Cell Stress Reduction by a Novel Perfusion-Culture System Using Commercial Culture Dish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
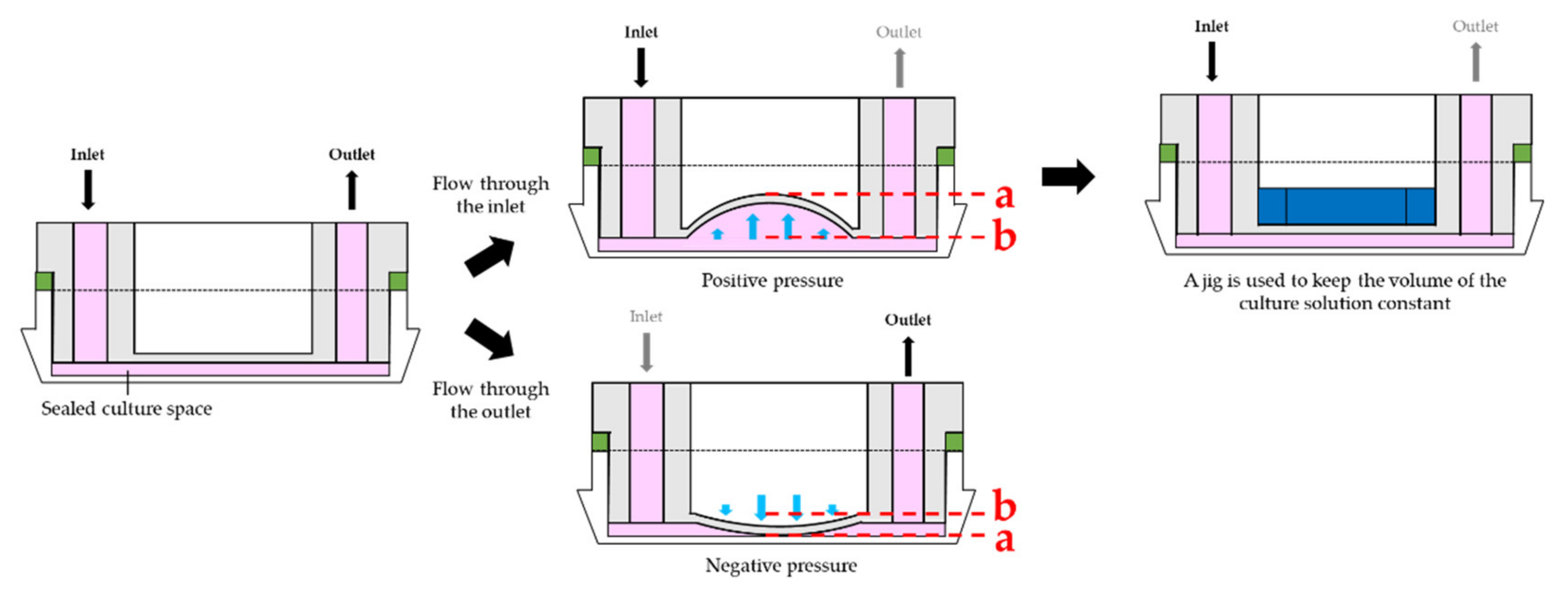
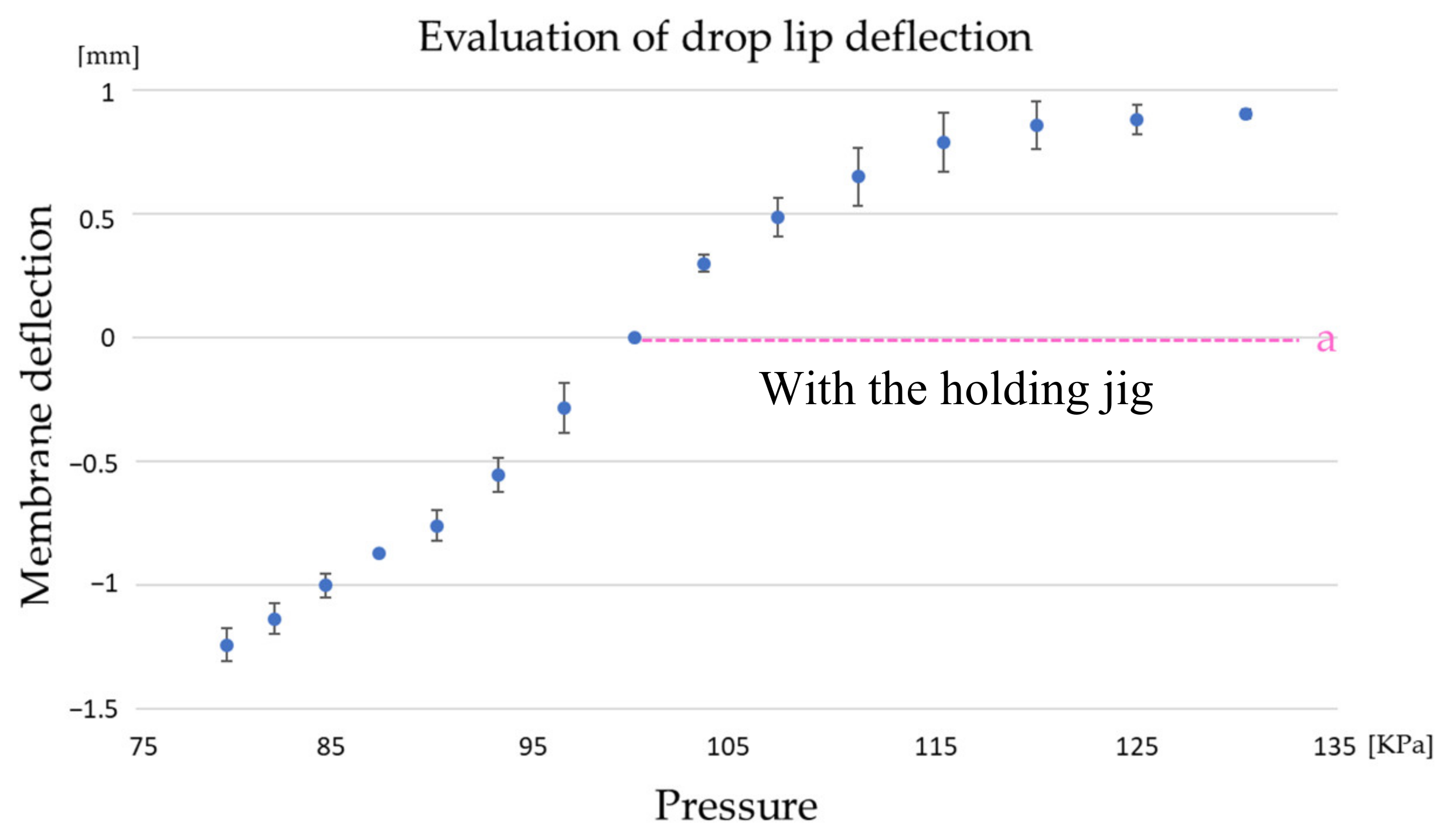
2.2. Study of Culture Media Pumping Methods and Evaluation of CD-Adapter Deflection
2.3. Evaluation of The Stress Marker DDIT3 Expression in Perfusion Culture and Manual Medium Change
3. Results and Discussion
3.1. Analysis of Culture Medium Pumping Methods and Evaluation of CD-Adapter Deflection
3.2. Evaluation of the Expression of the Stress Marker DDIT3 in Perfusion Culture and Manual Medium Change
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Unchern, S. Basic techniques in animal cell culture. In Proceedings of the Drug Delivery System Workshop, Bangkok, Thailand, 19–20 August 1999; pp. 1–30. [Google Scholar]
- Ciraldo, F.E.; Boccardi, E.; Melli, V.; Westhauser, F.; Boccaccini, A.R. Tackling bioactive glass excessive in vitro bioreactivity: Preconditioning approaches for cell culture tests. Acta Biomater. 2018, 75, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Diaz, M.F.; Price, K.M.; Ozuna, J.A.; Zhang, S.; Sevick-Muraca, E.M.; Hagan, J.P.; Wenzel, P.L. Fluid shear stress activates YAP1 to promote cancer cell motility. Nat. Commun. 2017, 8, 14122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.K.; Kim, S.H.; Lee, G.M. Effect of low culture temperature on specific productivity and transcription level of anti-4-1BB antibody in recombinant Chinese hamster ovary cells. Biotechnol. Prog. 2003, 19, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Toh, Y.-C.; Voldman, J. Fluid shear stress primes mouse embryonic stem cells for differentiation in a self-renewing environment via heparan sulfate proteoglycans transduction. FASEB J. 2011, 25, 1208–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, A.; Ng, R.; Yang, S.