Community Composition of Alpine Dung Beetles Is Mostly Driven by Temperature and Habitat Type
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dung Beetle Collection

2.2. Data Analysis
3. Results
3.1. Temperature Trends in the Investigated Habitats
3.2. Species Shared among Habitats for the Overall Dataset and within Each Site
3.3. Species Habitat Preferences
3.4. Patterns of Diversity Variation among Habitats and Sites
3.5. Effects of Habitat and Time on Community Composition
4. Discussion
4.1. Diversity Patterns within and between Habitats
4.2. Habitat Preferences and the Effect of Temperature
4.3. Caveats
4.4. Management Implications
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tittensor, D.P.; Walpole, M.; Hill, S.L.L.; Boyce, D.G.; Britten, G.L.; Burgess, N.D.; Butchart, S.H.M.; Leadley, P.W.; Regan, E.C.; Alkemade, R.; et al. A Mid-Term Analysis of Progress toward International Biodiversity Targets. Science 2014, 346, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Forister, M.L.; Pelton, E.M.; Black, S.H. Declines in Insect Abundance and Diversity: We Know Enough to Act Now. Conserv. Sci. Pract. 2019, 1, e80. [Google Scholar] [CrossRef]
- Habel, J.C.; Samways, M.J.; Schmitt, T. Mitigating the Precipitous Decline of Terrestrial European Insects: Requirements for a New Strategy. Biodivers. Conserv. 2019, 28, 1343–1360. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Ssymank, A.; Sorg, M.; de Kroon, H.; Jongejans, E. Insect Biomass Decline Scaled to Species Diversity: General Patterns Derived from a Hoverfly Community. Proc. Natl. Acad. Sci. USA 2021, 118, e2002554117. [Google Scholar] [CrossRef] [PubMed]
- Rusch, A.; Bommarco, R.; Chiverton, P.; Öberg, S.; Wallin, H.; Wiktelius, S.; Ekbom, B. Response of Ground Beetle (Coleoptera, Carabidae) Communities to Changes in Agricultural Policies in Sweden over Two Decades. Agric. Ecosyst. Environ. 2013, 176, 63–69. [Google Scholar] [CrossRef]
- Jovičić, S.; Burgio, G.; Diti, I.; Krašić, D.; Markov, Z.; Radenković, S.; Vujić, A. Influence of Landscape Structure and Land Use on Merodon and Cheilosia (Diptera: Syrphidae): Contrasting Responses of Two Genera. J. Insect Conserv. 2017, 21, 53–64. [Google Scholar] [CrossRef]
- Fox, R. The Decline of Moths in Great Britain: A Review of Possible Causes. Insect Conserv. Divers. 2013, 6, 5–19. [Google Scholar] [CrossRef]
- Gossner, M.M.; Lewinsohn, T.M.; Kahl, T.; Grassein, F.; Boch, S.; Prati, D.; Birkhofer, K.; Renner, S.C.; Sikorski, J.; Wubet, T.; et al. Land-Use Intensification Causes Multitrophic Homogenization of Grassland Communities. Nature 2016, 540, 266–269. [Google Scholar] [CrossRef]
- Holter, P. Herbivore Dung as Food for Dung Beetles: Elementary Coprology for Entomologists. Ecol. Entomol. 2016, 41, 367–377. [Google Scholar] [CrossRef]
- Buse, J.; Hoenselaar, G.; Langenbach, F.; Langenbach, F.; Schleicher, P.; Twietmeyer, S.; Popa, F.; Heurich, M. Dung beetle richness is positively affected by the density of wild ungulate populations in forests. Biodivers. Conserv. 2021, 30, 3115–3131. [Google Scholar] [CrossRef]
