholar] [CrossRef]
- Yao, W.; Xie, X.; Zhao, P.; Bai, J. Global scale geochemical mapping program—Contributions from China. J. Geochem. Explor. 2014, 139, 9–20. [Google Scholar] [CrossRef]
- Gough, L.P. Geochemical Landscapes of Alaska: New Map Presentations and Interpretations for 23 Elements in Surficial Materials; US Geological Survey: Reston, VA, USA, 2005. [Google Scholar]
- Ajala, L.; Onwukeme, V.; Mgbemena, M. Speciation of some trace metals in floodplain soil of Eke-Mgbom, Afikpo, Nigeria. Am. Chem. Sci. J. 2014, 4, 963–974. [Google Scholar] [CrossRef]
- Holmgren, G.; Meyer, M.; Chaney, R.; Daniels, R. Cadmium, Lead, Zinc, Copper, and Nickel in Agricultural Soils of the United States of America. J. Environ. Qual. 1993, 22, 335–348. [Google Scholar] [CrossRef]
- Nachtergaele, F. Soil taxonomy—A basic system of soil classification for making and interpreting soil surveys. Geoderma 2001, 99, 336–337. [Google Scholar] [CrossRef]
- Hseu, Z.-Y.; Chen, Z.-S. Nickel in serpentine soils. In Nickel in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2018; pp. 181–198. [Google Scholar]
- Bear, F.E. Trace elements, progress report on research with particular reference to New Jersey soils. J. Agric. Food Chem. 1954, 2, 244–251. [Google Scholar] [CrossRef]
- Rinklebe, J.; Shaheen, S.M. Redox chemistry of nickel in soils and sediments: A review. Chemosphere 2017, 179, 265–278. [Google Scholar] [CrossRef]
- Uchimiya, M.; Bannon, D.; Nakanishi, H.; McBride, M.B.; Williams, M.A.; Yoshihara, T. Chemical speciation, plant uptake, and toxicity of heavy metals in agricultural soils. J. Agric. Food Chem. 2020, 68, 12856–12869. [Google Scholar] [CrossRef]
- Iyaka, Y.A. Nickel in soils: A review of its distribution and impacts. Sci. Res. Essays 2011, 6, 6774–6777. [Google Scholar]
- Derome, J.; Lindross, A.-J. Copper and nickel mobility in podzolic forest soils subjected to heavy metal and sulphur deposition in western Finland. Chemosphere 1998, 36, 1131–1136. [Google Scholar] [CrossRef]
- Adamo, P.; Dudka, S.; Wilson, M.; McHardy, W. Chemical and mineralogical forms of Cu and Ni in contaminated soils from the Sudbury mining and smelting region, Canada. Environ. Pollut. 1996, 91, 11–19. [Google Scholar] [CrossRef]
- Parzentny, H.R.; Róg, L. Distribution and mode of occurrence of co, ni, cu, zn, as, ag, cd, sb, pb in the feed coal, fly ash, slag, in the topsoil and in the roots of trees and undergrowth downwind of three power stations in poland. Minerals 2021, 11, 133. [Google Scholar] [CrossRef]
- Mirlean, N.; Roisenberg, A.; Chies, J.O. Metal contamination of vineyard soils in wet subtropics (southern Brazil). Environ. Pollut. 2007, 149, 10–17. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Pham, Q.V.; Nguyen, T.P.M.; Vu, V.T.; Do, T.H.; Hoang, M.T.; Thu Thuy Thi, N.; Minh, T.B. Distribution characteristics and ecological risks of heavy metals in bottom ash, fly ash, and particulate matter released from municipal solid waste incinerators in northern Vietnam. Environ. Geochem. Health 2023, 45, 2579–2590. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Sun, G.; Zhang, C.; Chen, Y.; Yang, W.; Shang, L. A new geochemical method for determining the sources of atmospheric particles: A case study from Gannan, Northeast China. Atmosphere 2019, 10, 632. [Google Scholar] [CrossRef]
- Heckman, J.; Angle, J.; Chaney, R. Soybean nodulation and nitrogen fixation on soil previously amended with sewage sludge. Biol. Fertil. Soils 1986, 2, 181–185. [Google Scholar] [CrossRef]
- Dellantonio, A.; Fitz, W.J.; Repmann, F.; Wenzel, W.W. Disposal of coal combustion residues in terrestrial systems: Contamination and risk management. J. Environ. Qual. 2010, 39, 761–775. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Kowalewska, A.; Mikołajczak, S.; Kołodziej, B.; Bryk, M.; Spychaj-Fabisiak, E.; Koliopoulos, T.; Babula, J. Phytoextraction of heavy metals after application of bottom ash and municipal sewage sludge considering the risk of environmental pollution. J. Environ. Manag. 2022, 306, 114517. [Google Scholar] [CrossRef]
- Asgari, K.; Cornelis, W.M. Heavy metal accumulation in soils and grains, and health risks associated with use of treated municipal wastewater in subsurface drip irrigation. Environ. Monit. Assess. 2015, 187, 410. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No. 1069/2009 and (EC) No. 1107/2009 and repealing Regulation (EC) No. 2003/2003. FAOLEX 2019, LEX-FAOC187729, 1–114.
