Phenol, Cyanide, and Thiocyanate in Aquatic Media: The Ecotoxicity of Individual Substances and Their Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Toxicity Tests Applied
2.1.1. Aliivibrio fischeri Test
2.1.2. Pseudomonas putida Test
2.1.3. Chlorella sp. Test
2.1.4. Lemna minor Test
2.1.5. Allium cepa Test
2.2. Analysis of Joint Toxic Activity
2.2.1. Determination of the Toxicity of Mixtures
2.2.2. Concentration Addition Model
3. Results and Discussion
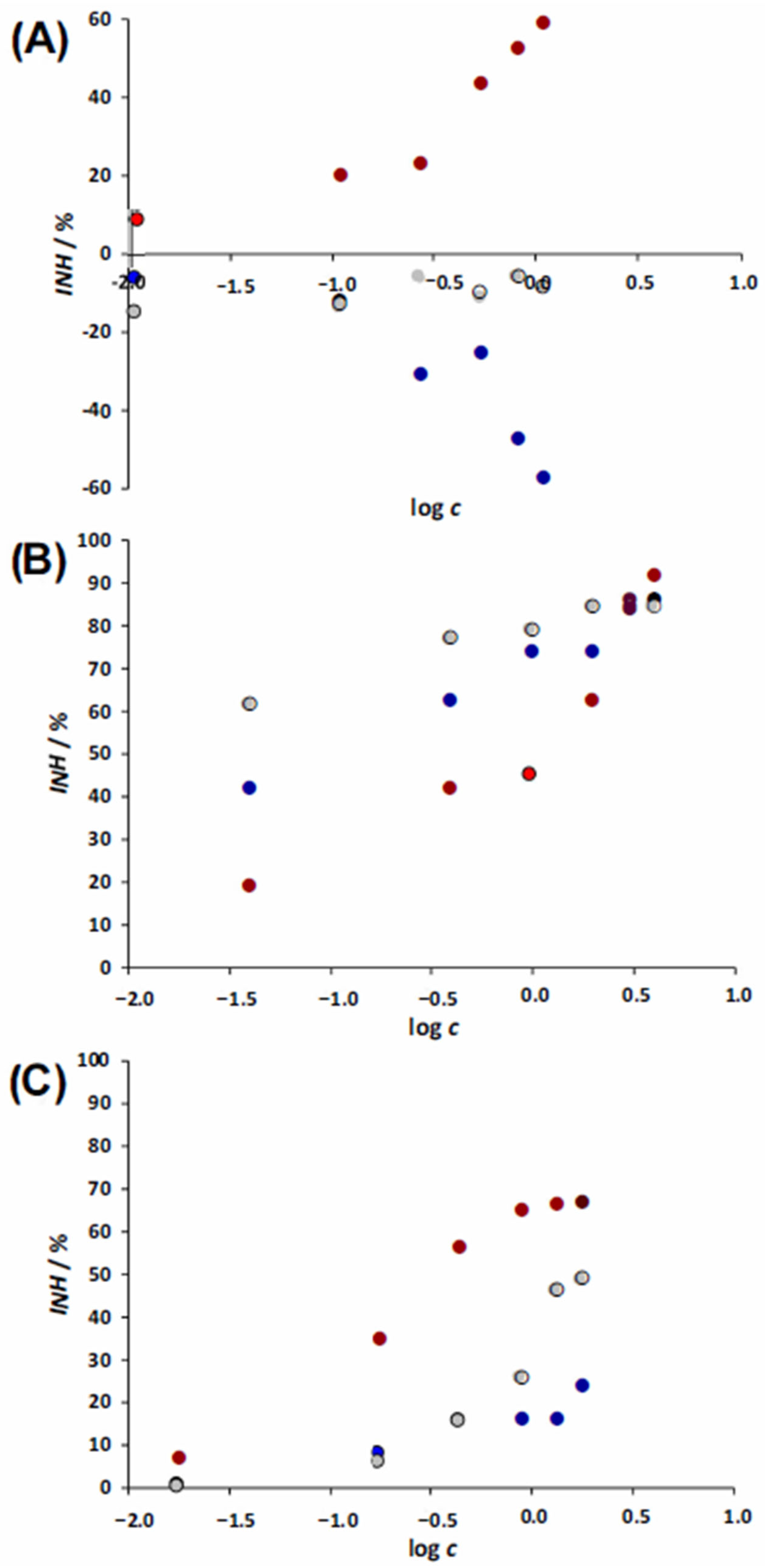
3.1. Toxicities Towards Aliivibrio fischeri, Pseudomonas putida, and Chlorella sp.
3.2. Toxicity Towards Lemna minor
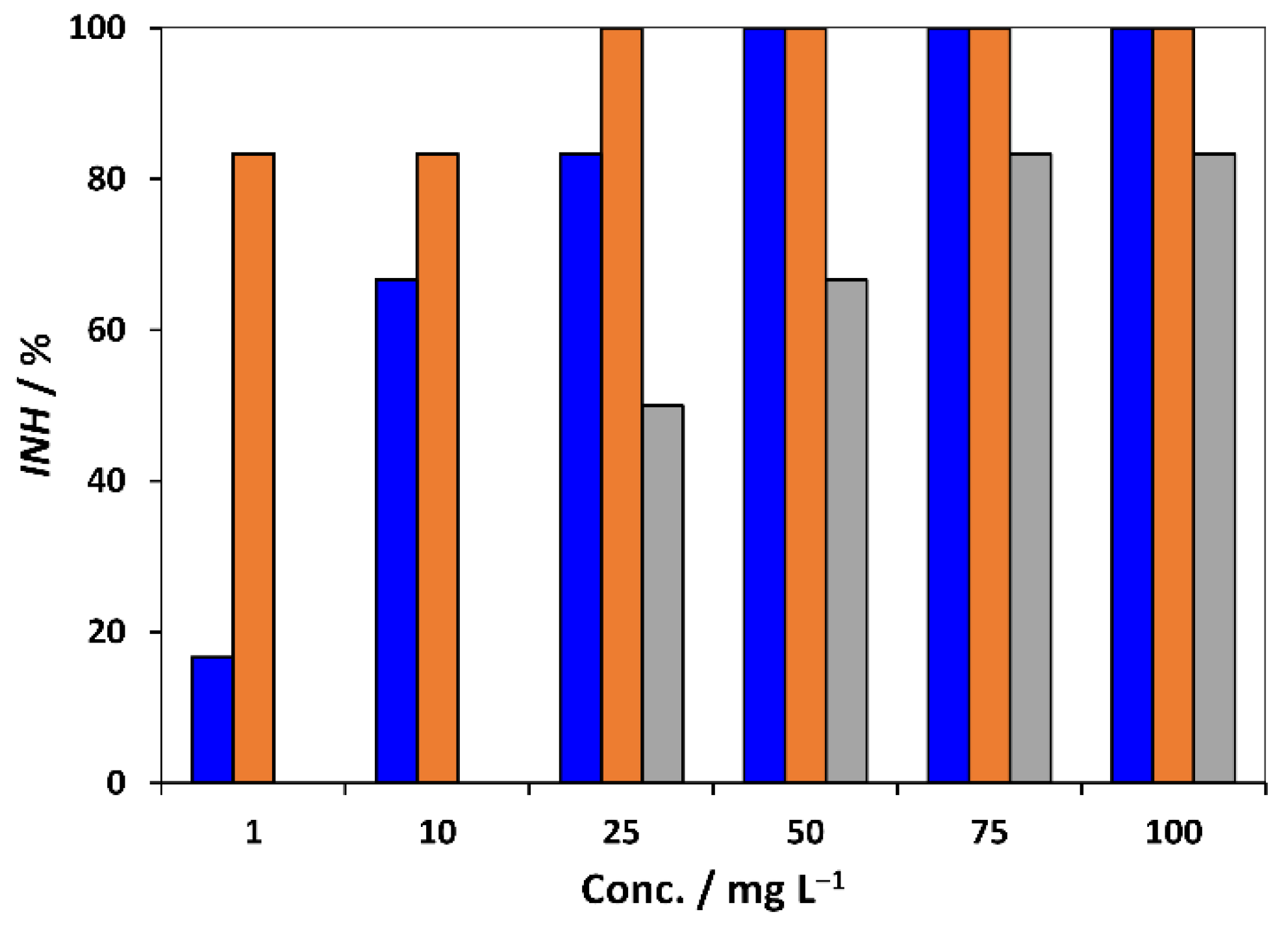
3.3. Toxicity Towards Allium cepa
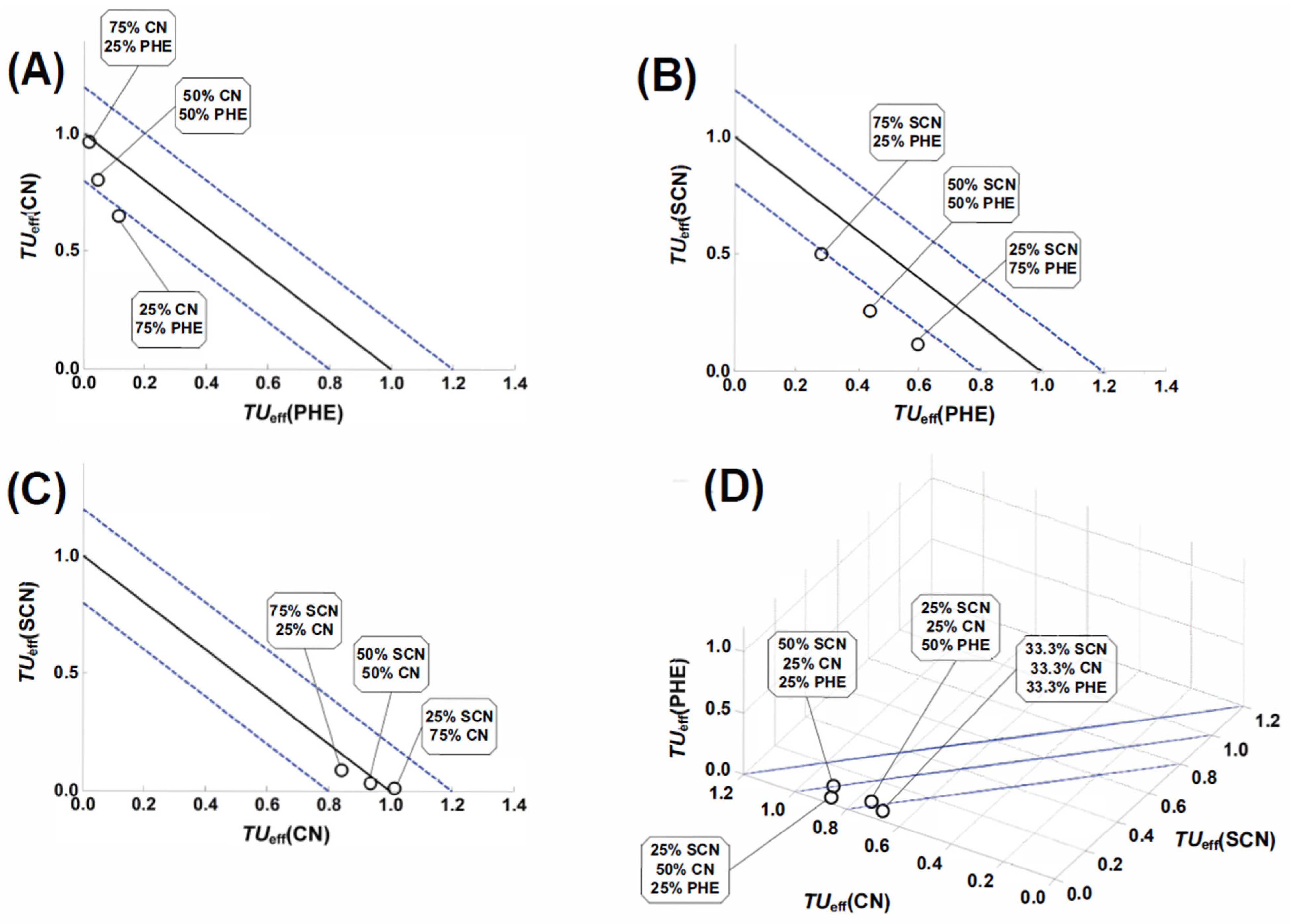
3.4. Joint Toxic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| BAT | Best Available Technique |
| CFU | Colony-forming units |
| CN | Cyanide |
| CO | Colorado |
| EC | Effective concentration, i.e., toxicity |
| INH | Inhibition |
| ISO | International Organization for Standardization |