-T. Long-Term Culturing of Undifferentiated Embryonic Stem Cells in Conditioned Media and Three-Dimensional Fibrous Matrices without Extracellular Matrix Coating. Stem Cells 2007, 25, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Libertini, G. Phylogeny of aging and related phenoptotic phenomena. Biochemistry 2015, 80, 1529–1546. [Google Scholar] [CrossRef] [PubMed]
- Sukho, P.; Cohen, A.; Hesselink, J.W.; Kirpensteijn, J.; Verseijden, F.; Bastiaansen-Jenniskens, Y.M. Adipose tissue-derived stem cell sheet application for tissue healing in vivo: A systematic review. Tissue Eng. Part B Rev. 2017, 24, 35–72. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Mercer, J.; Dutina, G.; Donahue, C.J.; Bauer, K.D.; Mather, J.P.; Etcheverry, T.; Ryll, T. Effects of temperature shift on cell cycle, apoptosis and nucleotide pools in CHO cell batch cultues. Cytotechnology 1997, 23, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.M.; Russell, J.B. Effect of ionophores and pH on growth of Streptococcus bovis in batch and continuous culture. Appl. Environ. Microbiol. 1990, 56, 1588–1593. [Google Scholar] [PubMed]
- Yu, H.; Meyvantsson, I.; Shkel, I.A.; Beebe, D.J. Diffusion dependent cell behavior in microenvironments. Lab Chip 2005, 5, 1089. [Google Scholar] [CrossRef] [PubMed]
- Mogi, K.; Kanetaka, N.; Sugii, Y.; Hishida, K. Measurement of concentration distribution in endothelial surface layer using super resolution LIF technique. Trans. JSME 2015, 82, 15-00404. [Google Scholar]
- Kondo, E.; Wada, K.-I.; Hosokawa, K.; Maeda, M. Microfluidic perfusion cell culture system confined in 35 mm culture dish for standard biological laboratories. J. Biosci. Bioeng. 2014, 118, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-B.; Chou, D.; Chang, Y.-H.; Li, K.-C.; Chiu, T.-K.; Ventikos, Y.; Wu, M.