The Stabilization of Liquid Smoke through Hydrodeoxygenation Over Nickel Catalyst Loaded on Sarulla Natural Zeolite
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Preparation and Activation of Sarulla Natural Zeolite
2.3. Characterization of Catalysts
2.4. Liquid Smoke Preparation
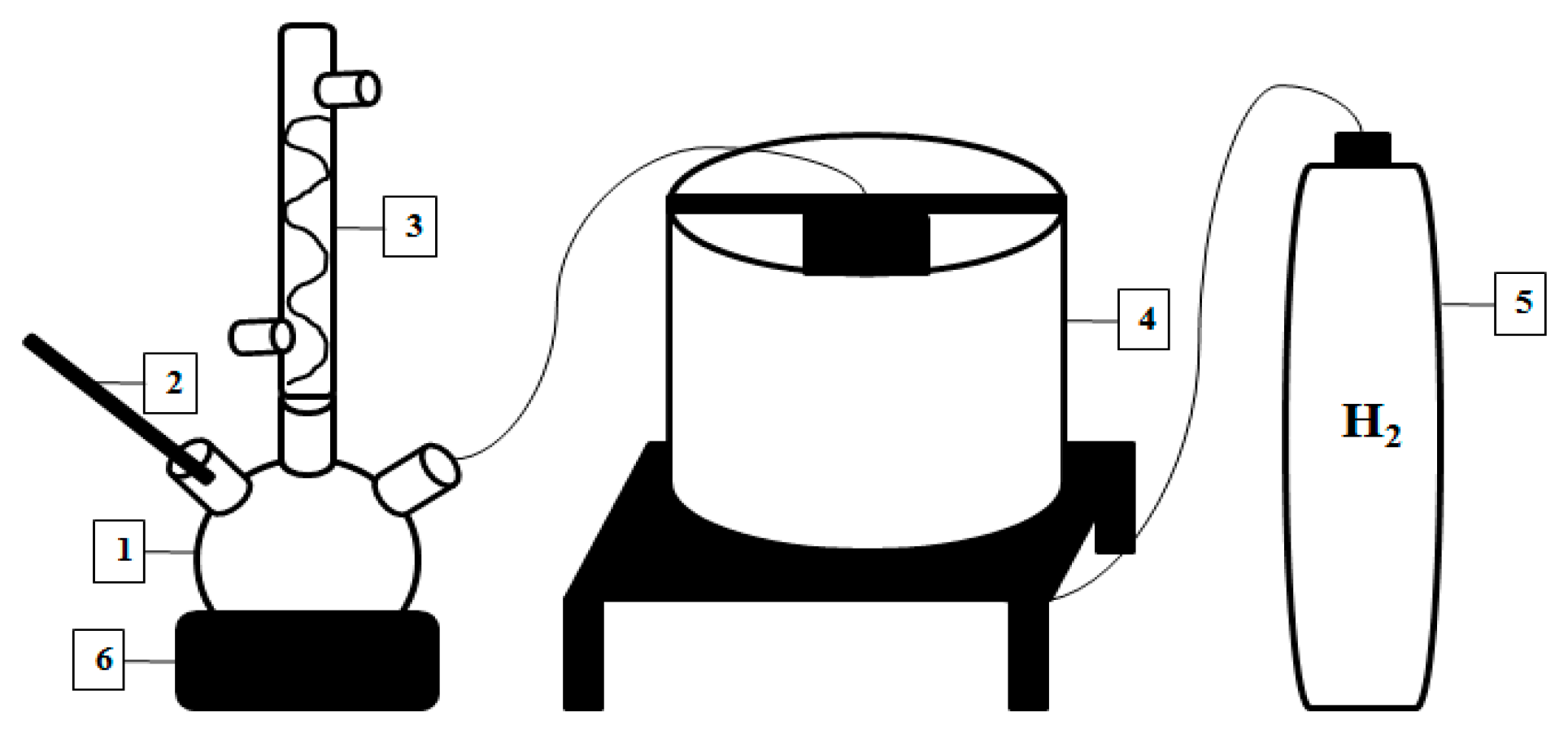
