Simultaneous Quantification of Eight Marker Components in Traditional Herbal Formula, Haepyoyijin-Tang Using HPLC–PDA
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Reagents
2.3. Preparation of HPYJT Water Decoction
2.4. Preparations of Test Solution and Stock Solution
2.5. HPLC–PDA Instruments and Analysis Conditions for the Phtochemical Determination
2.6. Validation of Analytical Procedure
3. Results and discussion
3.1. Optimizing Conditions for HPLC Analysis
3.2. Validation of the Analytical Procedure
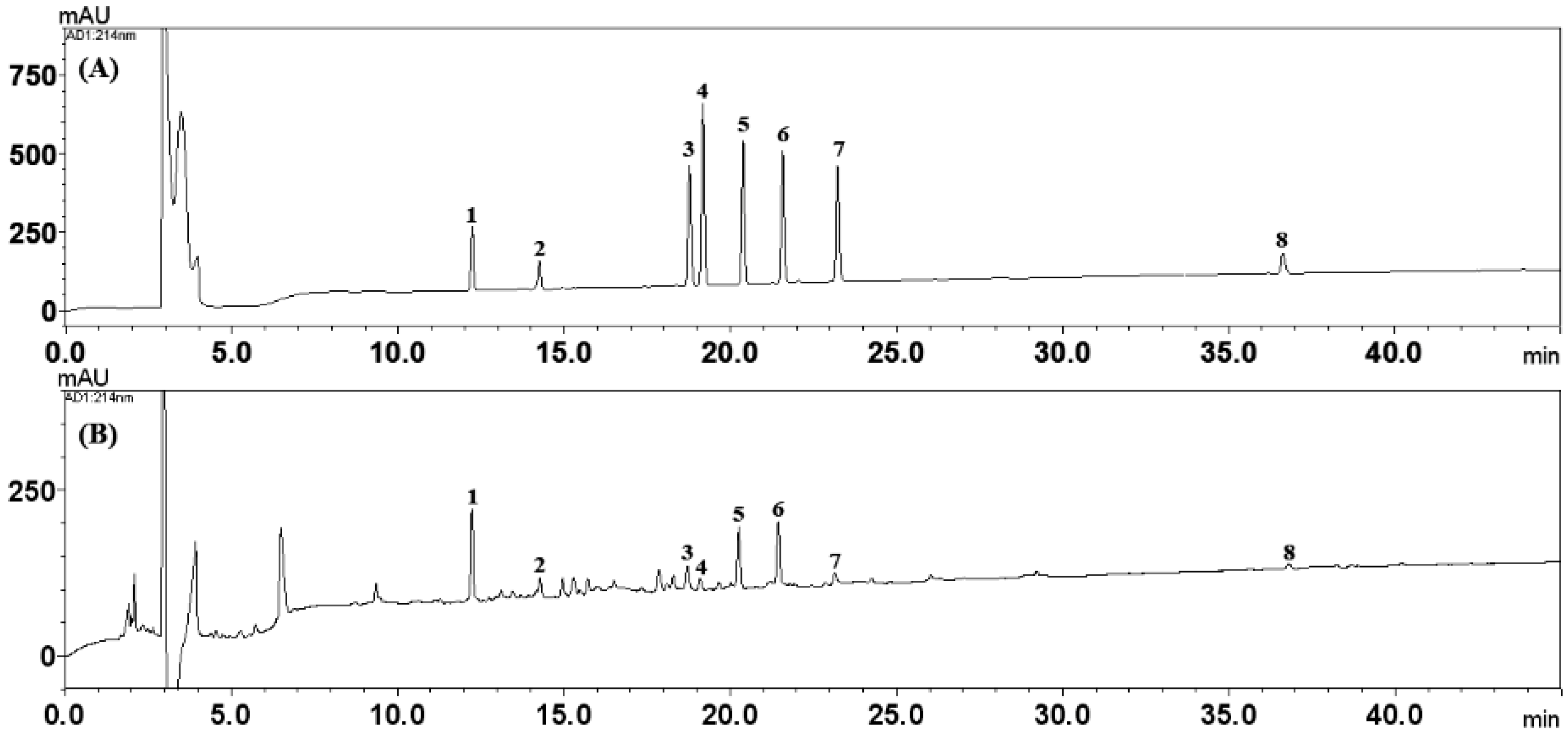
3.3. Simultaneous Determination of the Eight Marker Analytes in HPYJT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Heo, J. Dongui Bogam; Namsandang: Seoul, Korea, 2007; p. 481. [Google Scholar]
- Lee, K.T.; Park, D.I. The effects of Haepyoy jintang on the pulmonary injury caused by SO2 in rats. J. Korean Med. 1996, 17, 178–190. [Google Scholar]
- Suk, Y.H.; Min, S.Y.; Kim, J.H. Effect of Haepyo Jin-tang on Airway Mucin Secretion, Production, Gene Expression and Hypersecretion of Mucus. J. Korean Pediatr. Med. 2015, 29, 65–79. [Google Scholar] [CrossRef][Green Version]
- Han, J.H.; Jo, S.G.; Lee, M.J.; Baek, S.H.; Park, S.H. Contents of homogentisic acid and 3,4-dihydroxybenzaldehyde in the Pinellia ternate by various processing method and its safety estimate. J. Physiol. Pathol. Korean Med. 2004, 18, 846–853. [Google Scholar]
- Zhao, B.T.; Kim, E.J.; Son, K.H.; Son, J.K.; Min, B.S.; Woo, M.H. Quality evaluation and pattern recognition analyses of marker compounds from five medicinal drugs of Rutaceae family by HPLC/PDA. Arch. Pharm. Res. 2015, 38, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xu, M.-L.; Lee, C.-S.; Woo, M.-H.; Chang, H.-W.; Son, J.K. Cytotoxicity and DNA topoisomerases inhibitory activity of constituents from the sclerotium of Poria cocos. Arch. Pharm. Res. 2004, 27, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ye, M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J. Chromatogr. 