- Molina, M.; Aburto, F.; Calderón, R.; Cazanga, M.; Escudey, M. Trace element composition of selected fertilizers used in Chile: Phosphorus fertilizers as a source of long-term soil contamination. Soil Sediment Contam. 2009, 18, 497–511. [Google Scholar] [CrossRef]
- Liu, G.; Simonne, E.; Li, Y. Nickel Nutrition in Plants; FAS Extension University of Florida: Gainesville, FL, USA, 2011; Volume 6. [Google Scholar]
- Sawyer, D.C.; Barak, P. Mehlich III predicts that soils in Wisconsin and Illinois may cause nickel deficiency in crops. Plant Soil 2023, 497, 523–534. [Google Scholar] [CrossRef]
- Nyczepir, A.; Wood, B.; Reilly, C. Field Deficiency of Nickel in Trees: Symptoms and Causes. In Proceedings of the V International Symposium on Mineral Nutrition of Fruit Plants 721, Talca, Chile, 16–21 January 2005; pp. 83–98. [Google Scholar]
- Barker, A.V.; Pilbeam, D.J. Handbook of Plant Nutrition; CRC press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Heckman, J.; Angle, J.; Chaney, R. Residual Effects of Sewage Sludge on Soybean: I. Accumulation of Heavy Metals. J. Environ. Qual. 1987, 16, 113–117. [Google Scholar] [CrossRef]
- Siqueira Freitas, D.; Wurr Rodak, B.; Rodrigues dos Reis, A.; de Barros Reis, F.; Soares de Carvalho, T.; Schulze, J.; Carbone Carneiro, M.A.; Guimaraes Guilherme, L.R. Hidden nickel deficiency? Nickel fertilization via soil improves nitrogen metabolism and grain yield in soybean genotypes. Front. Plant Sci. 2018, 9, 614. [Google Scholar] [CrossRef]
- Westfall, D.; Mortvedt, J.; Peterson, G.; Gangloff, W. Efficient and environmentally safe use of micronutrients in agriculture. Commun. Soil Sci. Plant Anal. 2005, 36, 169–182. [Google Scholar] [CrossRef]
- Schaumlöffel, D. Nickel species: Analysis and toxic effects. J. Trace Elem. Med. Biol. 2012, 26, 1–6. [Google Scholar] [CrossRef]
- Buxton, S.; Garman, E.; Heim, K.E.; Lyons-Darden, T.; Schlekat, C.E.; Taylor, M.D.; Oller, A.R. Concise Review of Nickel Human Health Toxicology and Ecotoxicology. Inorganics 2019, 7, 89. [Google Scholar] [CrossRef]
- Spears, J. Nickel as a “newer trace element” in the nutrition of domestic animals. J. Anim. Sci. 1984, 59, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Spears, J.W. Boron, chromium, manganese, and nickel in agricultural animal production. Biol. Trace Elem. Res. 2019, 188, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Zhao, S.; Zheng, N.; Bu, D.; Beckers, Y.; Wang, J. Urea nitrogen induces changes in rumen microbial and host metabolic profiles in dairy cows. Livest. Sci. 2018, 210, 104–110. [Google Scholar] [CrossRef]
- Clough, T.J.; Cardenas, L.M.; Friedl, J.; Wolf, B. Nitrous oxide emissions from ruminant urine: Science and mitigation for intensively managed perennial pastures. Curr. Opin. Environ. Sustain. 2020, 47, 21–27. [Google Scholar] [CrossRef]
- Sharma, A.; Nagpal, A.K. Contamination of vegetables with heavy metals across the globe: Hampering food security goal. J. Food Sci. Technol. 2020, 57, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Mania, M.; Rebeniak, M.; Postupolski, J. Food as a source of exposure to nickel. Rocz. Państwowego Zakładu Hig. 2019, 70, 393–399. [Google Scholar]
- Smart, G.; Sherlock, J. Nickel in foods and the diet. Food Addit. Contam. 1987, 4, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Onianwa, P.; Lawal, J.; Ogunkeye, A.; Orejimi, B. Cadmium and nickel composition of Nigerian foods. J. Food Compos. Anal. 2000, 13, 961–969. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Z.; Yang, G.; Wang, Q. Health risk assessment of Chinese consumers to nickel via dietary intake of foodstuffs. Food Addit. Contam. Part A 2014, 31, 1861–1871. [Google Scholar] [CrossRef]
- Chain, E.P.o.C.i.t.F.; Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C. Update of the risk assessment of nickel in food and drinking water. EFSA J. 2020, 18, e06268. [Google Scholar]
- Seregin, I.V.; Kozhevnikova, A.D. Physiological role of nickel and its toxic effects on higher plants. Russ. J. Plant Physiol. 2006, 53, 257–277. [Google Scholar] [CrossRef]
- Massoura, S.T.; Echevarria, G.; Becquer, T.; Ghanbaja, J.; Leclerc-Cessac, E.; Morel, J.-L. Control of nickel availability by nickel bearing minerals in natural and anthropogenic soils. Geoderma 2006, 136, 28–37. [Google Scholar] [CrossRef]
- Rooney, C.P.; Zhao, F.-J.; McGrath, S.P. Phytotoxicity of nickel in a range of European soils: Influence of soil properties, Ni solubility and speciation. Environ. Pollut. 2007, 145, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Murray, P.; Hendershot, W.H. Trace metal speciation and bioavailability in urban soils. Environ. Pollut. 2000, 107, 137–144. [Google Scholar] [CrossRef]
