The Response of Denitrification to Increasing Water Temperature and Nitrate Availability: The Case of a Large Lowland River (Po River, Northern Italy) under a Climate Change Scenario
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Procedure
2.2. Measurement of Benthic Oxygen Fluxes along Temperature Gradients
2.3. Measurement of Denitrification along Temperature and NO3− Gradients
2.4. Sediment Characterization
2.5. Statistical Analysis
3. Results and Discussion
3.1. River Sediment Oxygen Demand along Seasonal Temperature Gradients
3.2. Influence of Water Temperature and NO3− Concentration on Partitioning between Dw and Dn
3.3. Climate Change-Related Factors Controlling Denitrification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. IPCC, 2021: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Whitehead, P.G.; Wilby, R.L.; Battarbee, R.W.; Kernan, M.; Wade, A.J. A Review of the Potential Impacts of Climate Change on Surface Water Quality. Hydrol. Sci. J. 2009, 54, 101–123. [Google Scholar] [CrossRef]
- Zhao, G.; Merder, J.; Ballard, T.C.; Michalak, A.M. Warming May Offset Impact of Precipitation Changes on Riverine Nitrogen Loading. Proc. Natl. Acad. Sci. USA 2023, 120, e2220616120. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, K.; Xie, Z.; Wang, Y. Seasonal Constraint of Dynamic Water Temperature on Riverine Dissolved Inorganic Nitrogen Transport in Land Surface Modeling. Atmos. Ocean. Sci. Lett. 2024, 17, 100485. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, B.; Yu, G.; Chen, W.; He, N.; Wang, T.; Miao, C. Coupled Effects of Biogeochemical and Hydrological Processes on C, N, and P Export during Extreme Rainfall Events in a Purple Soil Watershed in Southwestern China. J. Hydrol. 2014, 511, 692–702. [Google Scholar] [CrossRef]
- Ballard, T.C.; Sinha, E.; Michalak, A.M. Long-Term Changes in Precipitation and Temperature Have Already Impacted Nitrogen Loading. Environ. Sci. Technol. 2019, 53, 5080–5090. [Google Scholar] [CrossRef]
- Van Vliet, M.T.H.; Thorslund, J.; Strokal, M.; Hofstra, N.; Flörke, M.; Ehalt Macedo, H.; Nkwasa, A.; Tang, T.; Kaushal, S.S.; Kumar, R.; et al. Global River Water Quality under Climate Change and Hydroclimatic Extremes. Nat. Rev. Earth Environ. 2023, 4, 687–702. [Google Scholar] [CrossRef]