2009, 1216, 1954–1969. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.T.; Lee, K.R.; Lee, J.H.; Min, B.S.; Son, J.K.; Woo, M.H. Quality evaluation of Perillae Folium by HPLC/PDA. Arch. Pharm. Res. 2015, 38, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Okamura, N.; Miki, H.; Harada, T.; Yamashita, S.; Masaoka, Y.; Nakamoto, Y.; Tsuguma, M.; Yoshitomi, H.; Yagi, A. Simultaneous determination of ephedrine, pseudoephedrine, norephedrine and methylephedrine in Kampo medicines by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 1999, 20, 363–372. [Google Scholar] [CrossRef]
- Koo, J.-Y.; Hwang, E.-Y.; Cho, S.; Lee, J.-H.; Lee, Y.-M.; Hong, S.-P. Quantitative determination of amygdalin epimers from armeniacae semen by liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 814, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Shin, M.C.; Cho, C.W.; Lee, W.J.; Woo, E.R.; Kang, J.S. Specification and Analysis of Multiple Marker Compounds for Quality Control of Mori Cortex Radicis by HPLC. Bull. Korean Chem. Soc. 2015, 36, 117–122. [Google Scholar] [CrossRef]
- Chung, K.-O.; Kim, B.-Y.; Lee, M.-H.; Kim, Y.-R.; Chung, H.-Y.; Park, J.-H.; Moon, J.-O. In-vitro and in-vivo anti-inflammatory effect of oxyresveratrol from Morus alba L. J. Pharm. Pharmacol. 2003, 55, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shi, H.; Zhang, Q.; Liu, Y.; Wan, C.; Zhang, L. Simultaneous Determination of Five Components in Aster tataricus by Ultra Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. Sci. 2015, 54, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.X.; Hu, B.Q.; Zhang, M.; Zhang, C.F.; Xu, X.H. Simultaneous separation and determination of phenolic acids, pentapeptides, and triterpenoid saponins in the root of Aster tataricus by high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2015, 38, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2015; p. 292.
