Patient-Specific 3-Dimensional Printing Titanium Implant Biomechanical Evaluation for Complex Distal Femoral Open Fracture Reconstruction with Segmental Large Bone Defect: A Nonlinear Finite Element Analysis
Abstract
1. Introduction
2. Materials and Methods
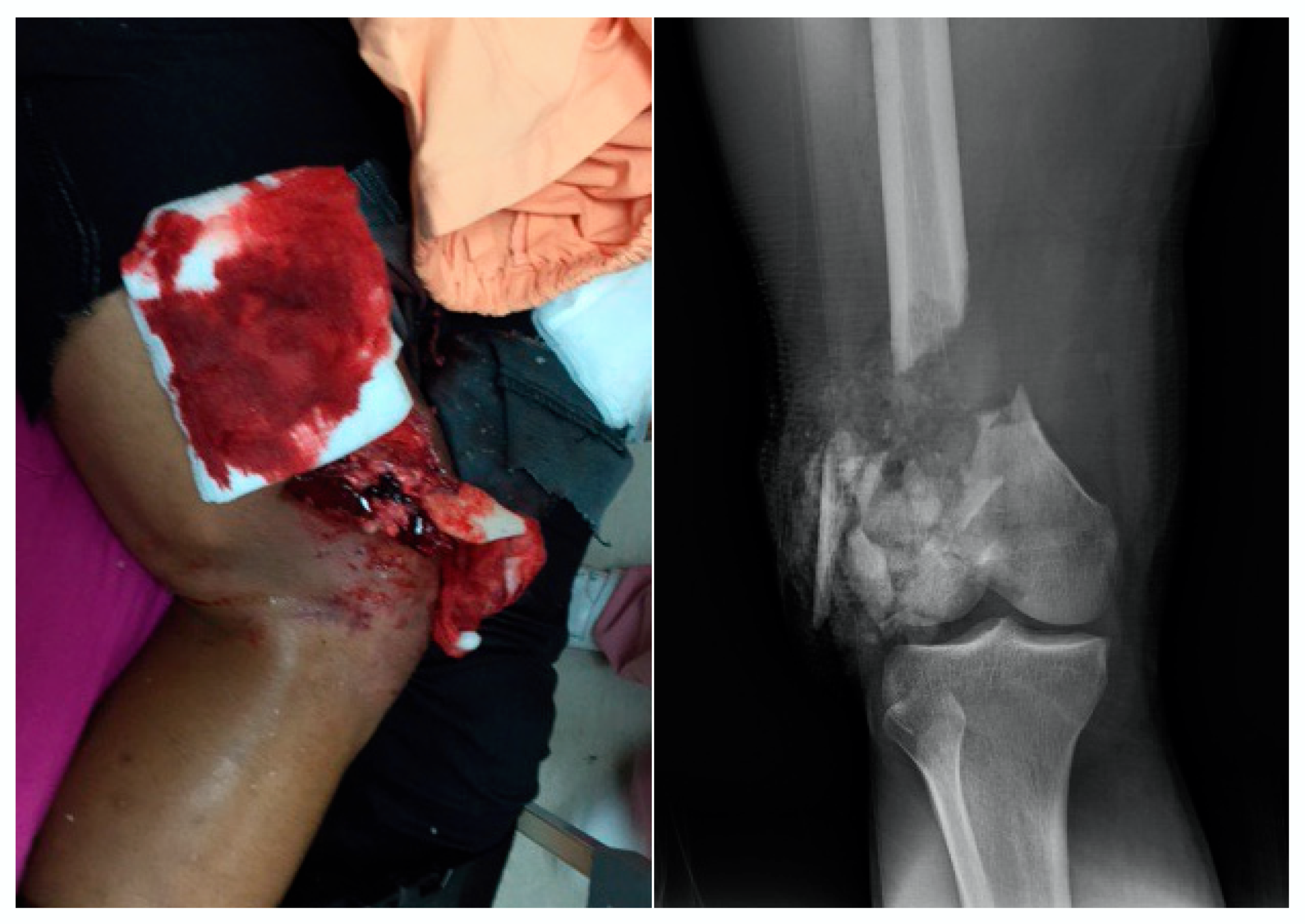
2.1. Case Report
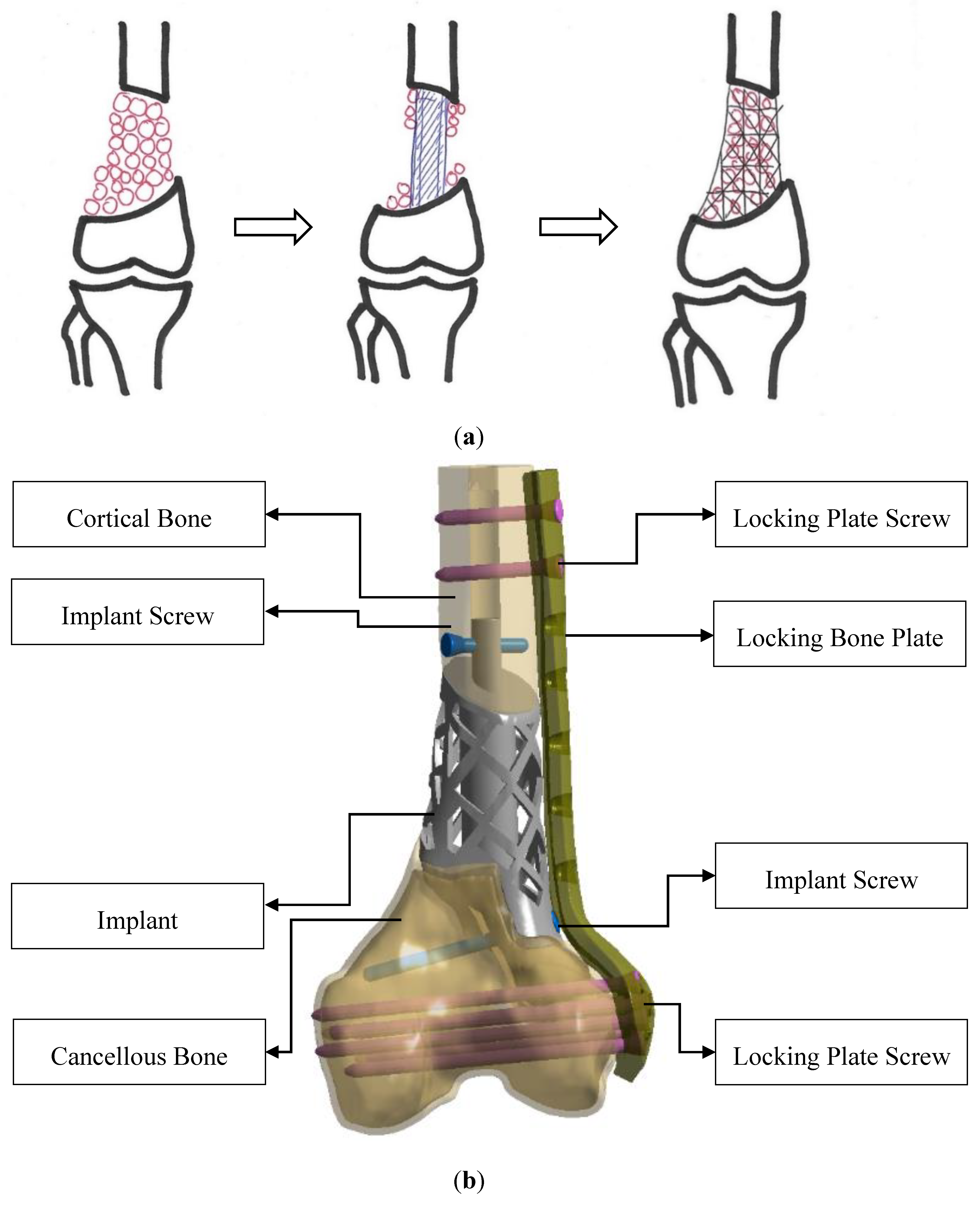
2.2. Model Design Concept and Construction
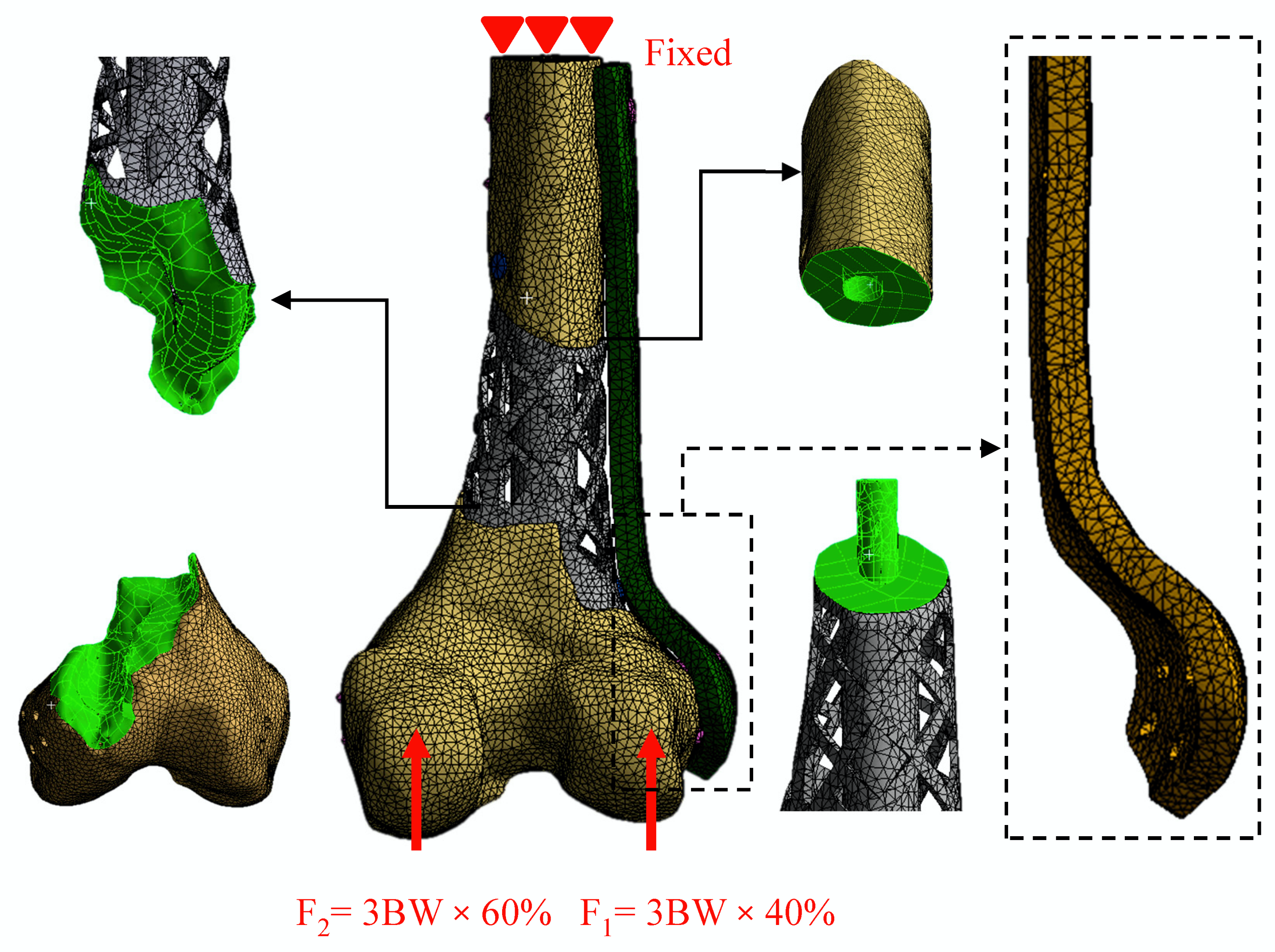
2.3. Non-Linear Finite Element Analysis
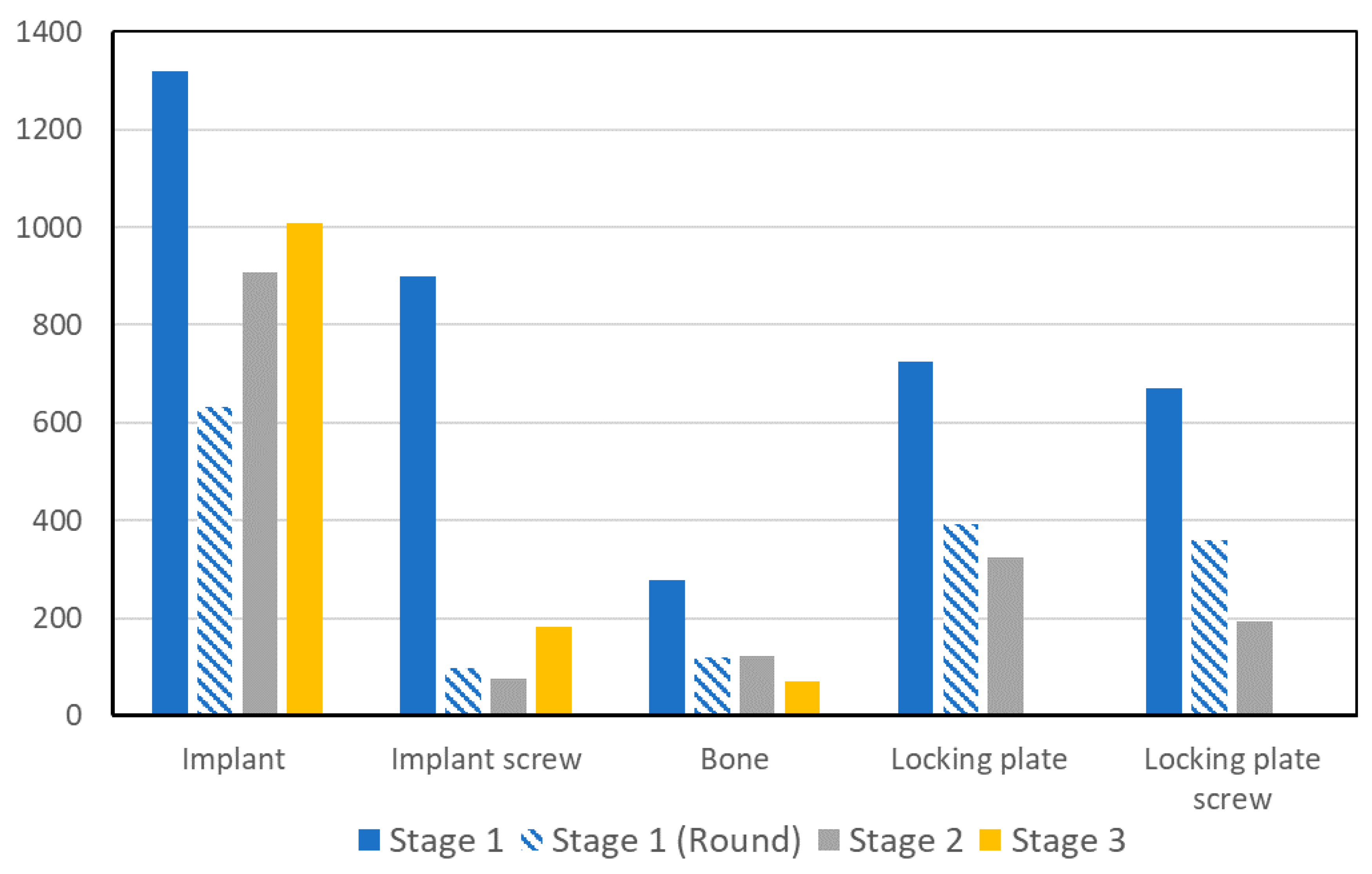
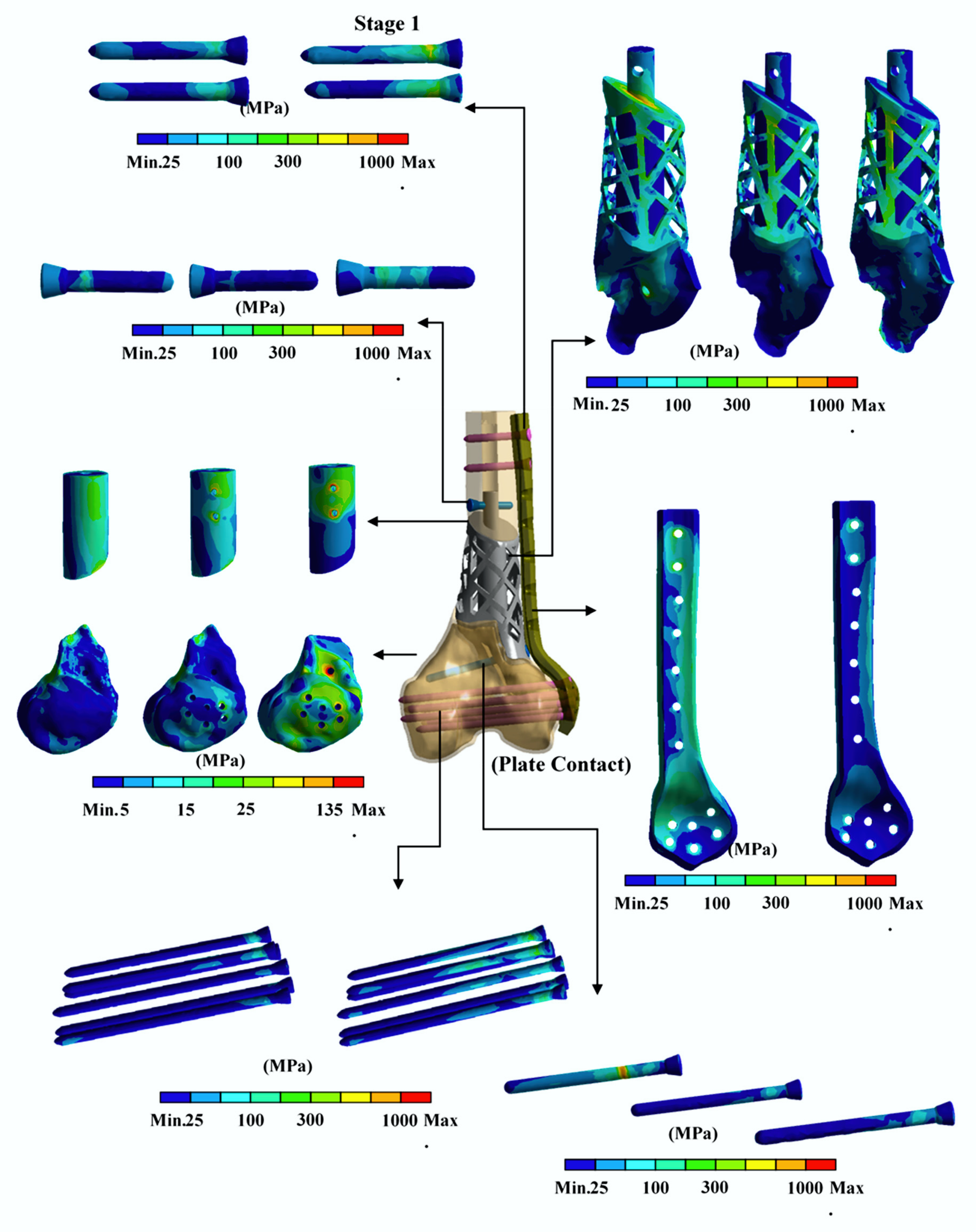
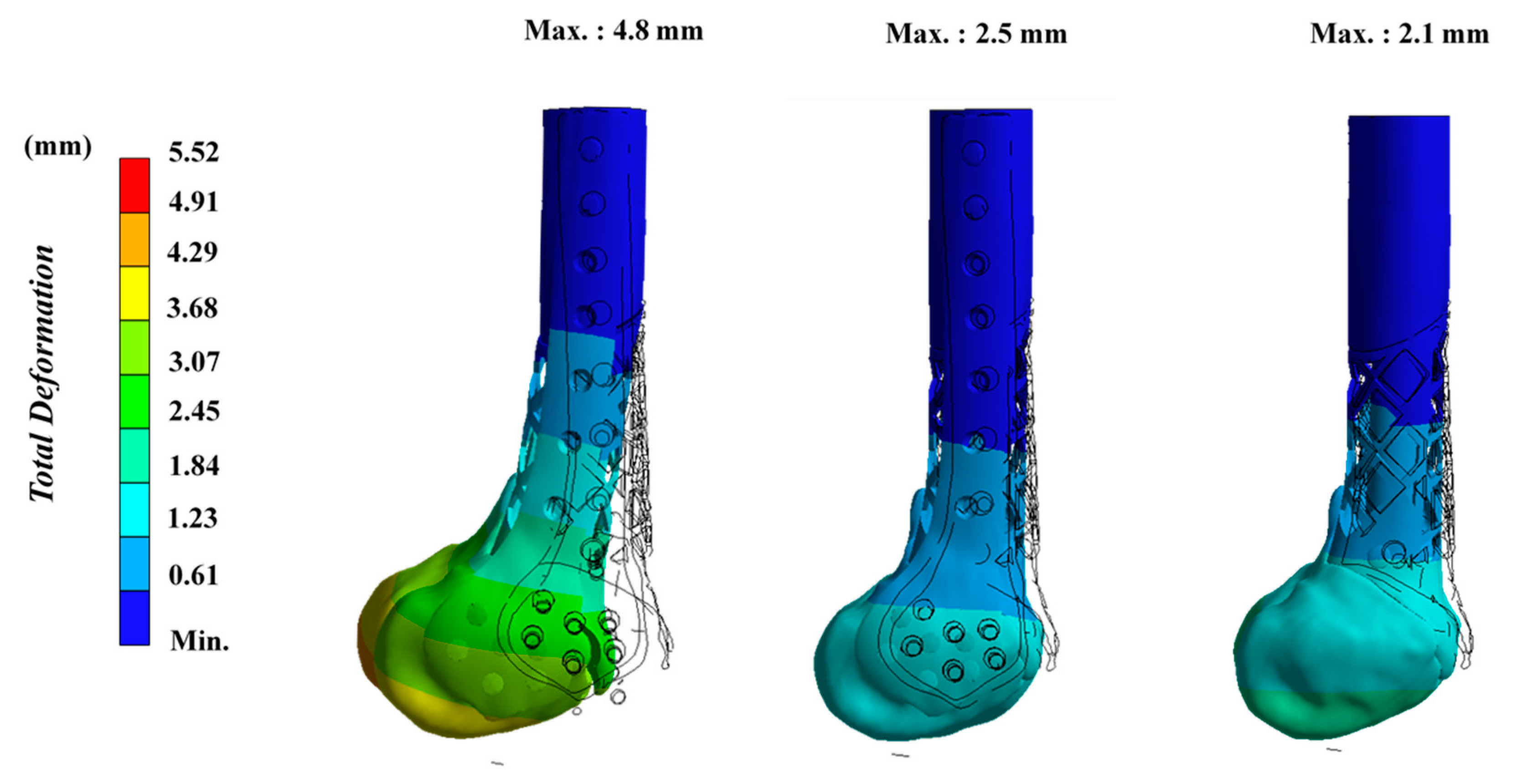
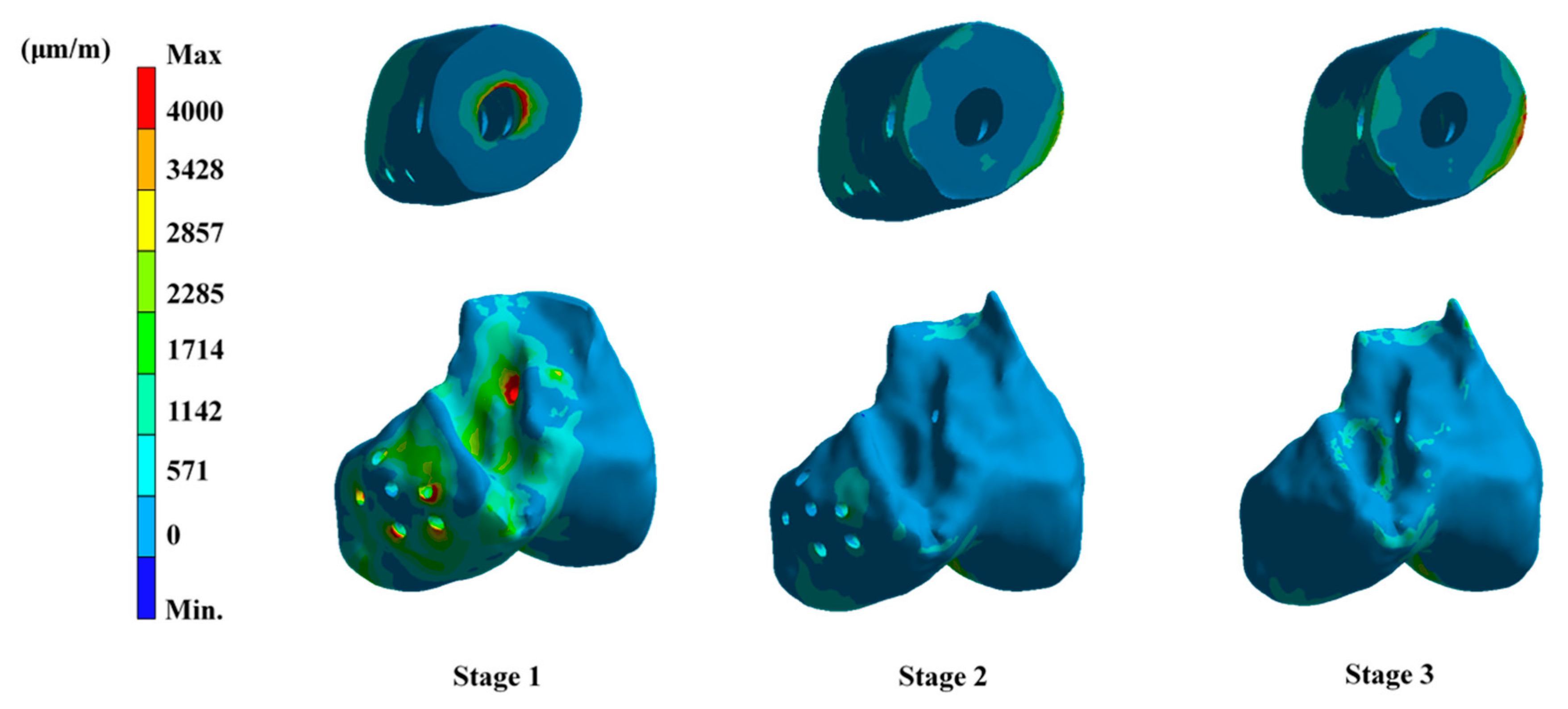