- Echevarria, G.; Massoura, S.T.; Sterckeman, T.; Becquer, T.; Schwartz, C.; Morel, J.L. Assessment and control of the bioavailability of nickel in soils. Environ. Toxicol. Chem. 2006, 25, 643–651. [Google Scholar] [CrossRef]
- Sajwani, K.S.; Ornes, W.H.; Youngblood, T.V.; Alva, A.K. Uptake of soil applied cadmium, nickel and selenium by bush beans. Water Air Soil Pollut. 1996, 91, 209–217. [Google Scholar] [CrossRef]
- Peralta-Videa, J.; Gardea-Torresdey, J.; Tiemann, K.; Gomez, E.; Arteaga, S.; Rascon, E.; Parsons, J. Uptake and effects of five heavy metals on seed germination and plant growth in alfalfa (Medicago sativa L.). Bull. Environ. Contam. Toxicol 2001, 66, 727–734. [Google Scholar]
- Krupa, Z.; Siedlecka, A.; Maksymiec, W.; Baszyński, T. In vivo response of photosynthetic apparatus of Phaseolus vulgaris L. to nickel toxicity. J. Plant Physiol. 1993, 142, 664–668. [Google Scholar] [CrossRef]
- Neumann, P.M.; Chamel, A. Comparative phloem mobility of nickel in nonsenescent plants. Plant Physiol. 1986, 81, 689–691. [Google Scholar] [CrossRef]
- Welch, R.M.; Shuman, L. Micronutrient nutrition of plants. Crit. Rev. Plant Sci. 1995, 14, 49–82. [Google Scholar] [CrossRef]
- Fismes, J.; Echevarria, G.; Leclerc-Cessac, E.; Morel, J.L. Uptake and transport of radioactive nickel and cadmium into three vegetables after wet aerial contamination. J. Environ. Qual. 2005, 34, 1497–1507. [Google Scholar] [CrossRef]
- Page, V.; Weisskopf, L.; Feller, U. Heavy metals in white lupin: Uptake, root-to-shoot transfer and redistribution within the plant. New Phytol. 2006, 171, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.S.A.; Ashraf, M. Essential Roles and Hazardous Effects of Nickel in Plants. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2011; pp. 125–167. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Found. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Kumar, O.; Singh, S.K.; Latare, A.M.; Yadav, S.N. Foliar fertilization of nickel affects growth, yield component and micronutrient status of barley (Hordeum vulgare L.) grown on low nickel soil. Arch. Agron. Soil Sci. 2018, 64, 1407–1418. [Google Scholar] [CrossRef]
- Hosseini, H.; Khoshgoftarmanesh, A. The effect of foliar application of nickel in the mineral form and urea-Ni complex on fresh weight and nitrogen metabolism of lettuce. Sci. Hortic. 2013, 164, 178–182. [Google Scholar] [CrossRef]
- de Queiroz Barcelos, J.P.; de Souza Osorio, C.R.W.; Leal, A.J.F.; Alves, C.Z.; Santos, E.F.; Reis, H.P.G.; dos Reis, A.R. Effects of foliar nickel (Ni) application on mineral nutrition status, urease activity and physiological quality of soybean seeds. Aust. J. Crop Sci. 2017, 11, 184–192. [Google Scholar] [CrossRef]
- The International Nickel Company. Nickel Compounds as Fungicides; ICB-39; The International Nickel Company, Inc.: New York, NY, USA, 1964. [Google Scholar]
- Ewart, A.J. On Bitter Pit and the Sensitivity of Apples to Poison; Ford & Son, Printers: Chicago, IL, USA, 1913. [Google Scholar]
- Brown, P.H.; Welch, R.M.; Cary, E.E. Nickel: A micronutrient essential for higher plants. Plant Physiol. 1987, 85, 801–803. [Google Scholar] [CrossRef]
- Macedo, F.G.; de Melo, W.J.; Cecílio Filho, A.B.; Santos, E.F.; Cruz, R.B.; Belloti, M. Nickel reduces blossom-end rot even under calcium deficiency conditions; evidence from physiological responses of the NI-CA interaction. J. Plant Nutr. 2023, 46, 2893–2904. [Google Scholar] [CrossRef]
- Bock, C.H.; Pisani, C.; Wood, B.W. Nickel and Plant Disease. In Mineral Nutrition and Plant Disease; Datnoff, L.E., Elmer, W.H., Rodrigues, F.A., Eds.; APS Press: St. Paul, MN, USA, 2023. [Google Scholar]
- Hausinger, R.P. Chapter Four—Five decades of metalloenzymology. In The Enzymes; Kaguni, L.S., Tamanoi, F., Eds.; Academic Press: New York, NY, USA, 2023; Volume 54, pp. 71–105. [Google Scholar]
- Mustafiz, A.; Ghosh, A.; Tripathi, A.K.; Kaur, C.; Ganguly, A.K.; Bhavesh, N.S.; Tripathi, J.K.; Pareek, A.; Sopory, S.K.; Singla-Pareek, S.L. A unique Ni2+-dependent and methylglyoxal-inducible rice glyoxalase I possesses a single active site and functions in abiotic stress response. Plant J. 2014, 78, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.L.; Persans, M.W.; Nieman, K.; Albrecht, C.; Peer, W.; Pickering, I.J.; Salt, D.E. Increased glutathione biosynthesis plays a role in nickel tolerance in thlaspi nickel hyperaccumulators. Plant Cell 2004, 16, 2176–2191. [Google Scholar] [CrossRef]
- Fabiano, C.C.; Tezotto, T.; Favarin, J.L.; Polacco, J.C.; Mazzafera, P. Essentiality of nickel in plants: A role in plant stresses. Front. Plant Sci. 2015, 6, 754. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Gajewska, E.; Skłodowska, M.; Słaba, M.; Mazur, J. Effect of nickel on antioxidative enzyme activities, proline and chlorophyll contents in wheat shoots. Biol. Plant. 2006, 50, 653–659. [Google Scholar] [CrossRef]