- Halffter, G.; Edmonds, W. The Nesting Behavior of Dung Beetles (Scarabaeinae); Publication 10; Instituto de Ecologia: Mexico City, Mexico, 1982; 176p. [Google Scholar]
- Kaleri, A.R.; Ma, J.; Abro, S.A.; Faqir, Y.; Nabi, F.; Hakeem, A.; Ahmed, A.; Ahmed, S.; Jakhar, A.M.; Shah, S.M.; et al. Dung Beetle Improves Soil Bacterial Diversity and Enzyme Activity and Enhances Growth and Antioxidant Content of Chinese Cabbage (Brassica rapa ssp. pekinensis). J. Soil Sci. Plant Nutr. 2021, 21, 3387–3401. [Google Scholar] [CrossRef]
- Stanbrook, R.; Harris, E.; Jones, M.; Wheater, C.P. The Effect of Dung Beetle Size on Soil Nutrient Mobilization in an Afrotropical Forest. Insects 2021, 12, 141. [Google Scholar] [CrossRef]
- Yamada, D.; Imura, O.; Shi, K.; Shibuya, T. Effect of Tunneler Dung Beetles on Cattle Dung Decomposition, Soil Nutrients and Herbage Growth. Grassl. Sci. 2007, 53, 121–129. [Google Scholar] [CrossRef]
- Milotić, T.; Baltzinger, C.; Eichberg, C.; Eycott, A.E.; Heurich, M.; Müller, J.; Noriega, J.A.; Menendez, R.; Stadler, J.; Ádám, R.; et al. Functionally Richer Communities Improve Ecosystem Functioning: Dung Removal and Secondary Seed Dispersal by Dung Beetles in the Western Palaearctic. J. Biogeogr. 2019, 46, 70–82. [Google Scholar] [CrossRef]
- Andresen, E. Dung Beetles in a Central Amazonian Rainforest and Their Ecological Role as Secondary Seed Dispersers. Ecol. Entomol. 2002, 27, 257–270. [Google Scholar] [CrossRef]
- Nervo, B.; Caprio, E.; Celi, L.; Lonati, M.; Lombardi, G.; Falsone, G.; Iussig, G.; Palestrini, C.; Said-Pullicino, D.; Rolando, A. Ecological Functions Provided by Dung Beetles Are Interlinked across Space and Time: Evidence from 15N Isotope Tracing. Ecology 2017, 98, 433–446. [Google Scholar] [CrossRef]
- Nichols, E.; Gómez, A. Dung Beetles and Fecal Helminth Transmission: Patterns, Mechanisms and Questions. Parasitology 2014, 141, 614–623. [Google Scholar] [CrossRef]
- Andresen, E.; Laurance, S.G.W. Possible Indirect Effects of Mammal Hunting on Dung Beetle Assemblages in Panama. Biotropica 2007, 39, 141–146. [Google Scholar] [CrossRef]
- Barbero, E.; Palestrini, C.; Rolando, A. Dung Beetle Conservation: Effects of Habitat and Resource Selection (Coleoptera: Scarabaeoidea). J. Insect Conserv. 1999, 3, 75–84. [Google Scholar] [CrossRef]
- Gebert, F.; Steffan-Dewenter, I.; Moretto, P.; Peters, M.K. Climate Rather than Dung Resources Predict Dung Beetle Abundance and Diversity along Elevational and Land Use Gradients on Mt. Kilimanjaro. J. Biogeogr. 2020, 47, 371–381. [Google Scholar] [CrossRef]
- Hanski, I.; Cambefort, Y. Dung Beetle Ecology; Princeton University Press: Princeton, NJ, USA, 1991; ISBN 978-0-691-08739-9. [Google Scholar]
- Nichols, E.; Uriarte, M.; Bunker, D.E.; Favila, M.E.; Slade, E.M.; Vulinec, K.; Larsen, T.; Vaz-de-Mello, F.Z.; Louzada, J.; Naeem, S.; et al. Trait-Dependent Response of Dung Beetle Populations to Tropical Forest Conversion at Local and Regional Scales. Ecology 2013, 94, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Korasaki, V.; Braga, R.F.; Zanetti, R.; Moreira, F.M.S.; Vaz-de-Mello, F.Z.; Louzada, J. Conservation Value of Alternative Land-Use Systems for Dung Beetles in Amazon: Valuing Traditional Farming Practices. Biodivers. Conserv. 2013, 22, 1485–1499. [Google Scholar] [CrossRef]