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Keating, J.F.; Simpson, A.H.; Robinson, C.M. The management of fractures with bone loss. J. Bone Jt. Surg. Br. 2005, 87, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Thakeb, M.F.; Mahran, M.A.; El-Motassem, H.M. Bone transport for the management of severely comminuted fractures without bone loss. Strateg. Trauma Limb Reconstr. 2016, 11, 19–24. [Google Scholar]
- Pipitone, P.S.; Rehman, S. Management of traumatic bone loss in the lower extremity. Orthop. Clin. N. Am. 2014, 45, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Giotikas, D.; Tarazi, N.; Spalding, L.; Nabergoj, M.; Krkovic, M. Results of the Induced Membrane Technique in the Management of Traumatic Bone Loss in the Lower Limb: A Cohort Study. J. Orthop. Trauma 2019, 33, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Jacek, B.; Maciej, P.; Tomasz, P.; Agata, B.; Wiesław, K.; Radosław, W.; Filip, G. 3D printed models in mandibular reconstruction with bony free flaps. J. Mater. Sci. Mater. Med. 2018, 29, 23. [Google Scholar] [CrossRef] [PubMed]
- Mangano, C.; Bianchi, A.; Mangano, C.; Dana, J.; Colombo, M.; Solop, I.; Admakin, O. Custom-made 3D printed subperiosteal titanium implants for the prosthetic restoration of the atrophic posterior mandible of elderly patients: A case series. 3D Print Med. 2020, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tetsworth, K.; Block, S.; Glatt, V. Putting 3D modelling and 3D printing into practice: Virtual surgery and preoperative planning to reconstruct complex post-traumatic