| OECD | Organisation for Economic Co-operation and Development |
| PHE | Phenol |
| MA | Massachusetts |
| SCN | Thiocyanate |
| TU | Toxicity unit |
| USA | United States of America |
| WA | Washington |
References
- Gaur, V.; Mathur, A. Determination of LC50 of phenolic compounds (phenol & m-cresol) for a fish, Labeo rohita. Int. J. Med. Lab. Res. 2019, 4, 55–63. [Google Scholar] [CrossRef]
- Tutić, A.; Miloloža, M.; Cvetnić, M.; Martinjak, V.; Furač, L.; Markić, M.; Ukić, Š.; Bolanča, T.; Kučić Grgić, D. An Overview of Coking Wastewater Characteristics and Treatment Technologies. Kem. Ind. 2023, 75, 349–358. [Google Scholar] [CrossRef]
- Singh, N.; Balomajumder, C. Simultaneous removal of phenol and cyanide from aqueous solution by adsorption onto surface modified activated caFrbon prepared from coconut shell. J. Water Process Eng. 2016, 9, 233–245. [Google Scholar] [CrossRef]
- Singh, H.; Mishra, B.K. Degradation of cyanide, aniline and phenol in pre-treated coke oven wastewater by peroxide assisted electro-oxidation process. Water Sci. Technol. 2018, 78, 2214–2227. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Priority Pollutant List. 2014. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/priority-pollutant-list-epa.pdf (accessed on 8 January 2025).
- Kim, Y.M. Acclimatization of communities of ammonia oxidizing bacteria to seasonal changes in optimal conditions in a coke wastewater treatment plant. Bioresour. Technol. 2013, 147, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Sarkar, A.; Nair, U.G. Comparative characterization of cyanide-containing steel industrial wastewater. Water Sci. Technol. 2021, 83, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, C.A.; Samaras, P.; Sakellaropoulos, G.P. Comparative study of phenol and cyanide containing wastewater in CSTR and SBR activated sludge reactors. Bioresour. Technol. 2009, 100, 31–37. [Google Scholar] [CrossRef]