- Costa, F.C.; Pessoa, K.K.T.; Liberal, C.N.; Filgueiras, B.K.C.; Salomão, R.P.; Iannuzzi, L. What Is the Importance of Open Habitat in a Predominantly Closed Forest Area to the Dung Beetle (Coleoptera, Scarabaeinae) Assemblage? Rev. Bras. Entomol. 2013, 57, 329–334. [Google Scholar] [CrossRef]
- Giménez Gómez, V.C.; Verdú, J.R.; Gómez-Cifuentes, A.; Vaz-de-Mello, F.Z.; Zurita, G.A. Influence of Land Use on the Trophic Niche Overlap of Dung Beetles in the Semideciduous Atlantic Forest of Argentina. Insect Conserv. Divers. 2018, 11, 554–564. [Google Scholar] [CrossRef]
- Nichols, E.; Spector, S.; Louzada, J.; Larsen, T.; Amezquita, S.; Favila, M.E. Ecological Functions and Ecosystem Services Provided by Scarabaeinae Dung Beetles. Biol. Conserv. 2008, 141, 1461–1474. [Google Scholar] [CrossRef]
- Silva, P.G.d.; Hernández, M.I.M. Spatial Variation of Dung Beetle Assemblages Associated with Forest Structure in Remnants of Southern Brazilian Atlantic Forest. Rev. Bras. Entomol. 2016, 60, 73–81. [Google Scholar] [CrossRef]
- Chamberlain, D.; Tocco, C.; Longoni, A.; Mammola, S.; Palestrini, C.; Rolando, A. Nesting Strategies Affect Altitudinal Distribution and Habitat Use in Alpine Dung Beetle Communities. Ecol. Entomol. 2015, 40, 372–380. [Google Scholar] [CrossRef]
- Martello, F.; Andriolli, F.; de Souza, T.B.; Dodonov, P.; Ribeiro, M.C. Edge and Land Use Effects on Dung Beetles (Coleoptera: Scarabaeidae: Scarabaeinae) in Brazilian Cerrado Vegetation. J. Insect Conserv. 2016, 20, 957–970. [Google Scholar] [CrossRef]
- Gómez-Cifuentes, A.; Munevar, A.; Gimenez, V.C.; Gatti, M.G.; Zurita, G.A. Influence of Land Use on the Taxonomic and Functional Diversity of Dung Beetles (Coleoptera: Scarabaeinae) in the Southern Atlantic Forest of Argentina. J. Insect Conserv. 2017, 21, 147–156. [Google Scholar] [CrossRef]
- Laurance, W.F.; Lovejoy, T.E.; Vasconcelos, H.L.; Bruna, E.M.; Didham, R.K.; Stouffer, P.C.; Gascon, C.; Bierregaard, R.O.; Laurance, S.G.; Sampaio, E. Ecosystem Decay of Amazonian Forest Fragments: A 22-Year Investigation. Conserv. Biol. 2002, 16, 605–618. [Google Scholar] [CrossRef]
- Shmida, A.; Wilson, M.V. Biological Determinants of Species Diversity. J. Biogeogr. 1985, 12, 1–20. [Google Scholar] [CrossRef]
- Martínez-Falcón, A.P.; Zurita, G.A.; Ortega-Martínez, I.J.; Moreno, C.E. Populations and Assemblages Living on the Edge: Dung Beetles Responses to Forests-Pasture Ecotones. PeerJ 2018, 6, e6148. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.J.; Pelissari, T.D.; Krinski, D.; Canale, G.; Vaz-de-Mello, F.Z. Abrupt Species Loss of the Amazonian Dung Beetle in Pastures Adjacent to Species-Rich Forests. J. Insect Conserv. 2017, 21, 487–494. [Google Scholar] [CrossRef]