skeletal deformities and defects. SICOT J. 2017, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Hamid, K.S.; Parekh, S.G.; Adams, S.B. Salvage of Severe Foot and Ankle Trauma with a 3D Printed Scaffold. Foot Ankle Int. 2016, 37, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Gesensway, D.; Putnam, M.D.; Mente, P.L.; Lewis, J.L. Design and biomechanics of a plate for the distal radius. J. Hand Surg Am. 1995, 20, 1021–1027. [Google Scholar] [CrossRef]
- Reeves, J.M.; Burkhart, T.A.; Dunning, C.E. The effect of static muscle forces on the fracture strength of the intact distal radius in vitro in response to simulated forward fall impacts. J. Biomech. 2014, 47, 2672–2678. [Google Scholar] [CrossRef] [PubMed]
- Completo, A.; Fonseca, F.; Simões, J. Experimental validation of intact and implanted distal femur finite element models. J. Biomech. 2007, 40, 2467–2476. [Google Scholar] [CrossRef] [PubMed]
- Conlisk, N.; Howie, C.R.; Pankaj, P. Optimum stem length for mitigation of periprosthetic fracture risk following primary total knee arthroplasty: A finite element study. Knee Surgery, Sports Traumatology. Arthroscopy 2018, 26, 1420–1428. [Google Scholar]
- Long, M.; Rack, H. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Cheng, K.; Liu, Y.; Yao, C.; Zhao, W.; Xu, X. A personalized mandibular implant with supporting and porous structures designed with topology optimization—A case study of canine. Rapid Prototyp. J. 2019, 25, 417–426. [Google Scholar] [CrossRef]