2.5. Liquid Smoke Hydrodeoxygenation (HDO) Process
3. Result and Discussion
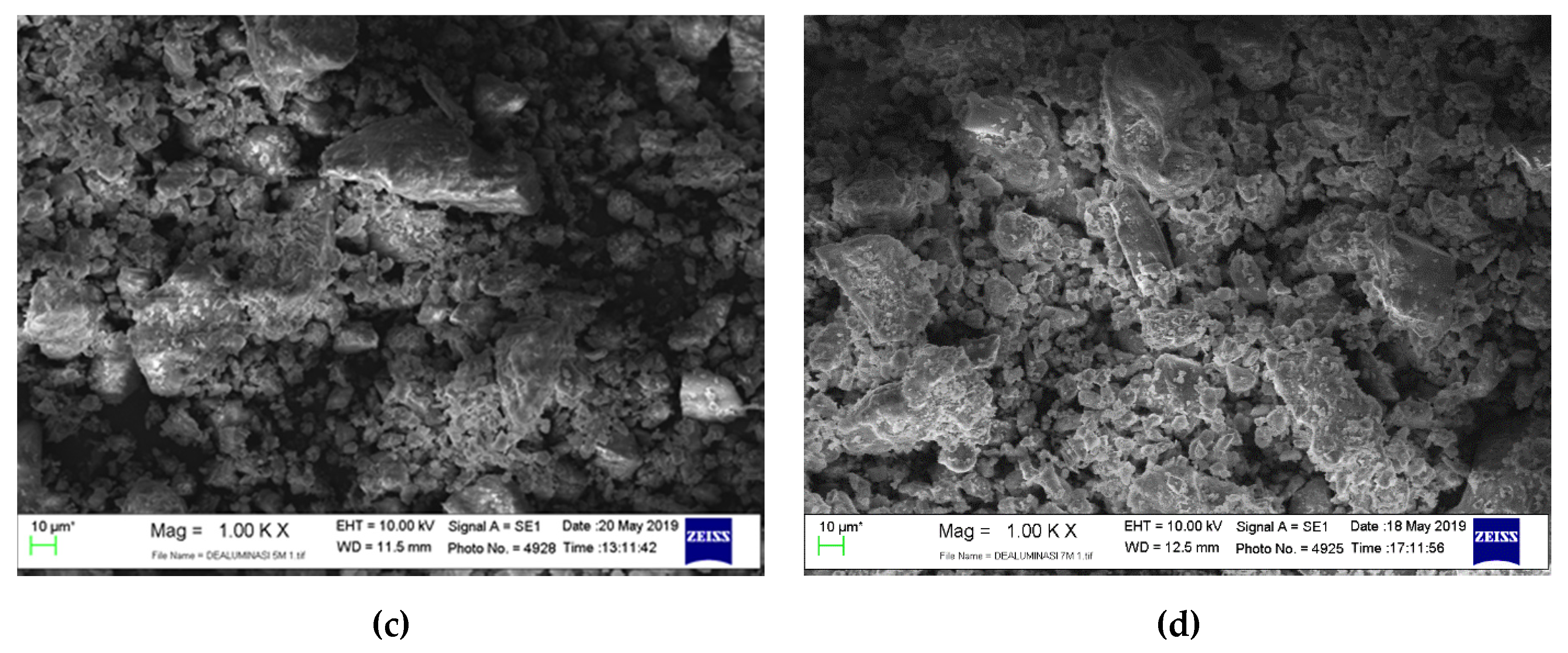
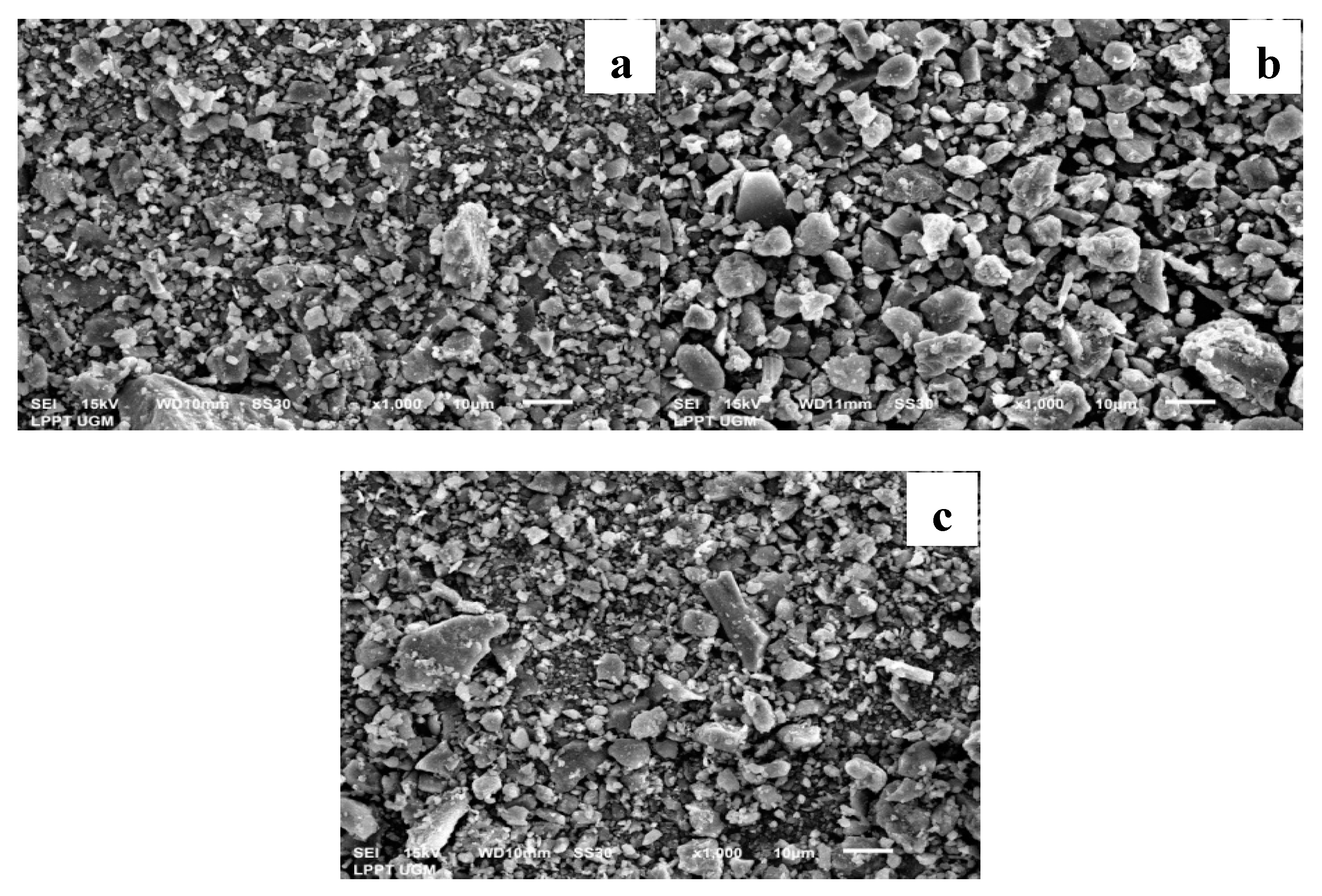