- Marsh, C.J.; Feitosa, R.M.; Louzada, J.; Ewers, R.M. Is β-Diversity of Amazonian Ant and Dung Beetles Communities Elevated at Rainforest Edges? J. Biogeogr. 2018, 45, 1966–1979. [Google Scholar] [CrossRef]
- Tocco, C.; Negro, M.; Rolando, A.; Palestrini, C. Does Natural Reforestation Represent a Potential Threat to Dung Beetle Diversity in the Alps? J. Insect Conserv. 2013, 17, 207–217. [Google Scholar] [CrossRef]
- Tocco, C.; Probo, M.; Lonati, M.; Lombardi, G.; Negro, M.; Nervo, B.; Rolando, A.; Palestrini, C. Pastoral Practices to Reverse Shrub Encroachment of Sub-Alpine Grasslands: Dung Beetles (Coleoptera, Scarabaeoidea) Respond More Quickly Than Vegetation. PLoS ONE 2013, 8, e83344. [Google Scholar] [CrossRef] [PubMed]
- Palestrini, C.; Roggero, A.; Gorret, R.; Tocco, C.; Negro, M.; Barbero, E. Scarabaeoidea coprofagi della Val Veni e della Val Ferret (Valle d’Aosta, Italia). Rev. Valdôtaine Hist. Nat. 2007, 61–62, 241–253. [Google Scholar]
- Dellacasa, G.; Dellacasa, M. Coleoptera. Aphodiidae-Aphodiinae; Edagricole Calderini: Bologna, Italy, 2010; ISBN 978-88-506-5203-7. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2024. Available online: http://qgis.osgeo.org (accessed on 21 July 2024).
- Wood, S.N. Fast Stable Restricted Maximum Likelihood and Marginal Likelihood Estimation of Semiparametric Generalized Linear Models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2011, 73, 3–36. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Simpson, G.L.; Singmann, H. Gratia: Graceful ’ggplot’-Based Graphics and Other Functions for GAMs Fitted Using “mgcv”. R Package Version 0.9.2. 2024. Available online: https://CRAN.R-project.org/package=gratia (accessed on 21 July 2024).
- Gao, C.; Dusa, A. ggVennDiagram: A ‘ggplot2’ Implement of Venn Diagram. R Package Version 1.5.2. 2024. Available online: https://CRAN.R-project.org/package=ggVennDiagram (accessed on 21 July 2024).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Bates, D.; Machler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Doube, B.M. A Functional Classification for Analysis of the Structure of Dung Beetle Assemblages. Ecol. Entomol. 1990, 15, 371–383. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A Distance-Based Framework for Measuring Functional Diversity from Multiple Traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Lenth, R.; Buerkner, P.; Herve, M.; Love, J.; Riebl, H.; Singmann, H. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.10.3. 2024. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 21 July 2024).
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. vegan: Community Ecology Package. R Package Version 2.6–6.1. 2024. Available online: https://CRAN.R-project.org/package=vegan (accessed on 21 July 2024).
- Blonder, B.; Morrow, C.B.; Brown, S.; Butruille, G.; Chen, D.; Laini, A.; Harris, D.J. Hypervolume: High Dimensional Geometry, Set Operations, Projection, and Inference Using Kernel Density Estimation, Support Vector Machines, and Convex Hulls. R package Version 3.1.4. 2024. Available online: https://CRAN.R-project.org/package=hypervolume (accessed on 21 July 2024).