- Yu, H.; Li, W.; Liu, X.; Song, Q.; Li, J.; Xu, J. Physiological and molecular bases of the nickel toxicity responses in tomato. Stress Biol. 2024, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Alfano, M.; Cavazza, C. Structure, function, and biosynthesis of nickel-dependent enzymes. Protein Sci. 2020, 29, 1071–1089. [Google Scholar] [CrossRef]
- Torres, E.; Kalcsits, L.; Nieto, L.G. Is calcium deficiency the real cause of bitter pit? A review. Front. Plant Sci. 2024, 15, 1383645. [Google Scholar] [CrossRef]
- Wood, B.W.; Reilly, C.C.; Nyczepir, A.P. Mouse-ear of pecan: A nickel deficiency. HortScience 2004, 39, 1238–1242. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Barman, M.; Datta, S.P.; Rattan, R.K.; Meena, M.C. Critical Limits of Deficiency of Nickel in Intensively Cultivated Alluvial Soils. J. Soil Sci. Plant Nutr. 2020, 20, 284–292. [Google Scholar] [CrossRef]
- Okoli, N.; Uzoho, B.; Ahukaemere, C.; Egboka, N.; Irokwe, I. Chemical fractionation and mobility of nickel in soils in relation to parent materials. Arch. Agron. Soil Sci. 2021, 67, 1075–1092. [Google Scholar] [CrossRef]
- Barrow, N.; Hartemink, A.E. The effects of pH on nutrient availability depend on both soils and plants. Plant Soil 2023, 487, 21–37. [Google Scholar] [CrossRef]
- Macedo, F.G.; Santos, E.F.; Lavres, J. Agricultural crop influences availability of nickel in the rhizosphere; a study on base cation saturations, Ni dosages and crop succession. Rhizosphere 2020, 13, 100182. [Google Scholar] [CrossRef]
- Deng, T.-H.-B.; van der Ent, A.; Tang, Y.-T.; Sterckeman, T.; Echevarria, G.; Morel, J.-L.; Qiu, R.-L. Nickel hyperaccumulation mechanisms: A review on the current state of knowledge. Plant Soil 2018, 423, 1–11. [Google Scholar] [CrossRef]
- Shalev, D.E. Studying peptide-metal ion complex structures by solution-state NMR. Int. J. Mol. Sci. 2022, 23, 15957. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Garland, T.R.; Wildung, R.E.; Drucker, H. Nickel in plants: II. Distribution and chemical form in soybean plants. Plant Physiol. 1978, 62, 566–570. [Google Scholar] [CrossRef]
- Montargès-Pelletier, E.; Chardot, V.; Echevarria, G.; Michot, L.J.; Bauer, A.; Morel, J.-L. Identification of nickel chelators in three hyperaccumulating plants: An X-ray spectroscopic study. Phytochemistry 2008, 69, 1695–1709. [Google Scholar] [CrossRef]
- Agrawal, B.; Czymmek, K.J.; Sparks, D.L.; Bais, H.P. Transient influx of nickel in root mitochondria modulates organic acid and reactive oxygen species production in nickel hyperaccumulator Alyssum murale. J. Biol. Chem. 2013, 288, 7351–7362. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, W.W.; Bunkowski, M.; Puschenreiter, M.; Horak, O. Rhizosphere characteristics of indigenously growing nickel hyperaccumulator and excluder plants on serpentine soil. Environ. Pollut. 2003, 123, 131–138. [Google Scholar] [CrossRef]
- Pianelli, K.; Mari, S.; Marquès, L.; Lebrun, M.; Czernic, P. Nicotianamine over-accumulation confers resistance to nickel in Arabidopsis thaliana. Transgenic Res. 2005, 14, 739–748. [Google Scholar] [CrossRef]
- Kim, S.; Takahashi, M.; Higuchi, K.; Tsunoda, K.; Nakanishi, H.; Yoshimura, E.; Mori, S.; Nishizawa, N.K. Increased nicotianamine biosynthesis confers enhanced tolerance of high levels of metals, in particular nickel, to plants. Plant Cell Physiol. 2005, 46, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Vacchina, V.; Mari, S.; Czernic, P.; Marquès, L.; Pianelli, K.; Schaumlöffel, D.; Lebrun, M.; Łobiński, R. Speciation of nickel in a hyperaccumulating plant by high-performance liquid chromatography−inductively coupled plasma mass spectrometry and electrospray MS/MS assisted by cloning using yeast complementation. Anal. Chem. 2003, 75, 2740–2745. [Google Scholar] [CrossRef] [PubMed]
- Colpas, G.J.; Hausinger, R.P. In Vivo and in Vitro Kinetics of Metal Transfer by the Klebsiella aerogenes Urease Nickel Metallochaperone, UreE* 210. J. Biol. Chem. 2000, 275, 10731–10737. [Google Scholar] [CrossRef]
- Hausinger, R.P. Metallocenter assembly in nickel-containing enzymes. JBIC J. Biol. Inorg. Chem. 1997, 2, 279–286. [Google Scholar] [CrossRef]
- Küpper, H.; Lombi, E.; Zhao, F.J.; Wieshammer, G.; McGrath, S.P. Cellular compartmentation of nickel in the hyperaccumulators Alyssum lesbiacum, Alyssum bertolonii and Thlaspi goesingense. J. Exp. Bot. 2001, 52, 2291–2300. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Garland, T.R.; Wildung, R.E. Nickel in plants: I. Uptake kinetics using intact soybean seedlings. Plant Physiol. 1978, 62, 563–565. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Aamer, M.; Nawaz, M.; Ali, A.; Khan, M.A.U.; Khan, T.A. Nickel toxicity in plants: Reasons, toxic effects, tolerance mechanisms, and remediation possibilities—A review. Environ. Sci. Pollut. Res. 2019, 26, 12673–12688. [Google Scholar] [CrossRef]