- Marañón, E.; Vázquez, I.; Rodríguez, J.; Castrillón, L.; Fernández, Y. Coke wastewater treatment by a three-step activated sludge system. Water. Air Soil Pollut. 2008, 192, 155–164. [Google Scholar] [CrossRef]
- Melcer, H.; Nutt, S.G. Nitrogen Control of Complex Industrial Wastewaters. J. Environ. Eng. 1988, 114, 166–178. [Google Scholar] [CrossRef]
- Barton, P.J.; Hammer, C.A.; Kennedy, D.C. Analysis of Cyanides in Coke Plant Wastewater Effluents. J. Water Pollut. Cont. Fed. 1978, 50, 234–239. [Google Scholar]
- European Commission. Best Available Techniques (BAT) Reference Document for Common Waste Water and Waste Gas Treatment/Management Systems in the Chemical Sector. Available online: https://eippcb.jrc.ec.europa.eu/sites/default/files/2019-11/CWW_Bref_2016_published.pdf (accessed on 7 April 2025).
- Bargieł, P.; Zabochnicka-Świątek, M.; Wolski, P. Treatment of Coking Wastewater Using Sorption Processes. J. Ecol. Eng. 2022, 23, 43–47. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 996, Phenol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Phenol (accessed on 7 January 2025).