- Cabrero-Sañudo, F.J.; Lobo, J.M. Biogeography of Aphodiinae Dung Beetles Based on the Regional Composition and Distribution Patterns of Genera. J. Biogeogr. 2009, 36, 1474–1492. [Google Scholar] [CrossRef]
- Negro, M.; Palestrini, C.; Giraudo, M.T.; Rolando, A. The Effect of Local Environmental Heterogeneity on Species Diversity of Alpine Dung Beetles (Coleoptera: Scarabaeidae). Eur. J. Entomol. 2013, 108, 91–98. [Google Scholar] [CrossRef]
- Noriega, J.A.; Realpe, E. Altitudinal Turnover of Species in a Neotropical Peripheral Mountain System: A Case Study with Dung Beetles (Coleoptera: Aphodiinae and Scarabaeinae). Environ. Entomol. 2018, 47, 1376–1387. [Google Scholar] [CrossRef]
- Negro, M.; Rolando, A.; Palestrini, C. The Impact of Overgrazing on Dung Beetle Diversity in the Italian Maritime Alps. Environ. Entomol. 2011, 40, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Perrin, W.; Moretti, M.; Vergnes, A.; Borcard, D.; Jay-Robert, P. Response of Dung Beetle Assemblages to Grazing Intensity in Two Distinct Bioclimatic Contexts. Agric. Ecosyst. Environ. 2020, 289, 106740. [Google Scholar] [CrossRef]
- Verdú, J.R.; Lobo, J.M.; Sánchez-Piñero, F.; Gallego, B.; Numa, C.; Lumaret, J.-P.; Cortez, V.; Ortiz, A.J.; Tonelli, M.; García-Teba, J.P.; et al. Ivermectin Residues Disrupt Dung Beetle Diversity, Soil Properties and Ecosystem Functioning: An Interdisciplinary Field Study. Sci. Total Environ. 2018, 618, 219–228. [Google Scholar] [CrossRef]