-H. Development of a pneumatically driven active cover lid for multi-well microplates for use in perfusion three-dimensional cell culture. Sci. Rep. 2015, 5, 18352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saalfrank, D.; Konduri, A.K.; Latifi, S.; Habibey, R.; Golabchi, A.; Martiniuc, A.V.; Knoll, A.; Ingebrandt, S.; Blau, A. Incubator-independent cell-culture perfusion platform for continuous long-term microelectrode array electrophysiology and time-lapse imaging. R. Soc. Open Sci. 2015, 2, 150031. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, R.; Adachi, S.; Okonogi, A.; Anzai, Y.; Kamiyama, T.; Katano, K.; Hoshi, N.; Natsume, T.; Mogi, K. Fabrication of a Novel Culture Dish Adapter with a Small Recess Structure for Flow Control in a Closed Environment. Appl. Sci. 2019, 9, 269. [Google Scholar] [CrossRef] [Green Version]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vihervaara, A.; Duarte, F.M.; Lis, J.T. Molecular mechanisms driving transcriptional stress responses. Nat. Rev. Genet. 2018, 19, 385–397. [Google Scholar] [CrossRef] [PubMed]




| Gene | Primer-Forward | Primer-Reverse |
|---|---|---|
| β-Actin | 5′-TGGATCAGCAAGCAGGAGTATG-3′ | 5′-GCATTTGCGGTGGACGAT-3′ |
| DDIT3 | 5′-TCAGAGCTGGAACCTGAGGA-3′ | 5′-CTGCCATCTCTGCAGTTGGA-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuda, R.; Adachi, S.; Okonogi, A.; Anzai, Y.; Kamiyama, T.; Katano, K.; Hoshi, N.; Mogi, K.; Natsume, T. Cell Stress Reduction by a Novel Perfusion-Culture System Using Commercial Culture Dish. Appl. Sci. 2020, 10, 95. https://doi.org/10.3390/app10010095
Yasuda R, Adachi S, Okonogi A, Anzai Y, Kamiyama T, Katano K, Hoshi N, Mogi K, Natsume T. Cell Stress Reduction by a Novel Perfusion-Culture System Using Commercial Culture Dish. Applied Sciences. 2020; 10(1):95. https://doi.org/10.3390/app10010095
Chicago/Turabian StyleYasuda, Reiko, Shungo Adachi, Atsuhito Okonogi, Yohei Anzai, Tadataka Kamiyama, Keiji Katano, Nobuhiko Hoshi, Katsuo Mogi, and Tohru Natsume. 2020. "Cell Stress Reduction by a Novel Perfusion-Culture System Using Commercial Culture Dish" Applied Sciences 10, no. 1: 95. https://doi.org/10.3390/app10010095