- Piña-Ochoa, E.; Álvarez-Cobelas, M. Denitrification in Aquatic Environments: A Cross-System Analysis. Biogeochemistry 2006, 81, 111–130. [Google Scholar] [CrossRef]
- Birgand, F.; Skaggs, R.W.; Chescheir, G.M.; Gilliam, J.W. Nitrogen Removal in Streams of Agricultural Catchments—A Literature Review. Crit. Rev. Environ. Sci. Technol. 2007, 37, 381–487. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, B.; Sun, X.; Yang, L. Hot Spots and Hot Moments of Nitrogen Removal from Hyporheic and Riparian Zones: A Review. Sci. Total Environ. 2021, 762, 144168. [Google Scholar] [CrossRef]
- Knowles, R. Denitrification. Microbiol. Rev. 1982, 46, 43–70. [Google Scholar] [CrossRef]
- Seitzinger, S.; Harrison, J.A.; Böhlke, J.K.; Bouwman, A.F.; Lowrance, R.; Peterson, B.; Tobias, C.; Drecht, G.V. Denitrification across landscapes and waterscapes: A synthesis. Ecol. Appl. 2006, 16, 2064–2090. [Google Scholar] [CrossRef] [PubMed]
- Wallenstein, M.D.; Myrold, D.D.; Firestone, M.; Voytek, M. Environmental controls on denitrifying communities and denitrification rates: Insights from molecular methods. Ecol. Appl. 2006, 16, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Silvennoinen, H.; Liikanen, A.; Torssonen, J.; Stange, C.F.; Martikainen, P.J. Denitrification and N2O Effluxes in the Bothnian Bay (Northern Baltic Sea) River Sediments as Affected by Temperature under Different Oxygen Concentrations. Biogeochemistry 2008, 88, 63–72. [Google Scholar] [CrossRef]
- Jickells, T.D.; Weston, K. Nitrogen Cycle—External Cycling. In Treatise on Estuarine and Coastal Science; Elsevier: Amsterdam, The Netherlands, 2011; pp. 261–278. ISBN 978-0-08-087885-0. [Google Scholar]
- Lu, C.; Zhang, J.; Tian, H.; Crumpton, W.G.; Helmers, M.J.; Cai, W.-J.; Hopkinson, C.S.; Lohrenz, S.E. Increased Extreme Precipitation Challenges Nitrogen Load Management to the Gulf of Mexico. Commun. Earth Environ. 2020, 1, 21. [Google Scholar] [CrossRef]