- Brooks, R.R.; Lee, J.; Reeves, R.D.; Jaffre, T. Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. J. Geochem. Explor. 1977, 7, 49–57. [Google Scholar] [CrossRef]
- Boyd, R.S. Ecology of Metal Hyperaccumulation. New Phytol. 2004, 162, 563–567. [Google Scholar] [CrossRef]
- Boyd, R.S.; Davis, M.A.; Wall, M.A.; Balkwill, K. Nickel defends the South African hyperaccumulator Senecio coronatus (Asteraceae) against Helix aspersa (Mollusca: Pulmonidae). Chemoecology 2002, 12, 91–97. [Google Scholar] [CrossRef]
- van der Pas, L.; Ingle, R.A. Towards an Understanding of the Molecular Basis of Nickel Hyperaccumulation in Plants. Plants 2019, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Nishida, S.; Kato, A.; Tsuzuki, C.; Yoshida, J.; Mizuno, T. Induction of Nickel Accumulation in Response to Zinc Deficiency in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 9420–9430. [Google Scholar] [CrossRef] [PubMed]
- Nishida, S.; Tsuzuki, C.; Kato, A.; Aisu, A.; Yoshida, J.; Mizuno, T. AtIRT1, the Primary Iron Uptake Transporter in the Root, Mediates Excess Nickel Accumulation in Arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 1433–1442. [Google Scholar] [CrossRef]
- Ingle, R.A.; Mugford, S.T.; Rees, J.D.; Campbell, M.M.; Smith, J.A.C. Constitutively High Expression of the Histidine Biosynthetic Pathway Contributes to Nickel Tolerance in Hyperaccumulator Plants. Plant Cell 2005, 17, 2089–2106. [Google Scholar] [CrossRef] [PubMed]
- White, J.F.; Kingsley, K.L.; Zhang, Q.; Verma, R.; Obi, N.; Dvinskikh, S.; Elmore, M.T.; Verma, S.K.; Gond, S.K.; Kowalski, K.P. Endophytic microbes and their potential applications in crop management. Pest Manag. Sci. 2019, 75, 2558–2565. [Google Scholar] [CrossRef]
- Vivas, A.; Biró, B.; Németh, T.; Barea, J.M.; Azcón, R. Nickel-tolerant Brevibacillus brevis and arbuscular mycorrhizal fungus can reduce metal acquisition and nickel toxicity effects in plant growing in nickel supplemented soil. Soil Biol. Biochem. 2006, 38, 2694–2704. [Google Scholar] [CrossRef]
- Ważny, R.; Rozpądek, P.; Domka, A.; Jędrzejczyk, R.J.; Nosek, M.; Hubalewska-Mazgaj, M.; Lichtscheidl, I.; Kidd, P.; Turnau, K. The effect of endophytic fungi on growth and nickel accumulation in Noccaea hyperaccumulators. Sci. Total Environ. 2021, 768, 144666. [Google Scholar] [CrossRef]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Vangronsveld, J. Phytoremediation: Plant–endophyte partnerships take the challenge. Curr. Opin. Biotechnol. 2009, 20, 248–254. [Google Scholar] [CrossRef]
- Weyens, N.; Croes, S.; Dupae, J.; Newman, L.; van der Lelie, D.; Carleer, R.; Vangronsveld, J. Endophytic bacteria improve phytoremediation of Ni and TCE co-contamination. Environ. Pollut. 2010, 158, 2422–2427. [Google Scholar] [CrossRef]
- Chen, J.; Li, N.; Han, S.; Sun, Y.; Wang, L.; Qu, Z.; Dai, M.; Zhao, G. Characterization and bioremediation potential of nickel-resistant endophytic bacteria isolated from the wetland plant Tamarix chinensis. FEMS Microbiol. Lett. 2020, 367, fnaa098. [Google Scholar] [CrossRef]
- Lodewyckx, C.; Taghavi, S.; Mergeay, M.; Vangronsveld, J.; Clijsters, H.; Lelie, D.v.d. The effect of recombinant heavy metal-resistant endophytic bacteria on heavy metal uptake by their host plant. Int. J. Phytoremediat. 2001, 3, 173–187. [Google Scholar] [CrossRef]
- Madhaiyan, M.; Poonguzhali, S.; Sa, T. Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 2007, 69, 220–228. [Google Scholar] [CrossRef]
- Visioli, G.; Vamerali, T.; Mattarozzi, M.; Dramis, L.; Sanangelantoni, A.M. Combined endophytic inoculants enhance nickel phytoextraction from serpentine soil in the hyperaccumulator Noccaea caerulescens. Front. Plant Sci. 2015, 6, 638. [Google Scholar] [CrossRef] [PubMed]
- Weyens, N.; Truyens, S.; Saenen, E.; Boulet, J.; Dupae, J.; Taghavi, S.; van der Lelie, D.; Carleer, R.; Vangronsveld, J. Endophytes and Their Potential to Deal with Co-Contamination of Organic Contaminants (Toluene) and Toxic Metals (Nickel) During Phytoremediation. Int. J. Phytoremediat. 2011, 13, 244–255. [Google Scholar] [CrossRef] [PubMed]
- White, J.F.; Kingsley, K.L.; Verma, S.K.; Kowalski, K.P. Rhizophagy cycle: An oxidative process in plants for nutrient extraction from symbiotic microbes. Microorganisms 2018, 6, 95. [Google Scholar] [CrossRef]
- Goldstein, W.; White, J. Seed endophytes, rhizophagy, nutrient density, nitrogen efficiency and fixation in corn. In Proceedings of the 2022 Organic Seed Alliance Growers Conference, Virtual, 4–11 February 2022. [Google Scholar]
