Concentration Quantification of Oil Samples by Three-Dimensional Concentration-Emission Matrix (CEM) Spectroscopy
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Samples and Reagents
2.2. Apparatus
2.3. Weathering Experiments
3. Results and Discussion
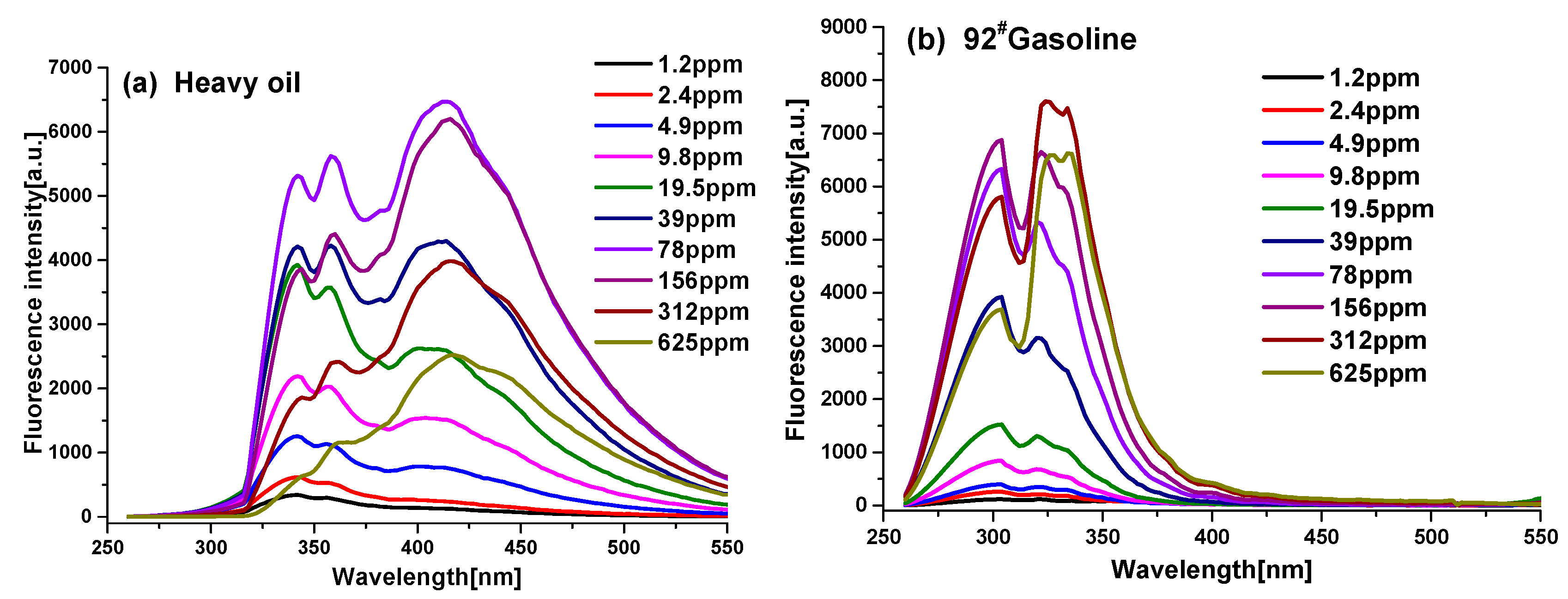
3.1. Fluorescence Spectral Properties of Oils at Different Concentrations
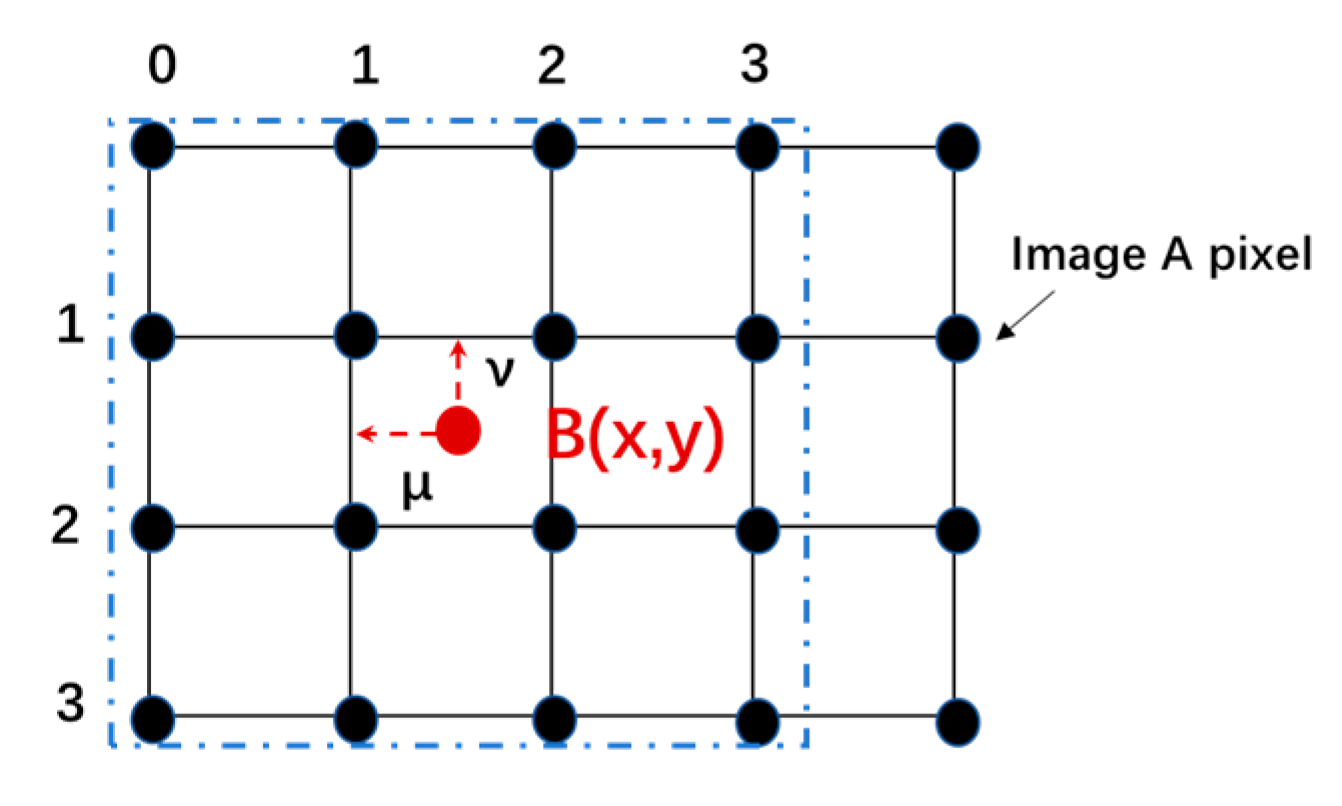
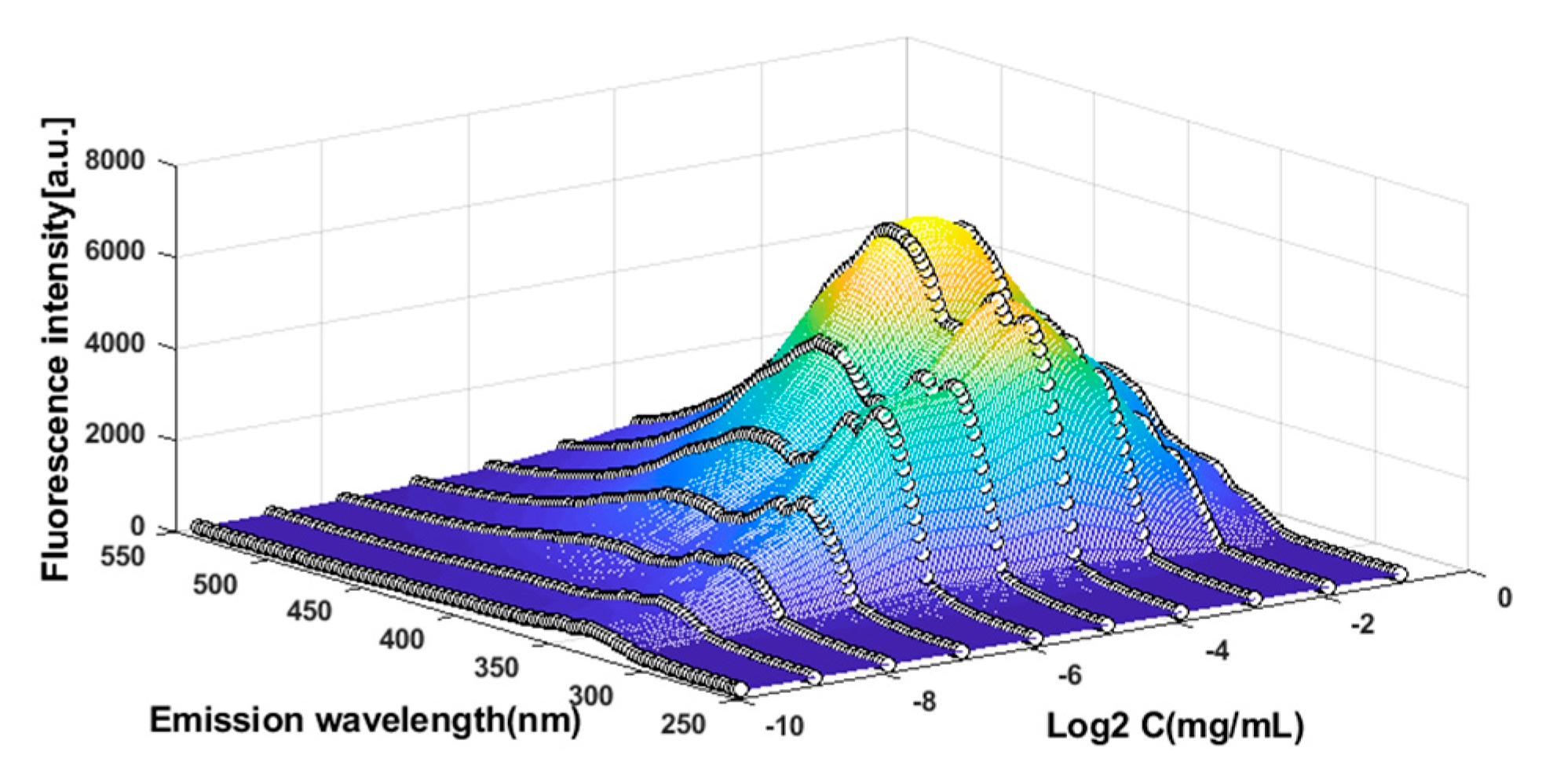
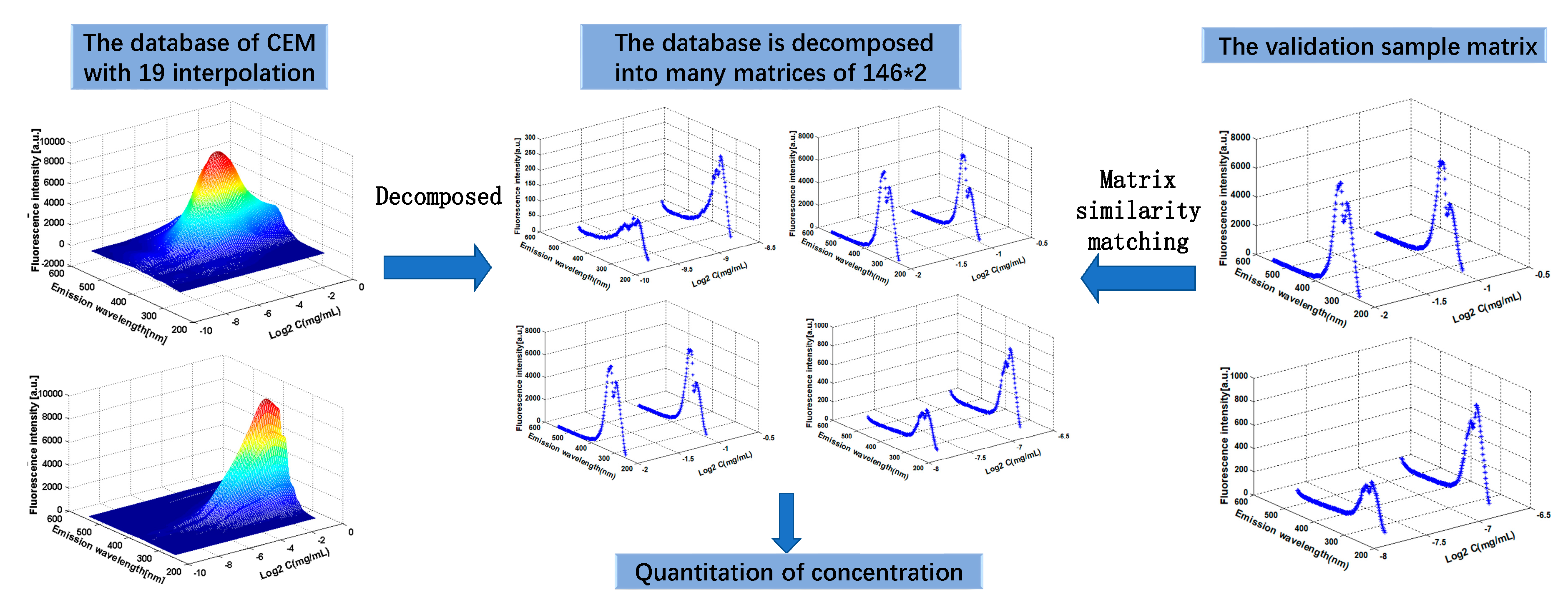
3.2. The Construction of CEM Spectra
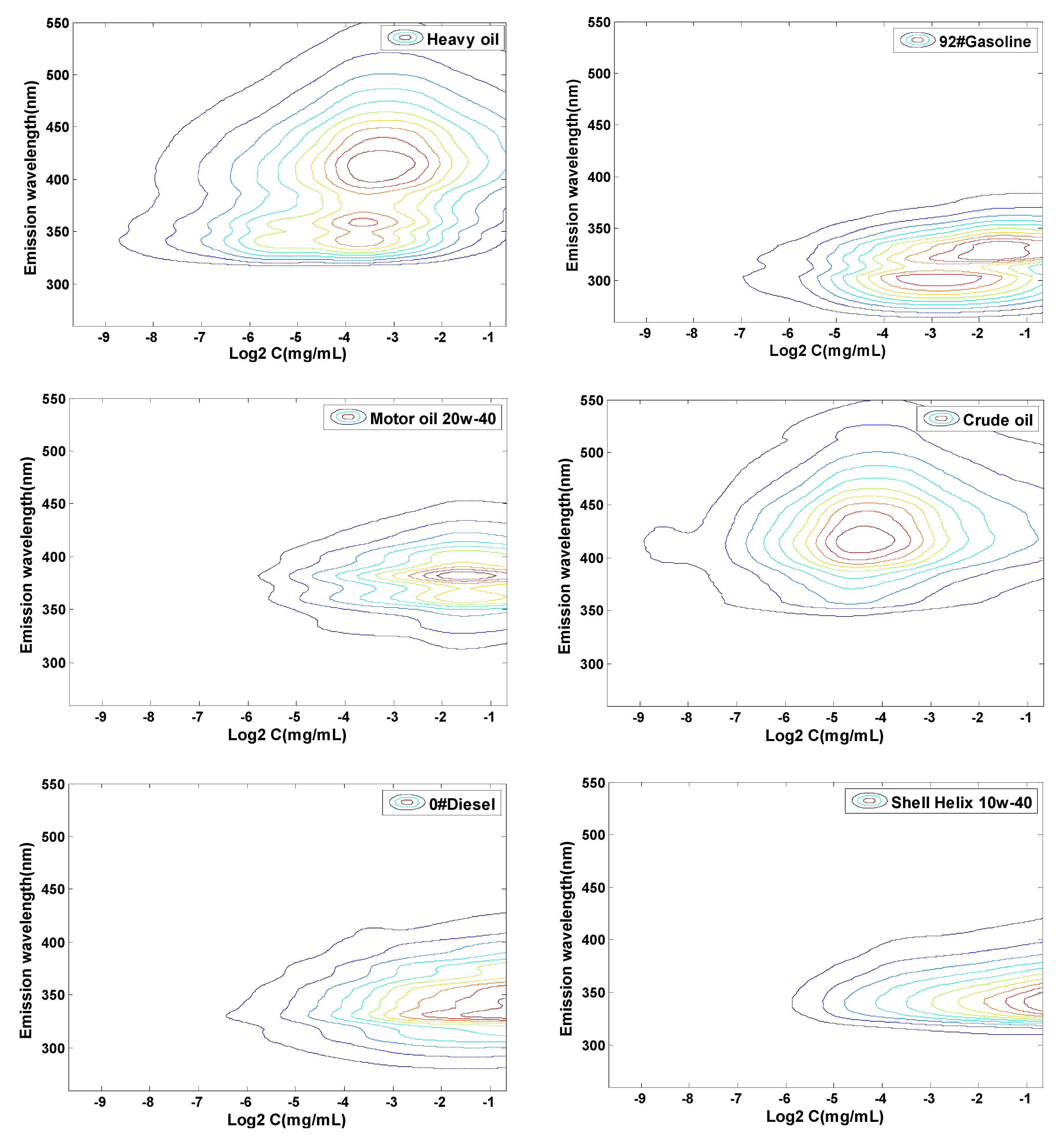
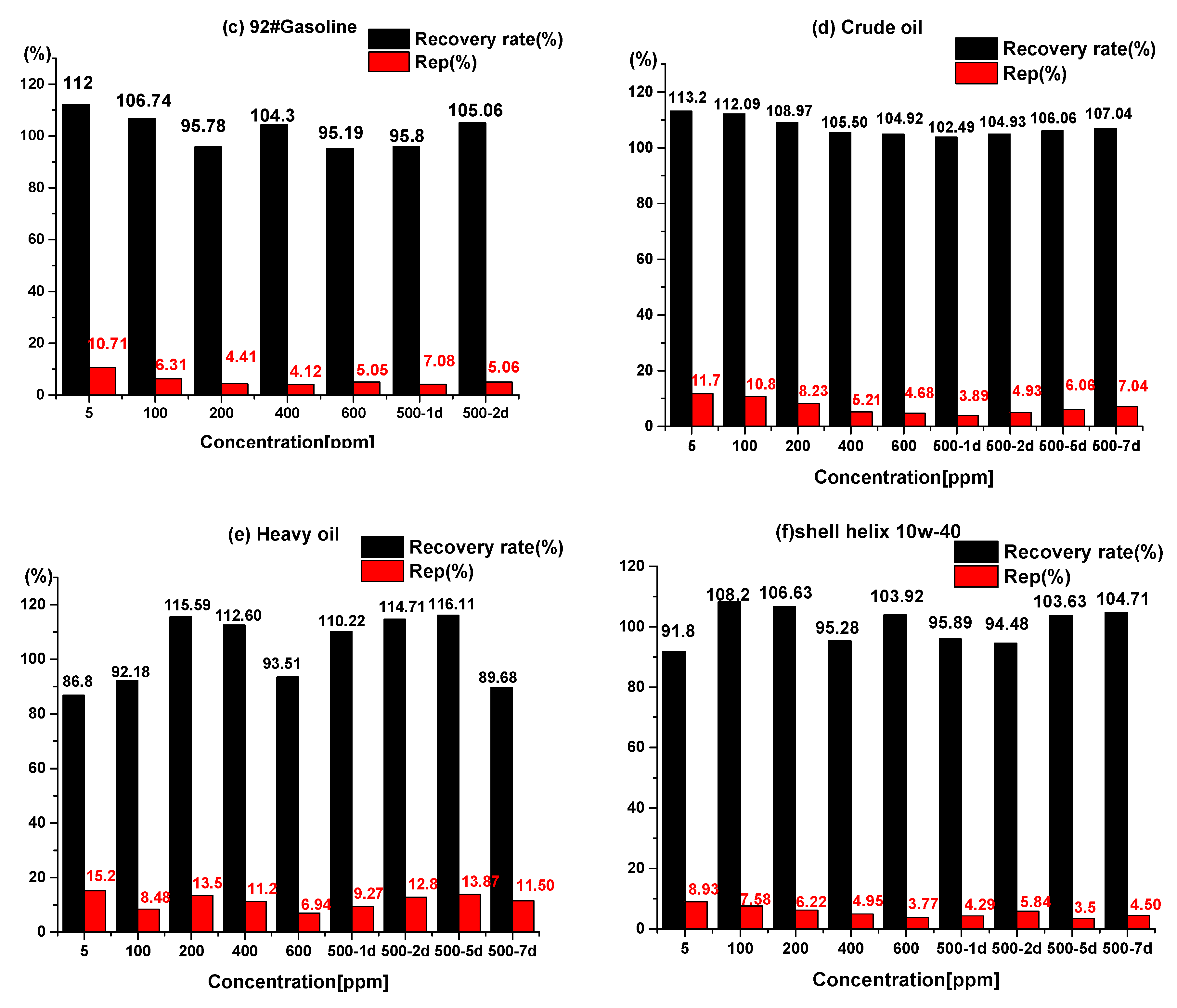
3.3. Quantitative Analysis of Concentration Based on CEM
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.; He, J.; Song, C. Oil Fingerprinting by Three-Dimensional (3D) Fluorescence Spectroscopy and Gas Chromatography–Mass Spectrometry (GC–MS). Environ. Forensics 2009, 10, 324–330. [Google Scholar] [CrossRef]