3.1. Catalyst Morphology
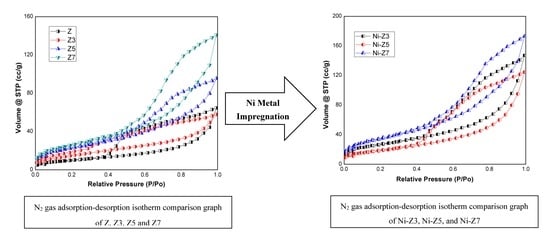
3.2. Specific Surface Area, Pore Volume and Size
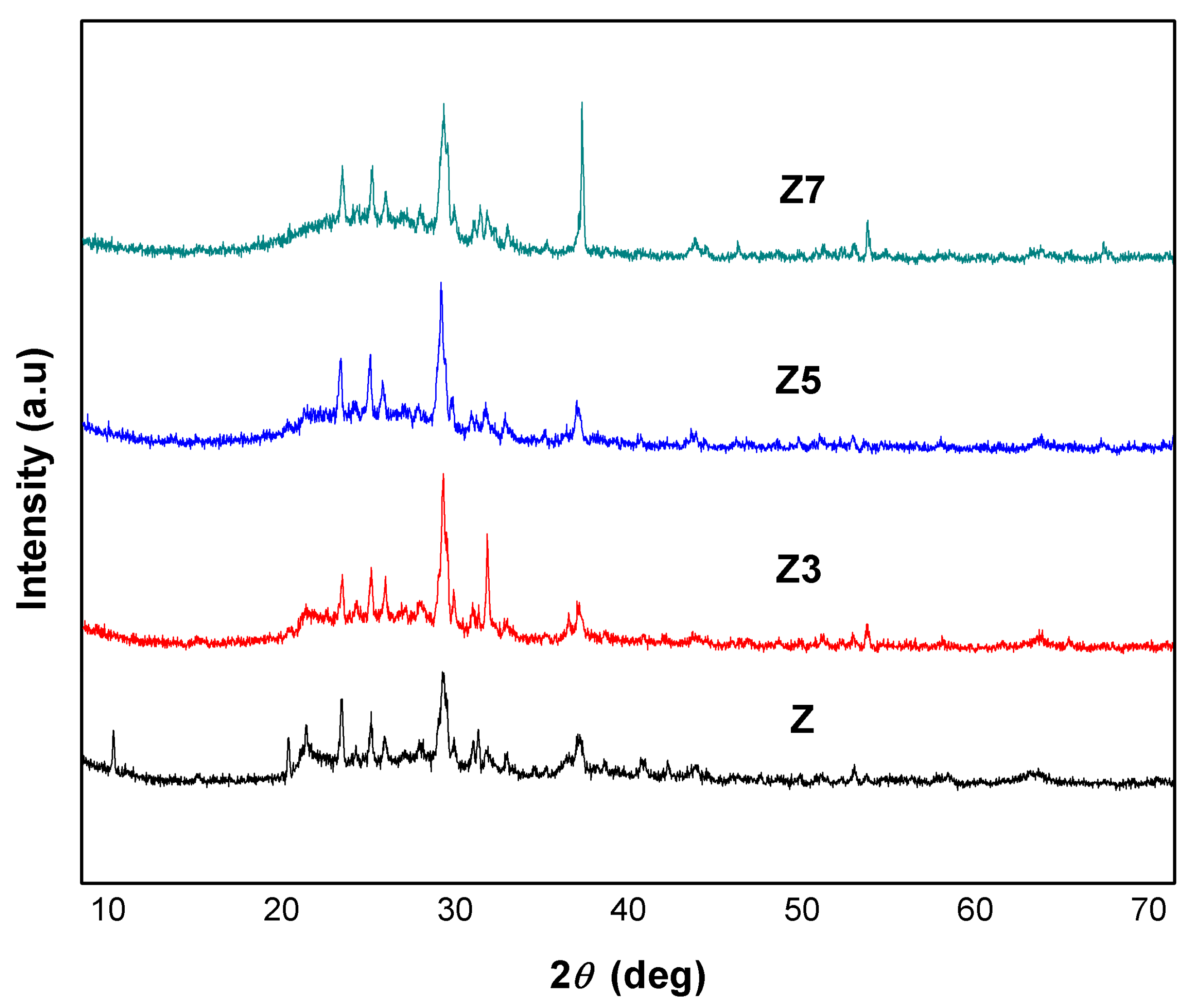