- Buse, J.; Šlachta, M.; Sladecek, F.X.J.; Pung, M.; Wagner, T.; Entling, M.H. Relative Importance of Pasture Size and Grazing Continuity for the Long-Term Conservation of European Dung Beetles. Biol. Conserv. 2015, 187, 112–119. [Google Scholar] [CrossRef]
- Durães, R.; Martins, W.P.; Vaz-de-Mellos, F.Z. Dung Beetle (Coleoptera: Scarabaeidae) Assemblages across a Natural Forest-Cerrado Ecotone in Minas Gerais, Brazil. Neotrop. Entomol. 2005, 34, 721–731. [Google Scholar] [CrossRef]
- Villada-Bedoya, S.; Cultid-Medina, C.A.; Escobar, F.; Guevara, R.; Zurita, G. Edge Effects on Dung Beetle Assemblages in an Andean Mosaic of Forest and Coffee Plantations. Biotropica 2017, 49, 195–205. [Google Scholar] [CrossRef]
- Nervo, B.; Roggero, A.; Isaia, M.; Chamberlain, D.; Rolando, A.; Palestrini, C. Integrating Thermal Tolerance, Water Balance and Morphology: An Experimental Study on Dung Beetles. J. Therm. Biol. 2021, 101, 103093. [Google Scholar] [CrossRef]
- deCastro-Arrazola, I.; Andrew, N.R.; Berg, M.P.; Curtsdotter, A.; Lumaret, J.-P.; Menéndez, R.; Moretti, M.; Nervo, B.; Nichols, E.S.; Sánchez-Piñero, F.; et al. A Trait-Based Framework for Dung Beetle Functional Ecology. J. Anim. Ecol. 2023, 92, 44–65. [Google Scholar] [CrossRef]
- Nervo, B.; Roggero, A.; Chamberlain, D.; Caprio, E.; Rolando, A.; Palestrini, C. Physiological, Morphological and Ecological Traits Drive Desiccation Resistance in North Temperate Dung Beetles. BMC Zool. 2021, 6, 26. [Google Scholar] [CrossRef]