- Soana, E.; Gervasio, M.P.; Granata, T.; Colombo, D.; Castaldelli, G. Climate Change Impacts on Eutrophication in the Po River (Italy): Temperature-Mediated Reduction in Nitrogen Export but No Effect on Phosphorus. J. Environ. Sci. 2024, 143, 148–163. [Google Scholar] [CrossRef]
- Gervasio, M.P.; Soana, E.; Granata, T.; Colombo, D.; Castaldelli, G. An Unexpected Negative Feedback between Climate Change and Eutrophication: Higher Temperatures Increase Denitrification and Buffer Nitrogen Loads in the Po River (Northern Italy). Environ. Res. Lett. 2022, 17, 084031. [Google Scholar] [CrossRef]
- Pavan, V.; Antolini, G.; Barbiero, R.; Berni, N.; Brunier, F.; Cacciamani, C.; Cagnati, A.; Cazzuli, O.; Cicogna, A.; De Luigi, C.; et al. High Resolution Climate Precipitation Analysis for North-Central Italy, 1961–2015. Clim. Dyn. 2019, 52, 3435–3453. [Google Scholar] [CrossRef]
- Persiano, S.; Ferri, E.; Antolini, G.; Domeneghetti, A.; Pavan, V.; Castellarin, A. Changes in Seasonality and Magnitude of Sub-Daily Rainfall Extremes in Emilia-Romagna (Italy) and Potential Influence on Regional Rainfall Frequency Estimation. J. Hydrol. Reg. Stud. 2020, 32, 100751. [Google Scholar] [CrossRef]
- Soana, E.; Bartoli, M.; Milardi, M.; Fano, E.A.; Castaldelli, G. An Ounce of Prevention Is Worth a Pound of Cure: Managing Macrophytes for Nitrate Mitigation in Irrigated Agricultural Watersheds. Sci. Total Environ. 2019, 647, 301–312. [Google Scholar] [CrossRef]
- Ludwig, W.; Dumont, E.; Meybeck, M.; Heussner, S. River Discharges of Water and Nutrients to the Mediterranean and Black Sea: Major Drivers for Ecosystem Changes during Past and Future Decades? Prog. Oceanogr. 2009, 80, 199–217. [Google Scholar] [CrossRef]
- Blaas, H.; Kroeze, C. Excessive Nitrogen and Phosphorus in European Rivers: 2000–2050. Ecol. Indic. 2016, 67, 328–337. [Google Scholar] [CrossRef]
- Grilli, F.; Accoroni, S.; Acri, F.; Bernardi Aubry, F.; Bergami, C.; Cabrini, M.; Campanelli, A.; Giani, M.; Guicciardi, S.; Marini, M.; et al. Seasonal and Interannual Trends of Oceanographic Parameters over 40 Years in the Northern Adriatic Sea in Relation to Nutrient Loadings Using the EMODnet Chemistry Data Portal. Water 2020, 12, 2280. [Google Scholar] [CrossRef]