- Çehreli, M.; Şahin, S.; Akça, K. Role of mechanical environment and implant design on bone tissue differentiation: Current knowledge and future contexts. J. Dent. 2004, 32, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. Wolff’s Law and bone’s structural adaptations to mechanical usage: An overview for clinicians. Angle Orthod. 1994, 64, 175–188. [Google Scholar] [PubMed]
- Rubin, C.T.; Lanyon, L.E. Regulation of bone mass by mechanical strain magnitude. Calcif. Tissue Int. 1985, 37, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Pattin, C.; Caler, W.; Carter, D. Cyclic mechanical property degradation during fatigue loading of cortical bone. J. Biomech. 1996, 29, 69–79. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, K.W.; Wu, C.D.; Chien, C.-S.; Lee, C.-W.; Yang, T.-H.; Lin, C.-L. Patient-Specific 3-Dimensional Printing Titanium Implant Biomechanical Evaluation for Complex Distal Femoral Open Fracture Reconstruction with Segmental Large Bone Defect: A Nonlinear Finite Element Analysis. Appl. Sci. 2020, 10, 4098. https://doi.org/10.3390/app10124098
Wong KW, Wu CD, Chien C-S, Lee C-W, Yang T-H, Lin C-L. Patient-Specific 3-Dimensional Printing Titanium Implant Biomechanical Evaluation for Complex Distal Femoral Open Fracture Reconstruction with Segmental Large Bone Defect: A Nonlinear Finite Element Analysis. Applied Sciences. 2020; 10(12):4098. https://doi.org/10.3390/app10124098
Chicago/Turabian StyleWong, Kin Weng, Chung Da Wu, Chi-Sheng Chien, Cheng-Wei Lee, Tai-Hua Yang, and Chun-Li Lin. 2020. "Patient-Specific 3-Dimensional Printing Titanium Implant Biomechanical Evaluation for Complex Distal Femoral Open Fracture Reconstruction with Segmental Large Bone Defect: A Nonlinear Finite Element Analysis" Applied Sciences 10, no. 12: 4098. https://doi.org/10.3390/app10124098
APA StyleWong, K. W., Wu, C. D., Chien, C.-S., Lee, C.-W., Yang, T.-H., & Lin, C.-L. (2020). Patient-Specific 3-Dimensional Printing Titanium Implant Biomechanical Evaluation for Complex Distal Femoral Open Fracture Reconstruction with Segmental Large Bone Defect: A Nonlinear Finite Element Analysis. Applied Sciences, 10(12), 4098. https://doi.org/10.3390/app10124098