- Park, J.S.; Brown, M.T.; Han, T. Phenol toxicity to the aquatic macrophyte Lemna paucicostata. Aquat. Toxicol. 2012, 106–107, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Bousselma, A.; Abdessemed, D.; Tahraoui, H.; Zedame, F.; Amrane, A. Polyphenols and Flavonoids Contents of Fresh and Dried Apricots Extracted by Cold Soaking and Ultrasound-assisted Extraction. Kem. Ind. 2023, 72, 161–168. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Sundar Rajan, P.S.; Arun, J.; Brindhadevi, K.; Le, Q.H.; Pugazhendhi, A. A review on recent advancements in extraction, removal and recovery of phenols from phenolic wastewater: Challenges and future outlook. Environ. Res. 2023, 237, 117005. [Google Scholar] [CrossRef] [PubMed]
- Bezverbna, O.; Załęska-Radziwiłł, M. Ecotoxicological evaluation the effects of the safe concentration of wastewater containing phenol on aquatic ecosystems. J. Environ. Eng. Landsc. Manag. 2017, 26, 57–63. [Google Scholar] [CrossRef]
- Ramos, R.L.; Martins, M.F.; Lebron, Y.A.R.; Moreira, V.R.; Reis, B.G.; Grossi, L.B.; Amaral, M.C.S. Membrane distillation process for phenolic compounds removal from surface water. J. Environ. Chem. Eng. 2021, 9, 105588. [Google Scholar] [CrossRef]
- Shehi, A.; Shpati, K.; Dama, A.; Myrtaj, B.; Nuro, A. Determination of phenol levels in some surface water ecosystems of Tirana Area, Albania. J. Ecol. Eng. 2025, 26, 48–55. [Google Scholar] [CrossRef]
- Perrin, D.D. Dissociation constants of inorganic acids and bases in aqueous solution. Pure Appl. Chem. 1969, 20, 133–236. [Google Scholar] [CrossRef]
- Šoljić, Z. Kvalitativna Kemijska Analiza Anaorganskih Tvari; Fakultet kemijskog inženjerstva i tehnologije & Hinus: Zagreb, Croatia, 2005. [Google Scholar]
- Xylem Lab Solutions. Cyanide Analysis Guide; OI Analytical: Yellow Springs, OH, USA, 2023; Available online: https://www.ysi.com/file%20library/documents/brochures%20and%20catalogs/2968-03-xylem-cyanide-guide---web.pdf?srsltid=AfmBOopLExlZeag09iMJp7qffSIFrwJ9ZEd5cKxE8HIDOsCYvlb5GTWf (accessed on 7 January 2025).
- Ibnul, N.K.; Russell, J.; Dennen, K.; Tripp, C.P. Quantification of free and weakly bound cyanide in water using infrared spectroscopy. Talanta 2024, 266, 124939. [Google Scholar] [CrossRef]
- Sehrawat, A.; Sindhu, S.S.; Glick, B.R. Hydrogen cyanide production by soil bacteria: Biological control of pests and promotion of plant growth in sustainable agriculture. Pedosphere 2022, 32, 15–38. [Google Scholar] [CrossRef]
- Ward, E.W.B.; Lebeau, J.B. Autolytic production of hydrogen cyanide by certain snow mold fungi. Can. J. Bot. 1962, 40, 85–88. [Google Scholar] [CrossRef]
- Aranguri-Llerena, G.; Siche, R. Superior Plants with Significant Amounts of Cyanide and Their Toxicological Implications. Rev. Agric. Sci. 2020, 8, 354–366. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, G.; Ding, J.; Zou, H.; Shi, H.; Huang, C. Evaluation of the Removal of Potassium Cyanide and its Toxicity in Green Algae (Chlorella vulgaris). Bull. Environ. Contam. Toxicol. 2017, 100, 228–233. [Google Scholar] [CrossRef]
- Ryu, B.G.; Kim, J.; Yoo, G.; Lim, J.T.; Kim, W.; Han, J.I. Microalgae-mediated simultaneous treatment of toxic thiocyanate and production of biodiesel. Bioresour. Technol. 2014, 158, 166–173. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Flora, S.J.S. CHAPTER 19—Cyanide Toxicity and its Treatment. In Handbook of Toxicology of Chemical Warfare Agents; Gupta, R.C., Ed.; Academic Press: Chennai, India, 2009; pp. 255–270. [Google Scholar] [CrossRef]
- Marugán, J.; Bru, D.; Pablos, C.; Catalá, M. Comparative evaluation of acute toxicity by Aliivibrio fischeri and fern spore based bioassays in the follow-up of toxic chemicals degradation by photocatalysis. J. Hazard. Mater. 2012, 213–214, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Tez, S.; Oral, R.; Kocbas, F.; Koru, E.; Turkcu, E.; Pagano, G.; Trifuoggi, M. Comparative multi-species analysis of potassium cyanide toxicity. Mar. Pollut. Bull. 2022, 182, 113965. [Google Scholar] [CrossRef] [PubMed]
- Ketcheson, K.; Fingas, M. Chapter 39: Sodium cyanide: Properties, toxicity, uses and environmental impacts. In The Handbook of Hazardous Materials Spills Technology; Fingas, M., Ed.; McGraw-Hill: New York, NY, USA, 2002; pp. 39.1–39.22. [Google Scholar]
- Hampton Research. Safety Data Sheet According to Regulation (EC) No 1907/2006; HR2-693; Version 1.5; Hampton Research: Aliso Viejo, CA, USA, 2 June 2020; Available online: https://hamptonresearch.com/uploads/support_materials/2-693_SDS_-_Sigma_251410.pdf (accessed on 10 January 2025).