- Ha, Y.W.; Kim, Y.S. Preparative isolation of six major saponins from Platycodi Radix by high-speed counter-current chromatography. Phytochem. Anal. 2009, 20, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, C.-Y.; Wen, X.; Qi, L.-W.; Qi, L.-W. Simultaneous determination of 6-gingerol, 8-gingerol, 10-gingerol and 6-shogaol in rat plasma by liquid chromatography–mass spectrometry: Application to pharmacokinetics. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H. The Dispensatory on the Visual and Organoleptic Examination of Herbal Medicine; National Institute of Food and Drug Safety Evaluation: Seoul, Korea, 2013; pp. 24–696. [Google Scholar]
- Seo, C.-S.; Shin, H.-K. Quality assessment of traditional herbal formula, Hyeonggaeyeongyo-tang through simultaneous determination of twenty marker components by HPLC-PDA and LC-MS/MS. Saudi Pharm. J. 2020, 28, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.-S.; Lee, M.-Y. HPLC–PDA and LC–MS/MS Analysis for the Simultaneous Quantification of the 14 Marker Components in Sojadodamgangki-Tang. Appl. Sci. 2020, 10, 2804. [Google Scholar] [CrossRef]
- International Conference on Harmonisation. Guidance for Industry, Q2B, Validation of Analytical Procedures: Methodology; Food and Drug Administration: Rockville, MD, USA, 1996. [Google Scholar]
| Analyte | Linear Range (μg/mL) | Regression Equation a y = ax + b | r2 | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| MULA | 1.56–100.00 | y = 26254.19x + 7269.63 | 0.9998 | 0.35 | 1.06 |
| AMY | 1.56–100.00 | y = 6226.21x + 3363.42 | 0.9996 | 0.09 | 0.27 |
| LIQA | 1.56–100.00 | y = 14121.49x + 5547.89 | 0.9999 | 0.24 | 0.72 |
| LIQ | 0.78–50.00 | y = 18895.76x + 3098.35 | 0.9999 | 0.14 | 0.42 |
| NAR | 1.56–100.00 | y = 16761.20x + 6157.72 | 0.9999 | 0.48 | 1.46 |
| HES | 1.56–100.00 | y = 15716.44x + 6639.20 | 0.9999 | 0.31 | 0.95 |
| RA | 0.78–50.00 | y = 20714.33x + 830.82 | 1.0000 | 0.11 | 0.33 |
| GA | 1.56–100.00 | y = 8011.17x + 2326.31 | 0.9999 | 0.24 | 0.72 |
| Analyte | Spiked Conc. (μg/mL) | Found Conc. (μg/mL) | Recovery (%) | SD | RSD (%) |
|---|---|---|---|---|---|
| MULA | 6.00 | 5.92 | 98.68 | 1.21 | 1.22 |
| 15.00 | 15.08 | 100.52 | 1.50 | 1.50 | |
| 30.00 | 31.05 | 103.50 | 1.10 | 1.07 | |
| AMY | 6.00 | 5.88 | 97.99 | 2.49 | 2.54 |
| 15.00 | 14.99 | 99.94 | 2.35 | 2.36 | |
| 30.00 | 30.18 | 100.61 | 1.81 | 1.80 | |
| LIQA | 4.00 | 4.02 | 100.54 | 0.95 | 0.95 |
| 10.00 | 9.81 | 98.11 | 1.54 | 1.56 | |
| 20.00 | 20.45 | 102.27 | 0.71 | 0.69 | |
| LIQ | 2.00 | 1.97 | 98.39 | 2.14 | 2.17 |
| 5.00 | 4.90 | 97.98 | 1.01 | 1.03 | |
| 10.00 | 10.00 | 100.05 | 0.62 | 0.62 | |
| NAR | 4.00 | 3.97 | 99.25 | 2.57 | 2.59 |
| 10.00 | 9.72 | 97.18 | 1.08 | 1.11 | |
| 20.00 | 19.91 | 99.56 | 1.16 | 1.16 | |
| HES | 4.00 | 3.98 | 99.54 | 2.52 | 2.53 |
| 10.00 | 10.09 | 100.94 | 1.94 | 1.92 | |
| 20.00 | 19.84 | 99.22 | 1.33 | 1.34 | |
| RA | 2.00 | 2.01 | 100.30 | 1.38 | 1.37 |
| 5.00 | 4.93 | 98.66 | 1.12 | 1.13 | |
| 10.00 | 10.21 | 102.05 | 1.00 | 0.98 | |
| GA | 4.00 | 4.01 | 100.26 | 0.54 | 0.54 |
| 10.00 | 9.80 | 98.01 | 1.62 | 1.65 | |
| 20.00 | 20.65 | 103.26 | 1.11 | 1.07 |