- Taki, M.; Asahara, T.; Mandai, Y.; Uno, T.; Nagai, M. Discriminatory Analysis of Crude Oils Using Biomarkers. Energy Fuels 2009, 23, 5003–5011. [Google Scholar] [CrossRef]
- Matysik, S.; Schmitz, G. Application of gas chromatography–triple quadrupole mass spectrometry to the determination of sterol components in biological samples in consideration of the ionization mode. Biochimie 2013, 95, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Shun, P.; Zhou, Q.; Guang, M.; Wang, X.; Zhao, Y.; Cao, L. Fingerprint analysis of polycyclic aromatic hydrocarbons in crude oil by internal standard method. J. Anal. Test. 2008, 27, 344–348. [Google Scholar]
- Snape, I.; Harvey, P.M.; Ferguson, S.H.; Rayner, J.L.; Revill, A.T. Investigation of evaporation and biodegradation of fuel spills in Antarctica I. A chemical approach using GC-FID. Chemosphere 2005, 61, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guo, G.; Yan, Z.; Lu, G.; Wang, Q.; Li, F. The development of a method for the qualitative and quantitative determination of petroleum hydrocarbon components using thin-layer chromatography with flame ionization detection. J. Chromatogr. A 2010, 1217, 368–374. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Luan, X.; Zhang, D.; Zhang, J.; Zheng, R. Identification of similar oil source oil spills based on concentration parameter synchronous fluorescence spectra. Spectrosc. Spectr. Anal. 2010, 30, 2700–2705. [Google Scholar]
- Zhu, L.; Zhang, Q.; An, W. Identification of oil spill by high concentration synchronous fluorescence method. Spectrosc. Spectr. Anal. 2011, 31, 737–741. [Google Scholar]
- Abbas, O.; Rébufa, C.; Dupuy, N.; Permanyer, A.; Kister, J.; Azevedo, D.A. Application of chemometric methods to synchronous UV fluorescence spectra of petroleum oils. Fuel 2006, 85, 2653–2661. [Google Scholar] [CrossRef]