- Sigma-Aldrich. Safety Data Sheet According to Regulation (EC) No. 1907/2006; SIGALD-207799; Version 6.7; Merck: Darmstadt, Germany, 2 June 2023; Available online: https://www.sigmaaldrich.com/HR/en/sds/sigald/207799?userType=undefined (accessed on 10 January 2025).
- Thermo Fisher Scientific. SAFETY DATA SHEET ALFAAA13731; Version 3, Revision Date: 7 May 2024; Thermo Fisher Scientific: Waltham, MA, USA, 2010; Available online: https://assets.thermofisher.com/DirectWebViewer/private/document.aspx?prd=ALFAAA13731~~PDF~~MTR~~CGV4~~EN~~2024-04-30%2019:10:40~~Potassium%20thiocyanate~~ (accessed on 10 January 2025).
- González, P.S.; Maglione, G.A.; Giordana, M.; Paisio, C.E.; Talano, M.A.; Agostini, E. Evaluation of phenol detoxification by Brassica napus hairy roots, using Allium cepa test. Environ. Sci. Pollut. Res. Int. 2012, 19, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Levan, A.; Wangenheim, K.H. Potassium cyanide in the allium test. Hereditas 1952, 38, 297–313. [Google Scholar] [CrossRef]
- Kalčíková, G.; Skalar, T.; Marolt, G.; Kokalj, A.J. An environmental concentration of aged microplastics with adsorbed silver significantly affects aquatic organisms. Water Res. 2020, 175, 115644. [Google Scholar] [CrossRef]
- Putar, U.; Turk, K.; Jung, J.; Kim, C.; Kalčíková, G. The dual impact of tire wear microplastics on the growth and ecological interactions of duckweed Lemna minor. Environ. Pollut. 2025, 368, 125681. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Backhaus, T.; Faust, M. Predictive environmental risk assessment of chemical mixtures: A conceptual framework. Environ. Sci. Technol. 2012, 46, 2564–2573. [Google Scholar] [CrossRef]
- Qin, L.-T.; Liu, S.-S.; Zhang, J.; Xiao, Q.-F. A novel model integrated concentration addition with independent action for the prediction of toxicity of multi-component mixture. Toxicology 2011, 280, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, Z.; Li, Y.; Liu, M.; Wu, Y.; Chen, Z.; Zhu, B. Biodegradation of benzo[a]pyrene by a marine Chlorella vulgaris LH-1 with heterotrophic ability. Mar. Pollut. Bull. 2024, 198, 115848. [Google Scholar] [CrossRef] [PubMed]
- El-Naas, M.H.; Al-Muhtaseb, S.A.; Makhlouf, S. Biodegradation of phenol by Pseudomonas putida immobilized in polyvinyl alcohol (PVA) gel. J. Hazard. Mat. 2009, 164, 720–725. [Google Scholar] [CrossRef]
- Medić, A.B.; Karadžić, I.M. Pseudomonas in environmental bioremediation of hydrocarbons and phenolic compounds-key catabolic degradation enzymes and new analytical platforms for comprehensive investigation. World J. Microbiol. Biotechnol. 2022, 38, 165. [Google Scholar] [CrossRef]