- White, J.F., Jr.; Kingsley, K.L.; Verma, S.K.; Kowalski, K. Rhizophagy cycle: A nutritional symbiosis involving bacteria that alternate between root—endophytic and free—living soil phases. In Proceedings of the 10th International Symposium on Fungal Endophytes of Grasses, Salamanca, Spain, 18–21 June 2018. [Google Scholar]
- Ameer, M.A.A.; Hussein, H.N. Induction of Rhizophagy by yeast Saccharomyces cerevisiae in roots of lettuce Lactuca sativa. In Proceedings of the Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2020; p. 012116. [Google Scholar]
- Wood, B.W.; Reilly, C.C.; Bock, C.H.; Hotchkiss, M.W. Suppression of pecan scab by nickel. HortScience 2012, 47, 503–508. [Google Scholar] [CrossRef]
- Keil, H.L.F.H.P. Rust Eradication. U.S. Patent US-2971880-A, 14 February 1961. [Google Scholar]
- Macomber, L.; Hausinger, R.P. Mechanisms of nickel toxicity in microorganisms. Metallomics 2011, 3, 1153–1162. [Google Scholar] [CrossRef]
- Gerwien, F.; Skrahina, V.; Kasper, L.; Hube, B.; Brunke, S. Metals in fungal virulence. FEMS Microbiol. Rev. 2018, 42, fux050. [Google Scholar] [CrossRef]
- Dalton, D.A. Essentiality of nickel for plants. In Nickel in Soils and Plants; CRC Press: London, UK, 2018; pp. 1–20. [Google Scholar]
- Carlini, C.R.; Ligabue-Braun, R. Ureases as multifunctional toxic proteins: A review. Toxicon 2016, 110, 90–109. [Google Scholar] [CrossRef]
- Becker-Ritt, A.B.; Martinelli, A.H.S.; Mitidieri, S.; Feder, V.; Wassermann, G.E.; Santi, L.; Vainstein, M.H.; Oliveira, J.T.A.; Fiuza, L.M.; Pasquali, G.; et al. Antifungal activity of plant and bacterial ureases. Toxicon 2007, 50, 971–983. [Google Scholar] [CrossRef]
- Mishra, D.; Kar, M. Nickel in plant growth and metabolism. Bot. Rev. 1974, 40, 395–452. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Brocato, J.; Laulicht, F.; Costa, M. Mechanisms of Nickel Carcinogenesis. In Essential and Non-Essential Metals: Carcinogenesis, Prevention and Cancer Therapeutics; Mudipalli, A., Zelikoff, J.T., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 181–197. [Google Scholar]
- Das, K.K.; Das, S.N.; Dhundasi, S.A. Nickel, its adverse health effects & oxidative stress. Indian J. Med. Res. 2008, 128, 412–425. [Google Scholar] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- Kazantzis, K.; Thomidis, T. Effect of cultivar resistance and pre-harvest nickel applications on fruit rots, shot-hole, Alternaria leaf spot and fruit cracking. Eur. J. Hortic. Sci. 2023, 88, 12. [Google Scholar] [CrossRef]
- Reilly, C.; Crawford, M.; Buck, J. Nickel suppresses daylily rust, Puccinia hemerocallidis on susceptible daylilies, Hemerocallis ssp. in greenhouse and field trials. Phytopathology 2005, 95, 588. [Google Scholar]
- Ahmed, A.I.; Yadav, D.R.; Lee, Y.S. Applications of nickel nanoparticles for control of Fusarium wilt on lettuce and tomato. Int. J. Innov. Res. Sci. Eng. Technol 2016, 5, 7378–7385. [Google Scholar]
- Einhardt, A.M.; Ferreira, S.; Hawerroth, C.; Valadares, S.V.; Rodrigues, F.Á. Nickel potentiates soybean resistance against infection by Phakopsora pachyrhizi. Plant Pathol. 2020, 69, 849–859. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.-N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef]
- La Torre, A.; Iovino, V.; Caradonia, F. Copper in plant protection: Current situation and prospects. Phytopathol. Mediterr. 2018, 57, 201–236. [Google Scholar]
- Solanki, P.; Bhargava, A.; Chhipa, H.; Jain, N.; Panwar, J. Nano-fertilizers and their smart delivery system. In Nanotechnologies in Food and Agriculture; Mahendra, R., Caue, R., Luiz, M., Nelson, D., Eds.; Springer International Publishing Switzerland: Cham, Switzerland, 2015; pp. 81–101. [Google Scholar]
- Rabinovich, A.; Rouff, A.A. Effect of phenolic organics on the precipitation of struvite from simulated dairy wastewater. ACS EST Water 2021, 1, 910–918. [Google Scholar] [CrossRef]
- Rouff, A.A.; Juarez, K.M. Zinc interaction with struvite during and after mineral formation. Environ. Sci. Technol. 2014, 48, 6342–6349. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, A.; Rouff, A.A. Changes to struvite growth and morphology as impacted by low molecular weight organics. ACS EST Water 2023, 3, 2277–2285. [Google Scholar] [CrossRef]
- Taddeo, R.; Honkanen, M.; Kolppo, K.; Lepistö, R. Nutrient management via struvite precipitation and recovery from various agroindustrial wastewaters: Process feasibility and struvite quality. J. Environ. Manag. 2018, 212, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Goswami, O.; Rouff, A.A. Interaction of divalent metals with struvite: Sorption, reversibility, and implications for mineral recovery from wastes. Environ. Technol. 2023, 44, 2315–2326. [Google Scholar] [CrossRef]