- Zhou, Z.; Guo, L.; Shiller, A.M.; Lohrenz, S.E.; Asper, V.L.; Osburn, C.L. Characterization of oil components from the Deepwater Horizon oil spill in the Gulf of Mexico using fluorescence EEM and PARAFAC techniques. Mar. Chem. 2013, 148, 10–21. [Google Scholar] [CrossRef]
- Palombi, L.; Alderighi, D.; Cecchi, G.; Raimondi, V.; Toci, G.; Lognoli, D. A Fluorescence LIDAR Sensor for Hyperspectral Time-resolved remote sensing and mapping. Opt. Express 2013, 21, 14736. [Google Scholar] [CrossRef] [PubMed]
- Ryder, A.G. Quantitative Analysis of Crude Oils by Fluorescence Lifetime and Steady State Measurements Using 380-nm Excitation. Appl. Spectrosc. 2002, 56, 107–116. [Google Scholar] [CrossRef]
- Cui, Z.C.; Liu, W.Q.; Zhao, N.J.; Xiao, X.; Wang, Z.G.; Zhang, Y.J.; Sima, W.C.; Liu, J.G.; Wei, Q.N. Study on the Method for Rapid Determination of Oil Concentration in Water. Spectrosc. Spectr. Anal. 2008, 28, 1332–1335. [Google Scholar]