- Babu, G.R.V.; Wolfram, J.H.; Chapatwala, K.D. Conversion of sodium cyanide to carbon dioxide and ammonia by immobilized cells of Pseudomonas putida. J. Ind. Microbiol. Biotechnol. 1992, 9, 235–238. [Google Scholar] [CrossRef]
- Dwivedi, N.; Majunder, C.B.; Mondal, P.; Dwivedi, S. Biological treatment of cyanide containing wastewater. Res. J. Chem. Sci. 2011, 7, 15–21. [Google Scholar]
- Shigeoka, T.; Sato, Y.; Takeda, Y.; Yoshida, K.; Yamauchi, F. Acute toxicity of chlorophenols to green algae, Selenastrum capricornutum and Chlorella vulgaris, and quantitative structure-activity relationships. Environ. Toxicol. Chem. 1988, 7, 847–854. [Google Scholar] [CrossRef]
- Song, Z.; Huang, G. Toxic effects of pentachlorophenol on Lemna polyrhiza. Ecotoxicol. Environ. Saf. 2007, 66, 343–347. [Google Scholar] [CrossRef]
- Duan, W.; Meng, F.; Cui, H.; Lin, Y.; Wang, G.; Wu, J. Ecotoxicity of phenol and cresols to aquatic organisms: A review. Ecotoxicol. Environ. Saf. 2018, 157, 441–456. [Google Scholar] [CrossRef] [PubMed]

| Phenol | Cyanide Ion | Thiocyanate Ion | |
|---|---|---|---|
| molecular formula | C6H6O | CN− | SCN− |
| structure | 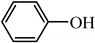 | N≡C− | N≡C−S− |
| molecular weight | 94.11 g mol−1 | 26.02 g mol−1 | 58.08 g mol−1 |
| Phenols, mg L−1 | Cyanides, mg L−1 | Thiocyanates, mg L−1 | Industry | Reference |
|---|---|---|---|---|
| 560 | 200 | 56 | coking | [4] |
| 384–534 | 12–24 | 367–642 | coking | [6] |
| 414–662 | 2.8–9 | 301–542 | steel | [7] |
| 400–1200 | 4–15 | 200–500 | coking | [8] |
| 100–221 | 11–41 | 198–427 | coking | [9] |
| 483 | 8.2 | 361 | coking | [10] |
| 2500 | 10–50 | 100–300 | coking | [11] |
| Test Organism | Optical Density | CFU/Cells mL−1 | pH | γ (O2)/mg L−1 | T/°C |
|---|---|---|---|---|---|
| Pseudomonas putida | 0.20 | 1.9 × 108 | 7.6 | 7.7 | 26.7 |
| Chlorella sp. | 0.02 | 3.6 × 105 | 8.2 | 7.9 | 24.4 |
| Toxicant | Toxicity Test | Duration | Dose–Response Line | Toxicity Values | |||
|---|---|---|---|---|---|---|---|
| Intercept | Slope | R2 | EC20/mM (mg L–1) | EC50/mM (mg L–1) | |||
| Phenol | Aliivibrio fischeri | 30 min | 0.770 | 1.746 | 0.9983 | 0.1638 (15.41) | 0.3621 (34.08) |
| Pseudomonas putida | 72 h | –0.331 | 0.741 | 0.9877 | 0.4314 (40.60) | 2.7984 (263.36) * | |
| Chlorella sp. | 72 h | –0.300 | 1.040 | 0.9919 | 0.5123 (48.21) | 1.9415 (182.71) * | |
| Lemna minor | 7 d | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Allium cepa | 21 d | 1.275 | 1.000 | 1.0000 | 0.0133 (1.25) | 0.0531 (5.00) | |
| Cyanide | Aliivibrio fischeri | 30 min | 1.803 | 1.079 | 0.9956 | 0.0059 (0.15) | 0.0214 (0.56) |
| Pseudomonas putida | 72 h | –0.056 | 0.776 | 0.9809 | 0.1982 (5.16) | 1.1808 (30.72) | |