- Nervo, B.; Laini, A.; Roggero, A.; Palestrini, C.; Rolando, A. Spatio-Temporal Modelling Suggests That Some Dung Beetle Species (Coleoptera: Geotrupidae) May Respond to Global Warming by Boosting Dung Removal. Sci. Total Environ. 2024, 908, 168127. [Google Scholar] [CrossRef]
- Menéndez, R.; Gutiérrez, D. Shifts in Habitat Associations of Dung Beetles in Northern Spain: Climate Change Implications. Écoscience 2004, 11, 329–337. [Google Scholar] [CrossRef]
- Menéndez, R.; González-Megías, A.; Jay-Robert, P.; Marquéz-Ferrando, R. Climate Change and Elevational Range Shifts: Evidence from Dung Beetles in Two European Mountain Ranges. Glob. Ecol. Biogeogr. 2014, 23, 646–657. [Google Scholar] [CrossRef]
- Numa, C.; Verdú, J.R.; Sánchez, A.; Galante, E. Effect of Landscape Structure on the Spatial Distribution of Mediterranean Dung Beetle Diversity. Divers. Distrib. 2009, 15, 489–501. [Google Scholar] [CrossRef]
- Romero-Alcaraz, E.; Ávila, J.M. Landscape Heterogeneity in Relation to Variations in Epigaeic Beetle Diversity of a Mediterranean Ecosystem. Implications for Conservation. Biodivers. Conserv. 2000, 9, 985–1005. [Google Scholar] [CrossRef]
- Boan, J.J.; McLaren, B.E.; Malcolm, J.R. Influence of Post-Harvest Silviculture on Understory Vegetation: Implications for Forage in a Multi-Ungulate System. For. Ecol. Manag. 2011, 262, 1704–1712. [Google Scholar] [CrossRef]