- Montanari, A.; Nguyen, H.; Rubinetti, S.; Ceola, S.; Galelli, S.; Rubino, A.; Zanchettin, D. Why the 2022 Po River Drought Is the Worst in the Past Two Centuries. Sci. Adv. 2023, 9, eadg8304. [Google Scholar] [CrossRef]
- Bonaldo, D.; Bellafiore, D.; Ferrarin, C.; Ferretti, R.; Ricchi, A.; Sangelantoni, L.; Vitelletti, M.L. The Summer 2022 Drought: A Taste of Future Climate for the Po Valley (Italy)? Reg. Environ. Chang. 2023, 23, 1. [Google Scholar] [CrossRef]
- Gervasio, M.P.; Soana, E.; Vincenzi, F.; Magri, M.; Castaldelli, G. Drought-Induced Salinity Intrusion Affects Nitrogen Removal in a Deltaic Ecosystem (Po River Delta, Northern Italy). Water 2023, 15, 2405. [Google Scholar] [CrossRef]
- Gervasio, M.P.; Soana, E.; Gavioli, A.; Vincenzi, F.; Castaldelli, G. Contrasting Effects of Climate Change on Denitrification and Nitrogen Load Reduction in the Po River (Northern Italy). Environ. Sci. Pollut. Res. 2024, 31, 48189–48204. [Google Scholar] [CrossRef]
- Vezzoli, R.; Mercogliano, P.; Pecora, S.; Zollo, A.L.; Cacciamani, C. Hydrological Simulation of Po River (North Italy) Discharge under Climate Change Scenarios Using the RCM COSMO-CLM. Sci. Total Environ. 2015, 521–522, 346–358. [Google Scholar] [CrossRef]
- Dalsgaard, T.; Nielsen, L.P.; Brotas, V.; Viaroli, P.; Underwood, G.; Nedwell, D.; Sundback, K.; Rysgaard, S.; Miles, A.; Bartoli, M.; et al. Protocol Handbook for NICE-Nitrogen Cycling in Estuaries: A Project under the EU Research Programme: Marine Science and Technology (MAST III); Ministry of Environment and Energy National Environmental Research Institute: Silkeborg, Denmark; Department of Lake and Estuarine Ecology: Silkeborg, Denmark, 2000; pp. 1–62.
- Owens, M.S.; Cornwell, J.C. The Benthic Exchange of O2, N2 and Dissolved Nutrients Using Small Core Incubations. J. Vis. Exp. 2016, 104, 54098. [Google Scholar] [CrossRef]
- Braga, F.; Zaggia, L.; Bellafiore, D.; Bresciani, M.; Giardino, C.; Lorenzetti, G.; Maicu, F.; Manzo, C.; Riminucci, F.; Ravaioli, M.; et al. Mapping Turbidity Patterns in the Po River Prodelta Using Multi-Temporal Landsat 8 Imagery. Estuar. Coast. Shelf Sci. 2017, 198, 555–567. [Google Scholar] [CrossRef]