| Chlorella sp. | 72 h | 0.0164 | 0.775 | 0.9797 | 0.1593 (4.14) | 0.9526 (24.79) | |
| Lemna minor | 7 d | 0.479 | 0.473 | 0.9234 | 0.0052 (0.14) * | 0.0972 (2.53) | |
| Allium cepa | 21 d | n.d. | n.d. | n.d. | <0.0384 (<1.00) | <0.0384 (<1.00) | |
| Thiocyanate | Aliivibrio fischeri | 30 min | 0.221 | 1.045 | 0.9928 | 0.1632 (9.48) | 0.6150 (35.72) |
| Pseudomonas putida | 72 h | –0.658 | 1.036 | 0.9441 | 1.1315 (65.72) | 4.3111 (250.39) * | |
| Chlorella sp. | 72 h | –0.376 | 1.301 | 0.9909 | 0.6705 (38.94) | 1.9458 (113.01) | |
| Lemna minor | 7 d | –0.641 | 0.710 | 0.9386 | 1.1338 (65.85) | 7.9801 (463.48) * | |
| Allium cepa | 21 d | 0.451 | 1.265 | 0.9456 | 0.1470 (8.54) | 0.4397 (25.54) | |
| Combination | Mixture Ratio | Intercept | Slope | R2 | EC50/mM |
|---|---|---|---|---|---|
| phenol and cyanide | 25:75 | 1.579 | 1.259 | 0.9953 | 0.0557 |
| 50:50 | 1.676 | 1.145 | 0.9938 | 0.0344 | |
| 75:25 | 1.742 | 1.117 | 0.9972 | 0.0275 | |
| phenol and thiocyanate | 25:75 | 0.745 | 1.376 | 0.9955 | 0.2875 |
| 50:50 | 0.602 | 1.213 | 0.9973 | 0.3188 | |
| 75:25 | 0.453 | 1.176 | 0.9947 | 0.4120 | |
| cyanide and thiocyanate | 25:75 | 1.744 | 1.132 | 0.9975 | 0.0288 |
| 50:50 | 1.481 | 1.059 | 0.9971 | 0.0400 | |
| 75:25 | 1.189 | 1.040 | 0.9953 | 0.0719 | |
| phenol and cyanide and thiocyanate | 25:25:50 | 1.972 | 1.763 | 0.9967 | 0.0619 |
| 25:50:25 | 2.173 | 1.521 | 0.9935 | 0.0151 | |
| 50:25:25 | 1.963 | 1.621 | 0.9963 | 0.0250 | |
| 33.3:33.3:33.3 | 1.811 | 1.330 | 0.9972 | 0.0236 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tutić, A.; Miloloža, M.; Cvetnić, M.; Ukić, Š.; Kučić Grgić, D. Phenol, Cyanide, and Thiocyanate in Aquatic Media: The Ecotoxicity of Individual Substances and Their Mixtures. Environments 2025, 12, 128. https://doi.org/10.3390/environments12040128
Tutić A, Miloloža M, Cvetnić M, Ukić Š, Kučić Grgić D. Phenol, Cyanide, and Thiocyanate in Aquatic Media: The Ecotoxicity of Individual Substances and Their Mixtures. Environments. 2025; 12(4):128. https://doi.org/10.3390/environments12040128
Chicago/Turabian StyleTutić, Ana, Martina Miloloža, Matija Cvetnić, Šime Ukić, and Dajana Kučić Grgić. 2025. "Phenol, Cyanide, and Thiocyanate in Aquatic Media: The Ecotoxicity of Individual Substances and Their Mixtures" Environments 12, no. 4: 128. https://doi.org/10.3390/environments12040128
APA StyleTutić, A., Miloloža, M., Cvetnić, M., Ukić, Š., & Kučić Grgić, D. (2025). Phenol, Cyanide, and Thiocyanate in Aquatic Media: The Ecotoxicity of Individual Substances and Their Mixtures. Environments, 12(4), 128. https://doi.org/10.3390/environments12040128