- Englmeier, J.; von Hoermann, C.; Rieker, D.; Benbow, M.E.; Benjamin, C.; Fricke, U.; Ganuza, C.; Haensel, M.; Lackner, T.; Mitesser, O.; et al. Dung-Visiting Beetle Diversity Is Mainly Affected by Land Use, While Community Specialization Is Driven by Climate. Ecol. Evol. 2022, 12, e9386. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Verdú, J.R.; Zunino, M. Effects of the Progressive Abandonment of Grazing on Dung Beetle Biodiversity: Body Size Matters. Biodivers. Conserv. 2018, 27, 189–204. [Google Scholar] [CrossRef]
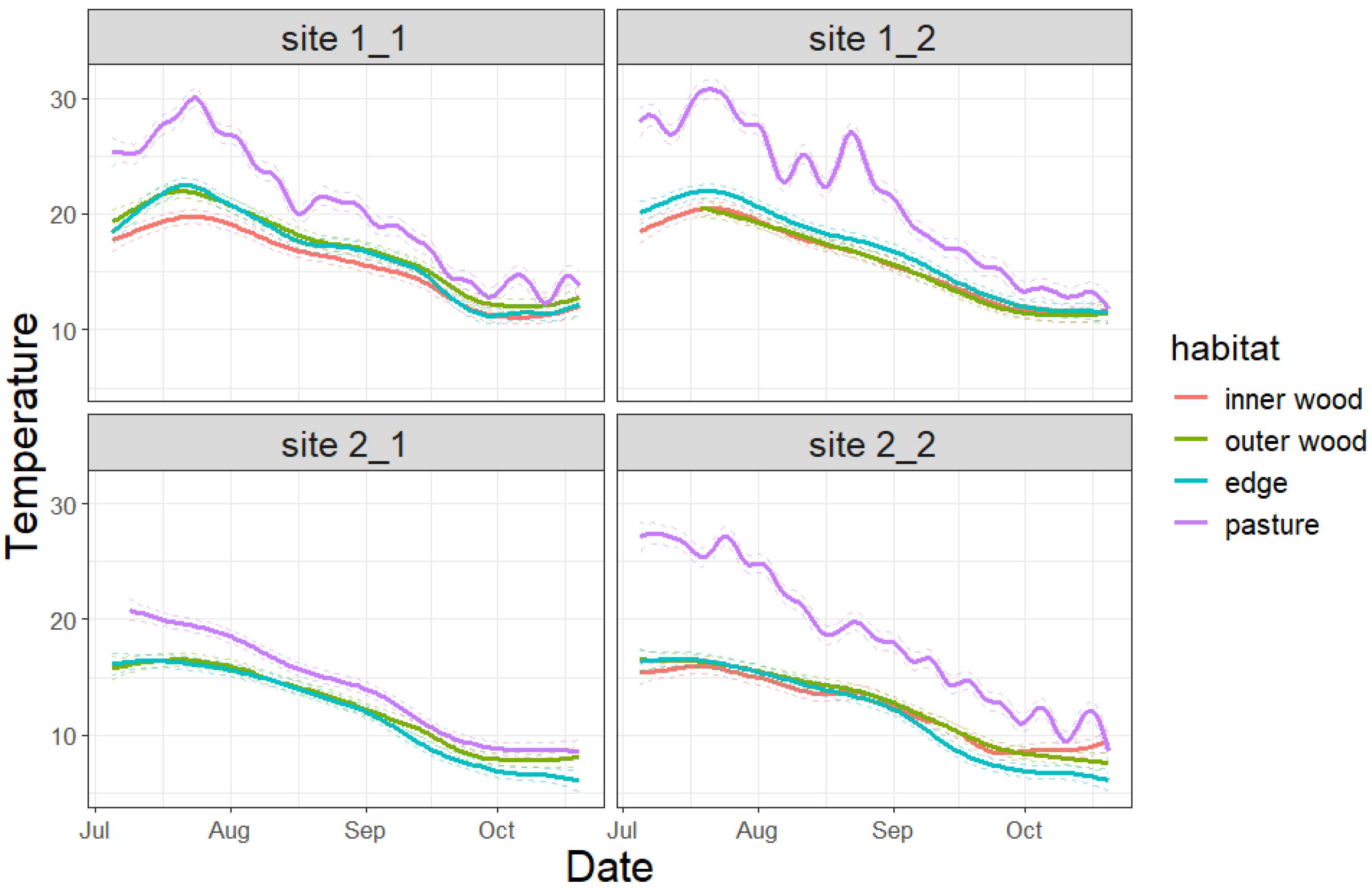

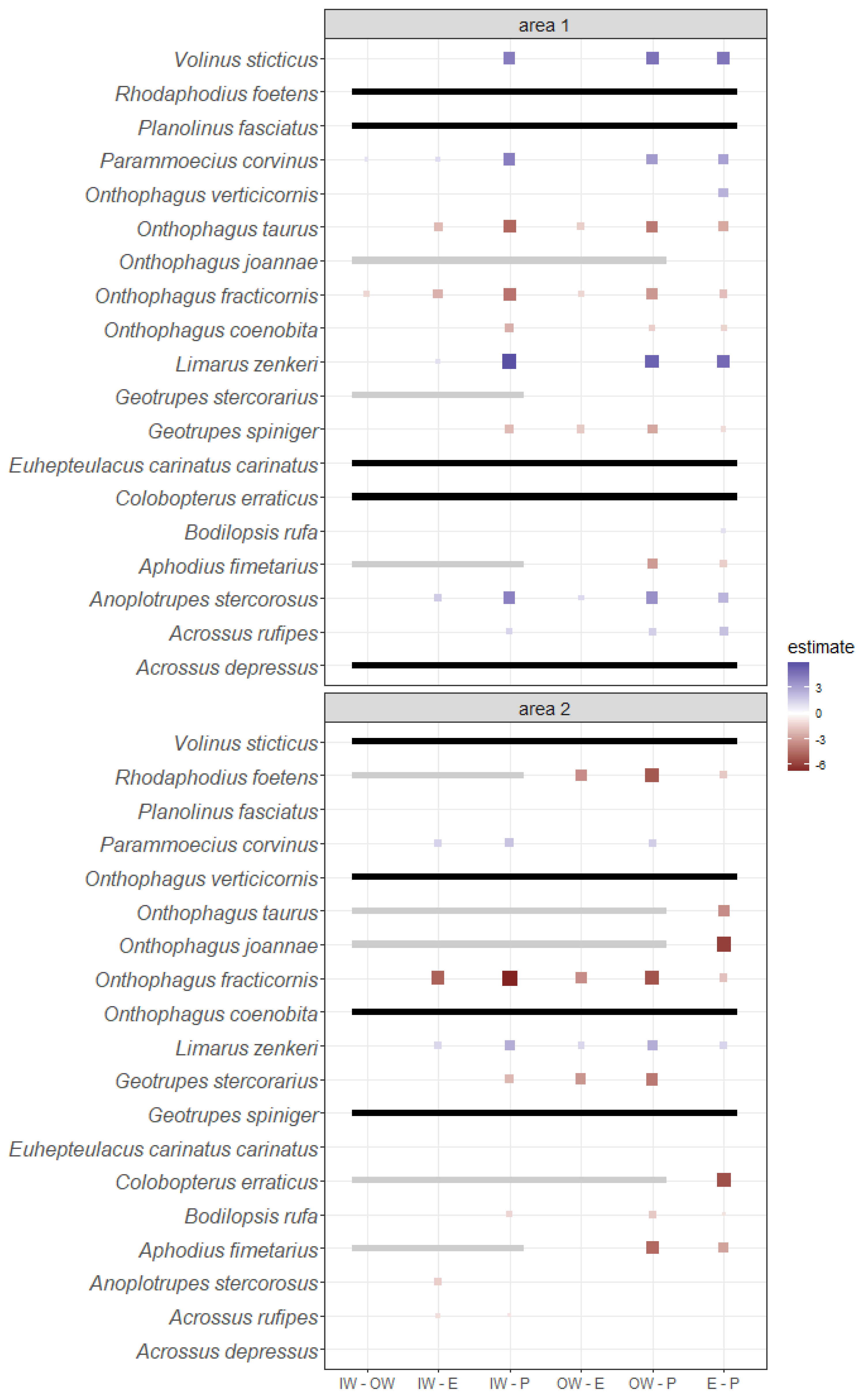

| Comparison | Site 1_1 | Site 1_2 | Site 2_1 | Site 2_2 |
|---|---|---|---|---|
| iw–ow | 49.9 | 45.7 | 42.4 | 31.0 |
| iw–edge | 28.3 | 16.7 | 34.8 | 17.4 |
| iw–pas | 0.0 | 0.0 | 23.9 | 0.0 |
| ow–edge | 44.9 | 42.9 | 64.2 | 45.6 |
| ow–pas | 4.2 | 8.4 | 35.8 | 0.0 |
| edge–pas | 21.0 | 12.8 | 41.9 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laini, A.; Roggero, A.; Carlin, M.; Palestrini, C.; Rolando, A. Community Composition of Alpine Dung Beetles Is Mostly Driven by Temperature and Habitat Type. Environments 2024, 11, 178. https://doi.org/10.3390/environments11080178
Laini A, Roggero A, Carlin M, Palestrini C, Rolando A. Community Composition of Alpine Dung Beetles Is Mostly Driven by Temperature and Habitat Type. Environments. 2024; 11(8):178. https://doi.org/10.3390/environments11080178
Chicago/Turabian StyleLaini, Alex, Angela Roggero, Mario Carlin, Claudia Palestrini, and Antonio Rolando. 2024. "Community Composition of Alpine Dung Beetles Is Mostly Driven by Temperature and Habitat Type" Environments 11, no. 8: 178. https://doi.org/10.3390/environments11080178