- Nielsen, L.P. Denitrification in Sediment Determined from Nitrogen Isotope Pairing. FEMS Microbiol. Lett. 1992, 86, 357–362. [Google Scholar] [CrossRef]
- Boldrin, A.; Langone, L.; Miserocchi, S.; Turchetto, M.; Acri, F. Po River Plume on the Adriatic Continental Shelf: Dispersion and Sedimentation of Dissolved and Suspended Matter during Different River Discharge Rates. Mar. Geol. 2005, 222–223, 135–158. [Google Scholar] [CrossRef]
- Menicucci, S.; De Felice, A.; Biagiotti, I.; Canduci, G.; Costantini, I.; Palermino, A.; Centurelli, M.; Leonori, I. Interannual Variation in the Zooplankton Community of the North Adriatic Sea under Short-Term Climatic Anomalies. Diversity 2024, 16, 291. [Google Scholar] [CrossRef]
- Armstrong, F.A.J.; Stearns, C.R.; Strickland, J.D.H. The Measurement of Upwelling and Subsequent Biological Process by Means of the Technicon Autoanalyzer® and Associated Equipment. Deep. Sea Res. Oceanogr. Abstr. 1967, 14, 381–389. [Google Scholar] [CrossRef]
- Kana, T.M.; Darkangelo, C.; Hunt, M.D.; Oldham, J.B.; Bennett, G.E.; Cornwell, J.C. Membrane Inlet Mass Spectrometer for Rapid High-Precision Determination of N2, O2, and Ar in Environmental Water Samples. Anal. Chem. 1994, 66, 4166–4170. [Google Scholar] [CrossRef]
- Robertson, E.K.; Bartoli, M.; Brüchert, V.; Dalsgaard, T.; Hall, P.O.J.; Hellemann, D.; Hietanen, S.; Zilius, M.; Conley, D.J. Application of the Isotope Pairing Technique in Sediments: Use, Challenges, and New Directions. Limnol. Ocean Methods 2019, 17, 112–136. [Google Scholar] [CrossRef]
- Steingruber, S.M.; Friedrich, J.; Gächter, R.; Wehrli, B. Measurement of Denitrification in Sediments with the 15N Isotope Pairing Technique. Appl. Environ. Microbiol. 2001, 67, 3771–3778. [Google Scholar] [CrossRef]
- Eyre, B.D.; Rysgaard, S.; Dalsgaard, T.; Christensen, P.B. Comparison of Isotope Pairing and N2:Ar Methods for Measuring Sediment Denitrification—Assumption, Modifications, and Implications. Estuaries 2002, 25, 1077–1087. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 2018, 3, 1–137. [Google Scholar]
- Deichmann, U.; Schuster, S.; Mazat, J.; Cornish-Bowden, A. Commemorating the 1913 Michaelis–Menten Paper Die Kinetik Der Invertinwirkung: Three Perspectives. FEBS J. 2014, 281, 435–463. [Google Scholar] [CrossRef]