| Analyte | Conc. (μg/mL) | Intraday (n = 5) | Interday (n = 5) | Repeatability (n = 6) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Measured Conc. (μg/mL) | Precision (%) | Accuracy (%) | Measured Conc. (μg/mL) | Precision (%) | Accuracy (%) | RSD (%) of Retention Time | RSD (%) of Peak Area | ||
| MULA | 25.00 | 25.25 | 0.30 | 101.02 | 25.17 | 0.94 | 100.69 | 0.11 | 0.51 |
| 50.00 | 50.79 | 0.75 | 101.57 | 51.72 | 2.94 | 103.44 | |||
| 100.00 | 101.10 | 0.64 | 101.10 | 102.38 | 1.18 | 102.38 | |||
| AMY | 25.00 | 24.93 | 0.98 | 99.71 | 24.88 | 1.01 | 99.50 | 0.04 | 0.71 |
| 50.00 | 50.22 | 0.39 | 100.43 | 50.60 | 1.24 | 101.20 | |||
| 100.00 | 100.53 | 1.42 | 100.53 | 100.55 | 1.32 | 100.55 | |||
| LIQA | 25.00 | 25.46 | 0.39 | 101.84 | 25.36 | 0.77 | 101.46 | 0.02 | 0.46 |
| 50.00 | 50.87 | 0.62 | 101.74 | 51.80 | 2.41 | 103.60 | |||
| 100.00 | 101.23 | 0.75 | 101.23 | 102.30 | 1.14 | 102.30 | |||
| LIQ | 12.50 | 12.69 | 0.83 | 101.53 | 12.61 | 0.83 | 100.92 | 0.02 | 0.50 |
| 25.00 | 25.35 | 0.93 | 101.41 | 25.88 | 2.83 | 103.52 | |||
| 50.00 | 50.34 | 0.60 | 100.69 | 50.82 | 1.01 | 101.63 | |||
| NAR | 25.00 | 25.39 | 0.49 | 101.57 | 25.30 | 0.69 | 101.20 | 0.04 | 0.45 |
| 50.00 | 50.69 | 0.39 | 101.38 | 51.11 | 1.09 | 102.22 | |||
| 100.00 | 101.00 | 0.28 | 101.00 | 101.93 | 0.91 | 101.93 | |||
| HES | 25.00 | 25.44 | 0.35 | 101.76 | 25.35 | 0.66 | 101.39 | 0.05 | 0.47 |
| 50.00 | 50.85 | 0.42 | 101.70 | 51.16 | 0.97 | 102.31 | |||
| 100.00 | 101.06 | 0.58 | 101.06 | 102.08 | 1.03 | 102.08 | |||
| RA | 12.50 | 12.38 | 0.34 | 99.02 | 12.24 | 1.07 | 97.94 | 0.05 | 0.47 |
| 25.00 | 24.88 | 0.75 | 99.51 | 25.10 | 1.85 | 100.38 | |||
| 50.00 | 49.98 | 0.62 | 99.96 | 50.09 | 0.58 | 100.18 | |||
| GA | 25.00 | 25.24 | 0.17 | 100.98 | 25.12 | 0.69 | 100.48 | 0.01 | 0.47 |
| 50.00 | 50.41 | 0.36 | 100.83 | 51.25 | 2.55 | 102.51 | |||
| 100.00 | 100.38 | 0.55 | 100.38 | 100.46 | 0.71 | 100.46 | |||
| Compound | Content (mg/g) | |
|---|---|---|
| Mean ± SD | RSD (%) | |
| MULA | 2.83 ± 0.06 | 2.06 |
| AMY | 2.77 ± 0.02 | 0.71 |
| LIQA | 1.49 ± 0.02 | 1.68 |
| LIQ | 0.44 ± 0.01 | 2.35 |
| NAR | 2.20 ± 0.03 | 1.49 |
| HES | 2.54 ± 0.06 | 2.33 |
| RA | 0.43 ± 0.01 | 2.26 |
| GA | 2.39 ± 0.04 | 1.61 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, C.-S.; Lee, M.-Y. Simultaneous Quantification of Eight Marker Components in Traditional Herbal Formula, Haepyoyijin-Tang Using HPLC–PDA. Appl. Sci. 2020, 10, 3888. https://doi.org/10.3390/app10113888
Seo C-S, Lee M-Y. Simultaneous Quantification of Eight Marker Components in Traditional Herbal Formula, Haepyoyijin-Tang Using HPLC–PDA. Applied Sciences. 2020; 10(11):3888. https://doi.org/10.3390/app10113888
Chicago/Turabian StyleSeo, Chang-Seob, and Mee-Young Lee. 2020. "Simultaneous Quantification of Eight Marker Components in Traditional Herbal Formula, Haepyoyijin-Tang Using HPLC–PDA" Applied Sciences 10, no. 11: 3888. https://doi.org/10.3390/app10113888
APA StyleSeo, C.-S., & Lee, M.-Y. (2020). Simultaneous Quantification of Eight Marker Components in Traditional Herbal Formula, Haepyoyijin-Tang Using HPLC–PDA. Applied Sciences, 10(11), 3888. https://doi.org/10.3390/app10113888