- Lu, X.; Xu, W.; Zeng, Q.; Liu, W.; Wang, F. Quantitative, morphological, and structural analysis of Ni incorporated with struvite during precipitation. Sci. Total Environ. 2022, 817, 152976. [Google Scholar] [CrossRef]
- Rabinovich, A.; Rouff, A.A.; Lew, B.; Ramlogan, M.V. Aerated fluidized bed treatment for phosphate recovery from dairy and swine wastewater. ACS Sustain. Chem. Eng. 2018, 6, 652–659. [Google Scholar] [CrossRef]
- Nepfumbada, C.; Tavengwa, N.T.; Masindi, V.; Foteinis, S.; Chatzisymeon, E. Recovery of phosphate from municipal wastewater as calcium phosphate and its subsequent application for the treatment of acid mine drainage. Resour. Conserv. Recycl. 2023, 190, 106779. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Lombi, E.; Wang, P.; Schjoerring, J.K.; Husted, S. Nanomaterials as fertilizers for improving plant mineral nutrition and environmental outcomes. Environ. Sci. Nano 2019, 6, 3513–3524. [Google Scholar] [CrossRef]
- Maity, D.; Gupta, U.; Saha, S. Biosynthesized metal oxide nanoparticles for sustainable agriculture: Next-generation nanotechnology for crop production, protection and management. Nanoscale 2022, 14, 13950–13989. [Google Scholar] [CrossRef] [PubMed]
- Shinde, N.A.; Kawar, P.G.; Dalvi, S.G. Chitosan-based nanoconjugates: A promising solution for enhancing crops drought-stress resilience and sustainable yield in the face of climate change. Plant Nano Biol. 2024, 7, 100059. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Dal Sasso, G.; Carmona, F.J.; Miguel-Rojas, C.; Pérez-de-Luque, A.; Masciocchi, N.; Guagliardi, A.; Delgado-López, J.M. Engineering biomimetic calcium phosphate nanoparticles: A green synthesis of slow-release multinutrient (NPK) nanofertilizers. ACS Appl. Bio Mater. 2020, 3, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Singh, U.; Adisa, I.O.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Effects of manganese nanoparticle exposure on nutrient acquisition in wheat (Triticum aestivum L.). Agronomy 2018, 8, 158. [Google Scholar] [CrossRef]
- Song, U.; Kim, J. Zinc oxide nanoparticles: A potential micronutrient fertilizer for horticultural crops with little toxicity. Hortic. Environ. Biotechnol. 2020, 61, 625–631. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, A.; Sharma, M.; Bhalla, N.; Estrela, P.; Jain, A.; Thakur, P.; Thakur, A. Nanomaterial fungicides: In vitro and in vivo antimycotic activity of cobalt and nickel nanoferrites on phytopathogenic fungi. Glob. Chall. 2017, 1, 1700041. [Google Scholar] [CrossRef] [PubMed]
- Phogat, N.; Ali Khan, S.; Shankar, S.; A Ansary, A.; Uddin, I. Fate of inorganic nanoparticles in agriculture. Adv. Mater. Lett. 2016, 7, 3–12. [Google Scholar] [CrossRef]
- Banerjee, A.; Sarkar, A.; Acharya, K.; Chakraborty, N. Nanotechnology: An emerging hope in crop improvement. Lett. Appl. NanoBioScience 2021, 10, 2784–2803. [Google Scholar]
- Wang, P.; Lombi, E.; Zhao, F.-J.; Kopittke, P.M. Nanotechnology: A New Opportunity in Plant Sciences. Trends in Plant Science 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Jośko, I.; Oleszczuk, P.; Futa, B. The effect of inorganic nanoparticles (ZnO, Cr2O3, CuO and Ni) and their bulk counterparts on enzyme activities in different soils. Geoderma 2014, 232, 528–537. [Google Scholar] [CrossRef]
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef]
- Zhou, P.; Jiang, Y.; Adeel, M.; Shakoor, N.; Zhao, W.; Liu, Y.; Li, Y.; Li, M.; Azeem, I.; Rui, Y.; et al. Nickel Oxide Nanoparticles Improve Soybean Yield and Enhance Nitrogen Assimilation. Environ. Sci. Technol. 2023, 57, 7547–7558. [Google Scholar] [CrossRef]
- Chouhan, D.; Dutta, A.; Kumar, A.; Mandal, P.; Choudhuri, C. Application of nickel chitosan nanoconjugate as an antifungal agent for combating Fusarium rot of wheat. Sci. Rep. 2022, 12, 14518. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, R.; Jayabaskaran, C.; Manikandan, A.; Anusuya, S. Synthesis of Nickel-Chitosan Nanoparticles for Controlling Blast Diseases in Asian Rice. Appl. Biochem. Biotechnol. 2023, 195, 2134–2148. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, L.; Mettenbrink, E.M.; DeAngelis, P.L.; Wilhelm, S. Nanoparticle Toxicology. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 269–289. [Google Scholar] [CrossRef] [PubMed]
- Klaine, S.J.; Koelmans, A.A.; Horne, N.; Carley, S.; Handy, R.D.; Kapustka, L.; Nowack, B.; von der Kammer, F. Paradigms to assess the environmental impact of manufactured nanomaterials. Environ. Toxicol. Chem. 2012, 31, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Auffan, M.; Santaella, C.; Thiéry, A.; Paillès, C.; Rose, J.; Achouak, W.; Thill, A.; Masion, A.; Wiesner, M.; Bottero, J.-Y. Ecotoxicity of Inorganic Nanoparticles: From Unicellular Organisms to Invertebrates. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 623–636. [Google Scholar]