- De Klein, J.J.M.; Overbeek, C.C.; Juncher Jørgensen, C.; Veraart, A.J. Effect of Temperature on Oxygen Profiles and Denitrification Rates in Freshwater Sediments. Wetlands 2017, 37, 975–983. [Google Scholar] [CrossRef]
- Johnson, M.F.; Albertson, L.K.; Algar, A.C.; Dugdale, S.J.; Edwards, P.; England, J.; Gibbins, C.; Kazama, S.; Komori, D.; MacColl, A.D.C.; et al. Rising Water Temperature in Rivers: Ecological Impacts and Future Resilience. WIREs Water 2024, 11, e1724. [Google Scholar] [CrossRef]
- Perkins, D.M.; Yvon-Durocher, G.; Demars, B.O.L.; Reiss, J.; Pichler, D.E.; Friberg, N.; Trimmer, M.; Woodward, G. Consistent Temperature Dependence of Respiration across Ecosystems Contrasting in Thermal History. Glob. Chang. Biol. 2012, 18, 1300–1311. [Google Scholar] [CrossRef]
- Saunders, D.L.; Kalff, J. Nitrogen Retention in Wetlands, Lakes and Rivers. Hydrobiologia 2001, 443, 205–212. [Google Scholar] [CrossRef]
- Boulêtreau, S.; Lyautey, E.; Dubois, S.; Compin, A.; Delattre, C.; Touron-Bodilis, A.; Mastrorillo, S.; Garabetian, F. Warming-Induced Changes in Denitrifier Community Structure Modulate the Ability of Phototrophic River Biofilms to Denitrify. Sci. Total Environ. 2014, 466–467, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Velthuis, M.; Veraart, A.J. Temperature Sensitivity of Freshwater Denitrification and N2O Emission—A Meta-Analysis. Glob. Biogeochem. Cycles 2022, 36, e2022GB007339. [Google Scholar] [CrossRef]
- Speir, S.L.; Tank, J.L.; Taylor, J.M.; Grose, A.L. Temperature and Carbon Availability Interact to Enhance Nitrous Oxide Production via Denitrification in Alluvial Plain River Sediments. Biogeochemistry 2023, 165, 191–203. [Google Scholar] [CrossRef]
- Veraart, A.J.; De Klein, J.J.M.; Scheffer, M. Warming Can Boost Denitrification Disproportionately Due to Altered Oxygen Dynamics. PLoS ONE 2011, 6, e18508. [Google Scholar] [CrossRef]
- Ward, B.B. Nitrification: An Introduction and Overview of the State of the Field. In Nitrification; Ward, B.B., Arp, D.J., Klotz, M.G., Eds.; ASM Press: Washington, DC, USA, 2014; pp. 1–8. ISBN 978-1-68367-116-9. [Google Scholar]