- He, L.M.; Kear-Padilla, L.L. Rapid in situ determination of total oil concentration in water using ultraviolet fluorescence and light scattering coupled with artificial neural networks. Anal. Chim. Acta 2003, 478, 245–258. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Z.; Guo, L. Chemical evolution of Macondo crude oil during laboratory degradation as characterized by fluorescence EEMs and hydrocarbon composition. Mar. Pollut. Bull. 2013, 66, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Patra, D. Distinguishing motor oils at higher concentration range by evaluating total fluorescence quantum yield as a novel sensing tool. Sens. Actuators B Chem. 2008, 129, 632–638. [Google Scholar] [CrossRef]
- Bahram, M.; Bro, R.; Stedmon, C.; Afkhami, A. Handling of Rayleigh and Raman scatter for PARAFAC modeling of fluorescence data using interpolation. J. Chem. 2006, 20, 99–105. [Google Scholar] [CrossRef]
- Keys, R.G. Cubic convolution interpolation for digital image processing. IEEE Trans. Acoust. Speech. Signal Process. 1981, 37, 1153–1160. [Google Scholar] [CrossRef] [Green Version]
- Zhai, H.L.; Li, B.Q.; Tian, Y.L.; Li, P.Z.; Zhang, X.Y. An application of wavelet moments to the similarity analysis of three-dimensional fingerprint spectra obtained by high-performance liquid chromatography coupled with diode array detector. Food Chem. 2014, 145, 625–631. [Google Scholar] [CrossRef]
- Escandar, G.M.; Bystol, A.J.; Campiglia, A.D. Spectrofluorimetric method for the determination of piroxicam and pyridoxine. Anal. Chim. Acta 2002, 466, 275–283. [Google Scholar] [CrossRef]
- Alarcón, F.; Báez, M.E.; Bravo, M.; Richter, P.; Escandar, G.M.; Olivieri, A.C.; Fuentes, E. Feasibility of the determination of polycyclic aromatic hydrocarbons in edible oils via unfolded partial least-squares/residual bilinearization and parallel factor analysis of fluorescence excitation emission matrices. Talanta 2013, 103, 361–370. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yang, R.; Zhao, N.; Zhu, W.; Huang, Y.; Zhang, R.; Chen, X.; Liu, J.; Liu, W.; Zuo, Z. Concentration Quantification of Oil Samples by Three-Dimensional Concentration-Emission Matrix (CEM) Spectroscopy. Appl. Sci. 2020, 10, 315. https://doi.org/10.3390/app10010315
Chen Y, Yang R, Zhao N, Zhu W, Huang Y, Zhang R, Chen X, Liu J, Liu W, Zuo Z. Concentration Quantification of Oil Samples by Three-Dimensional Concentration-Emission Matrix (CEM) Spectroscopy. Applied Sciences. 2020; 10(1):315. https://doi.org/10.3390/app10010315
Chicago/Turabian StyleChen, Yunan, Ruifang Yang, Nanjing Zhao, Wei Zhu, Yao Huang, Ruiqi Zhang, Xiaowei Chen, Jianguo Liu, Wenqing Liu, and Zhaolu Zuo. 2020. "Concentration Quantification of Oil Samples by Three-Dimensional Concentration-Emission Matrix (CEM) Spectroscopy" Applied Sciences 10, no. 1: 315. https://doi.org/10.3390/app10010315