- Iftikhar, M.; Noureen, A.; Jabeen, F.; Uzair, M.; Rehman, N.; Sher, E.K.; Katubi, K.M.; Américo-Pinheiro, J.H.P.; Sher, F. Bioinspired engineered nickel nanoparticles with multifunctional attributes for reproductive toxicity. Chemosphere 2023, 311, 136927. [Google Scholar] [CrossRef]
- Shah, G.A.; Ahmed, J.; Iqbal, Z.; Hassan, F.-u.; Rashid, M.I. Toxicity of NiO nanoparticles to soil nutrient availability and herbage N uptake from poultry manure. Sci. Rep. 2021, 11, 11540. [Google Scholar] [CrossRef]
- Hasegawa, T.; Sakurai, G.; Fujimori, S.; Takahashi, K.; Hijioka, Y.; Masui, T. Extreme climate events increase risk of global food insecurity and adaptation needs. Nat. Food 2021, 2, 587–595. [Google Scholar] [CrossRef]
- McNear, D.H., Jr.; Chaney, R.L.; Sparks, D.L. The hyperaccumulator Alyssum murale uses complexation with nitrogen and oxygen donor ligands for Ni transport and storage. Phytochemistry 2010, 71, 188–200. [Google Scholar] [CrossRef]
- Morris, J.W.; Scheckel, K.G.; McNear, D.H. Biogeochemistry of nickel in soils, plants, and the rhizosphere. In Nickel in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2018; pp. 51–86. [Google Scholar]
- de Oliveira, J.B.; Lavres, J.; Kopittke, P.M.; Chaney, R.L.; Harris, H.H.; Erskine, P.D.; Howard, D.L.; dos Reis, A.R.; van der Ent, A. Unravelling the fate of foliar-applied nickel in soybean: A comprehensive investigation. Plant Soil 2024, 1–20. [Google Scholar] [CrossRef]
- Caggiano, R.; Sabia, S.; Speranza, A. Trace elements and human health risks assessment of finer aerosol atmospheric particles (PM 1). Environ. Sci. Pollut. Res. 2019, 26, 36423–36433. [Google Scholar] [CrossRef]
- Prueitt, R.L.; Li, W.; Chang, Y.-C.; Boffetta, P.; Goodman, J.E. Systematic review of the potential respiratory carcinogenicity of metallic nickel in humans. Crit. Rev. Toxicol. 2020, 50, 605–639. [Google Scholar] [CrossRef] [PubMed]
- Bar-Tal, A.; Fine, P.; Yermiyahu, U.; Ben-Gal, A.; Hass, A. Practices that simultaneously optimize water and nutrient use efficiency: Israeli experiences in fertigation and irrigation with treated wastewater. In Managing Water and Fertilizer for Sustainable Agricultural Intensification; IFA, IWMI, IPNI and IPI: Paris, France, 2015; pp. 209–241. [Google Scholar]
- Pinto, I.S.; Neto, I.F.; Soares, H.M. Biodegradable chelating agents for industrial, domestic, and agricultural applications—A review. Environ. Sci. Pollut. Res. 2014, 21, 11893–11906. [Google Scholar] [CrossRef] [PubMed]
- Brusko, V.; Garifullin, B.; Geniyatullina, G.; Kuryntseva, P.; Galieva, G.; Galitskaya, P.; Selivanovskaya, S.; Dimiev, A.M. Novel Biodegradable Chelating Agents for Micronutrient Fertilization. J. Agric. Food Chem. 2023, 71, 14979–14988. [Google Scholar] [CrossRef]
- Apodaca, L. Nitrogen Statistics and Information|U.S. Geological Survey. Available online: https://www.usgs.gov/centers/national-minerals-information-center/nitrogen-statistics-and-information (accessed on 31 July 2024).
- Hu, Y.; Schmidhalter, U. Annual consumption and types of synthetic nitrogen fertilizers: Ammonia emission indicators for mitigation strategies in the European Union. Environ. Sustain. Indic. 2024, 22, 100365. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Zhao, Y.; Zhang, L.; Zhang, J.; Liu, M.; Zhou, M.; Luo, B. High-resolution ammonia emissions from nitrogen fertilizer application in China during 2005–2020. Atmosphere 2022, 13, 1297. [Google Scholar] [CrossRef]
- Motasim, A.M.; Samsuri, A.W.; Nabayi, A.; Akter, A.; Haque, M.A.; Abdul Sukor, A.S.; Adibah, A.M. Urea application in soil: Processes, losses, and alternatives—A review. Discov. Agric. 2024, 2, 42. [Google Scholar] [CrossRef]
- Good, A.G.; Beatty, P.H. Fertilizing nature: A tragedy of excess in the commons. PLoS Biol. 2011, 9, e1001124. [Google Scholar] [CrossRef]
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving nitrogen use efficiency in crops for sustainable agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Reiter, M.; Samtani, J.; Torres Quezada, E.; Singh, V.; Doughty, H.; Kuhar, T.P.; Sutton, K.; Wilson, J.; Langston, D.; Rideout, S. 2022–2023 Mid-Atlantic Commercial Vegetable Production Recommendations; Virginia Cooperative Extension: Fairfax, VA, USA, 2022. [Google Scholar]
- Topcu, Y.; Nambeesan, S.U.; van der Knaap, E. Blossom-end rot: A century-old problem in tomato (Solanum lycopersicum L.) and other vegetables. Mol. Hortic. 2022, 2, 1. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).