- Qu, W.; Suo, L.; Liu, R.; Liu, M.; Zhao, Y.; Xia, L.; Fan, Y.; Zhang, Q.; Gao, Z. Influence of Temperature on Denitrification and Microbial Community Structure and Diversity: A Laboratory Study on Nitrate Removal from Groundwater. Water 2022, 14, 436. [Google Scholar] [CrossRef]
- Brin, L.D.; Giblin, A.E.; Rich, J.J. Similar Temperature Responses Suggest Future Climate Warming Will Not Alter Partitioning between Denitrification and Anammox in Temperate Marine Sediments. Glob. Chang. Biol. 2017, 23, 331–340. [Google Scholar] [CrossRef]
- Verstraete, W.; Forcht, D.D. Biochemical Ecology of Nitrification and Denitrification [Soils]. In Advances in Microbial Ecology; Springer: Boston, MA, USA, 1977; pp. 135–214. [Google Scholar]
- Nogaro, G.; Burgin, A.J. Influence of Bioturbation on Denitrification and Dissimilatory Nitrate Reduction to Ammonium (DNRA) in Freshwater Sediments. Biogeochemistry 2014, 120, 279–294. [Google Scholar] [CrossRef]
- Racchetti, E.; Bartoli, M.; Soana, E.; Longhi, D.; Christian, R.R.; Pinardi, M.; Viaroli, P. Influence of Hydrological Connectivity of Riverine Wetlands on Nitrogen Removal via Denitrification. Biogeochemistry 2011, 103, 335–354. [Google Scholar] [CrossRef]
- Inwood, S.E.; Tank, J.L.; Bernot, M.J. Factors Controlling Sediment Denitrification in Midwestern Streams of Varying Land Use. Microb. Ecol. 2007, 53, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Soana, E.; Naldi, M.; Bonaglia, S.; Racchetti, E.; Castaldelli, G.; Brüchert, V.; Viaroli, P.; Bartoli, M. Benthic Nitrogen Metabolism in a Macrophyte Meadow (Vallisneria spiralis L.) under Increasing Sedimentary Organic Matter Loads. Biogeochemistry 2015, 124, 387–404. [Google Scholar] [CrossRef]
- Tesi, T.; Miserocchi, S.; Acri, F.; Langone, L.; Boldrin, A.; Hatten, J.A.; Albertazzi, S. Flood-Driven Transport of Sediment, Particulate Organic Matter, and Nutrients from the Po River Watershed to the Mediterranean Sea. J. Hydrol. 2013, 498, 144–152. [Google Scholar] [CrossRef]
- Stelzer, R.S.; Thad Scott, J.; Bartsch, L.A.; Parr, T.B. Particulate Organic Matter Quality Influences Nitrate Retention and Denitrification in Stream Sediments: Evidence from a Carbon Burial Experiment. Biogeochemistry 2014, 119, 387–402. [Google Scholar] [CrossRef]
- Seitzinger, S. Linkages between Organic Matter Mineralization and Denitrification in Eight Riparian Wetlands. Biogeochemistry 1994, 25, 19–39. [Google Scholar] [CrossRef]
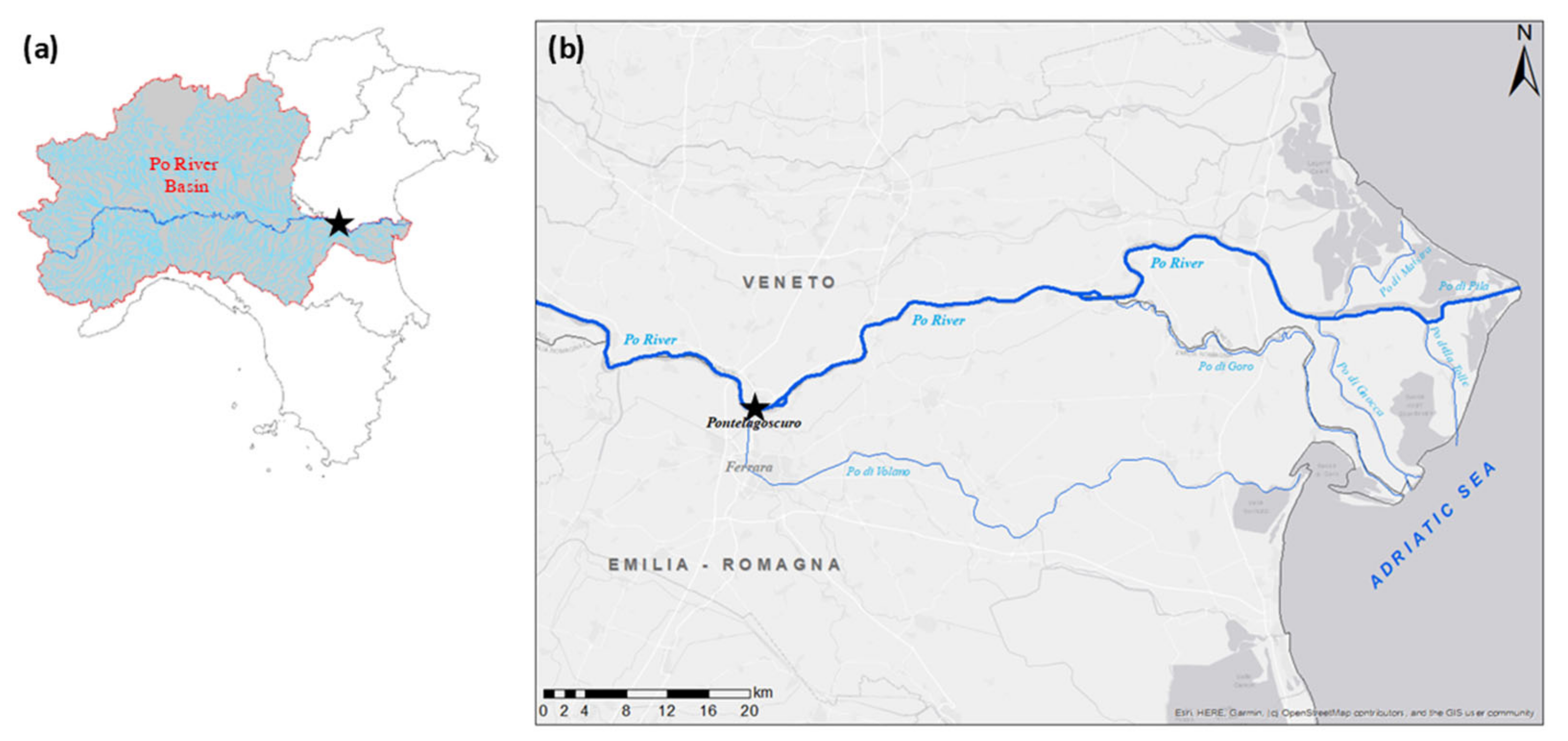
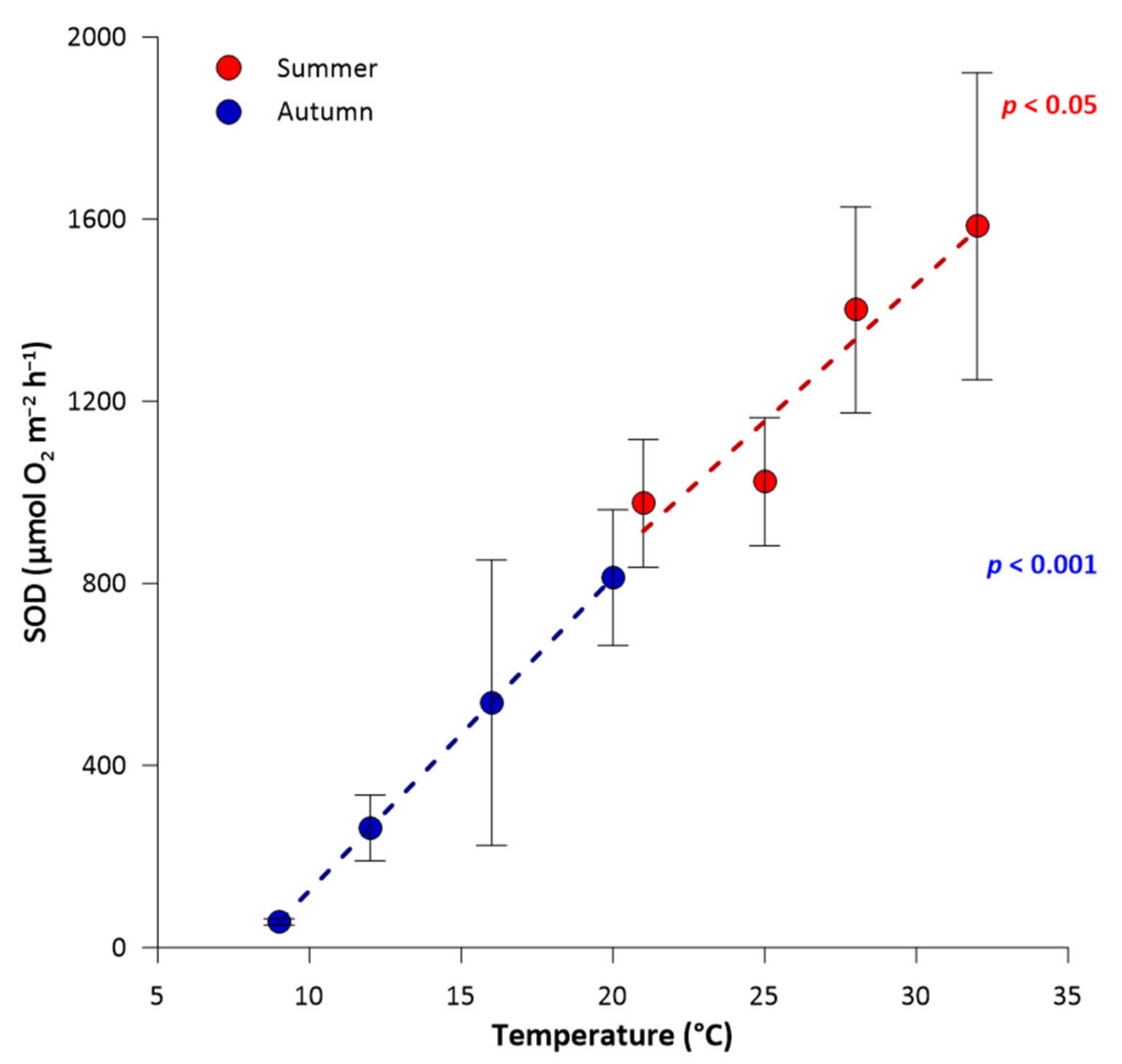


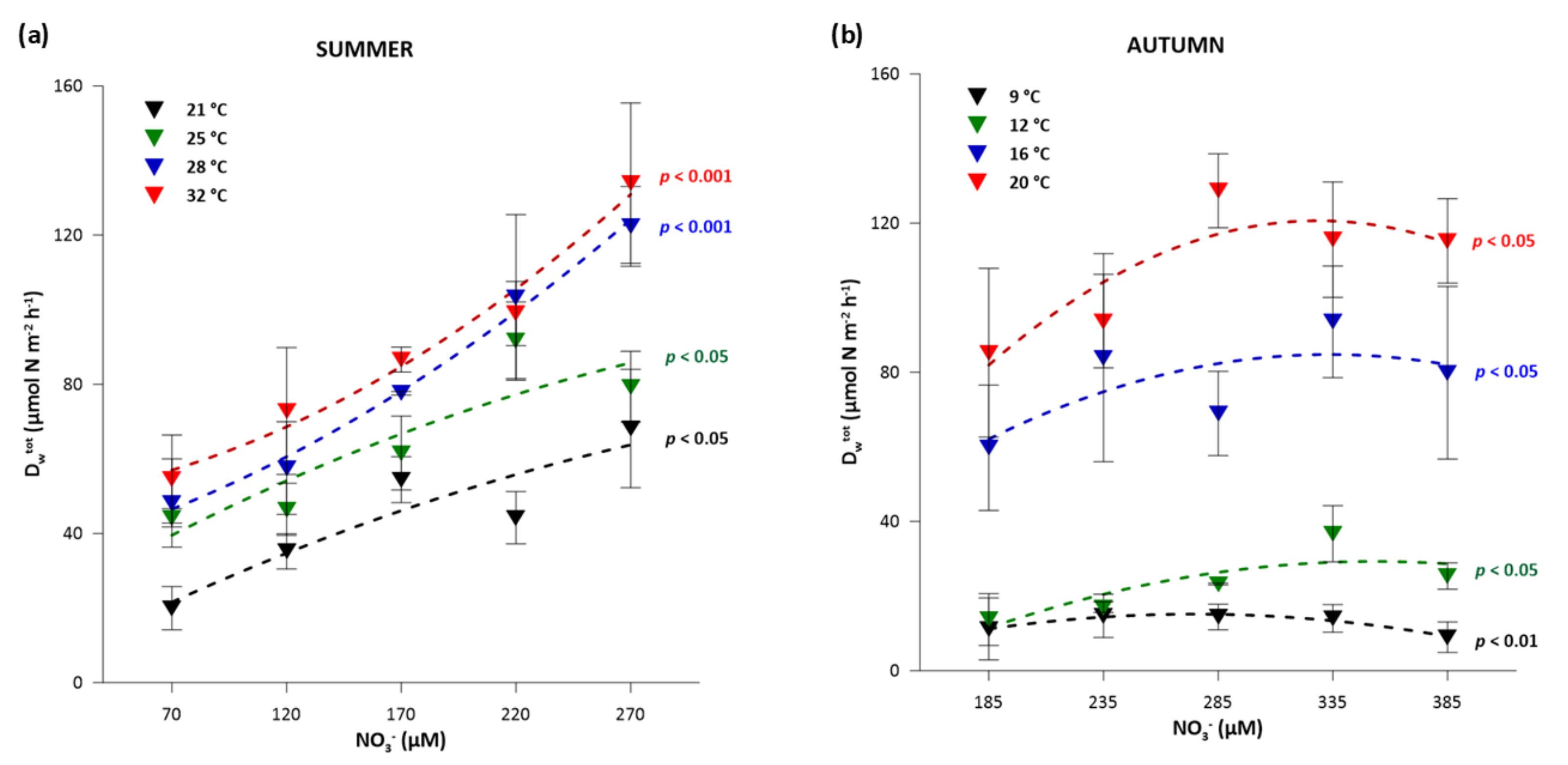
| Seasons | Temperature (°C) | O2 (mg L−1) | NO3− (µM) | OM (%) | Density (g mL−1) | Porosity (%) |
|---|---|---|---|---|---|---|
| Summer | 29 | 8.3 | 20 (2) | 0.4 (0.03) | 2.1 (0.1) | 40 (3) |
| Autumn | 14 | 10.2 | 135 (3) | 1.7 (0.4) | 1.8 (0.2) | 60 (10) |
| Season | Temperature (°C) | 15NO3− (µM) | Temperature × 15NO3− | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Summer | 33.0 | <0.001 | 115.7 | <0.001 | 3.6 | 0.05 |
| Autumn | 202.3 | <0.001 | 51.6 | <0.001 | 32.6 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gervasio, M.P.; Castaldelli, G.; Soana, E. The Response of Denitrification to Increasing Water Temperature and Nitrate Availability: The Case of a Large Lowland River (Po River, Northern Italy) under a Climate Change Scenario. Environments 2024, 11, 179. https://doi.org/10.3390/environments11080179
Gervasio MP, Castaldelli G, Soana E. The Response of Denitrification to Increasing Water Temperature and Nitrate Availability: The Case of a Large Lowland River (Po River, Northern Italy) under a Climate Change Scenario. Environments. 2024; 11(8):179. https://doi.org/10.3390/environments11080179
Chicago/Turabian StyleGervasio, Maria Pia, Giuseppe Castaldelli, and Elisa Soana. 2024. "The Response of Denitrification to Increasing Water Temperature and Nitrate Availability: The Case of a Large Lowland River (Po River, Northern Italy) under a Climate Change Scenario" Environments 11, no. 8: 179. https://doi.org/10.3390/environments11080179
APA StyleGervasio, M. P., Castaldelli, G., & Soana, E. (2024). The Response of Denitrification to Increasing Water Temperature and Nitrate Availability: The Case of a Large Lowland River (Po River, Northern Italy) under a Climate Change Scenario. Environments, 11(8), 179. https://doi.org/10.3390/environments11080179