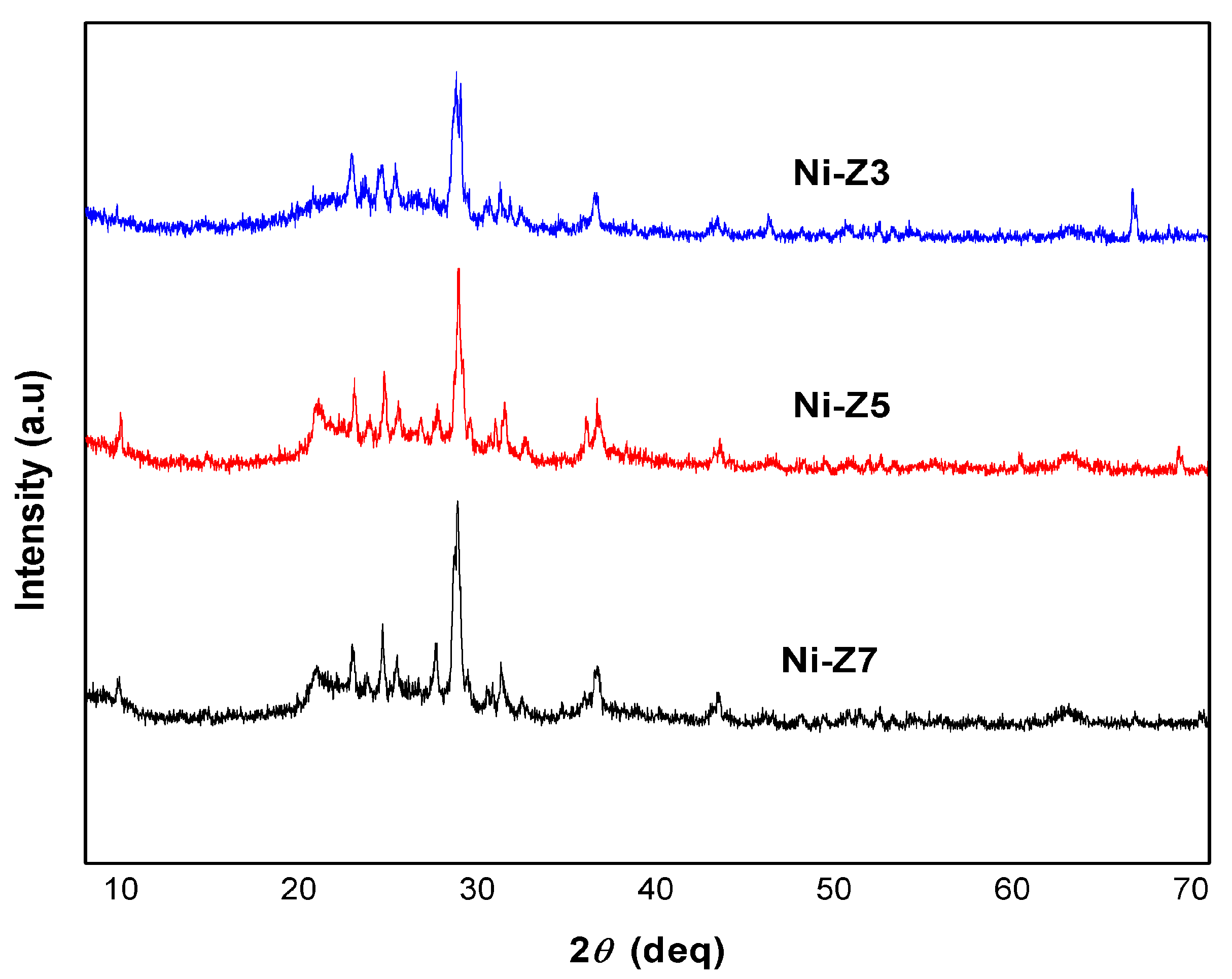
3.3. Catalyst Crystallinity
3.4. Hydrodeoxygenation (HDO) Process
3.5. Composition of Liquid Smoke of the HDO Product
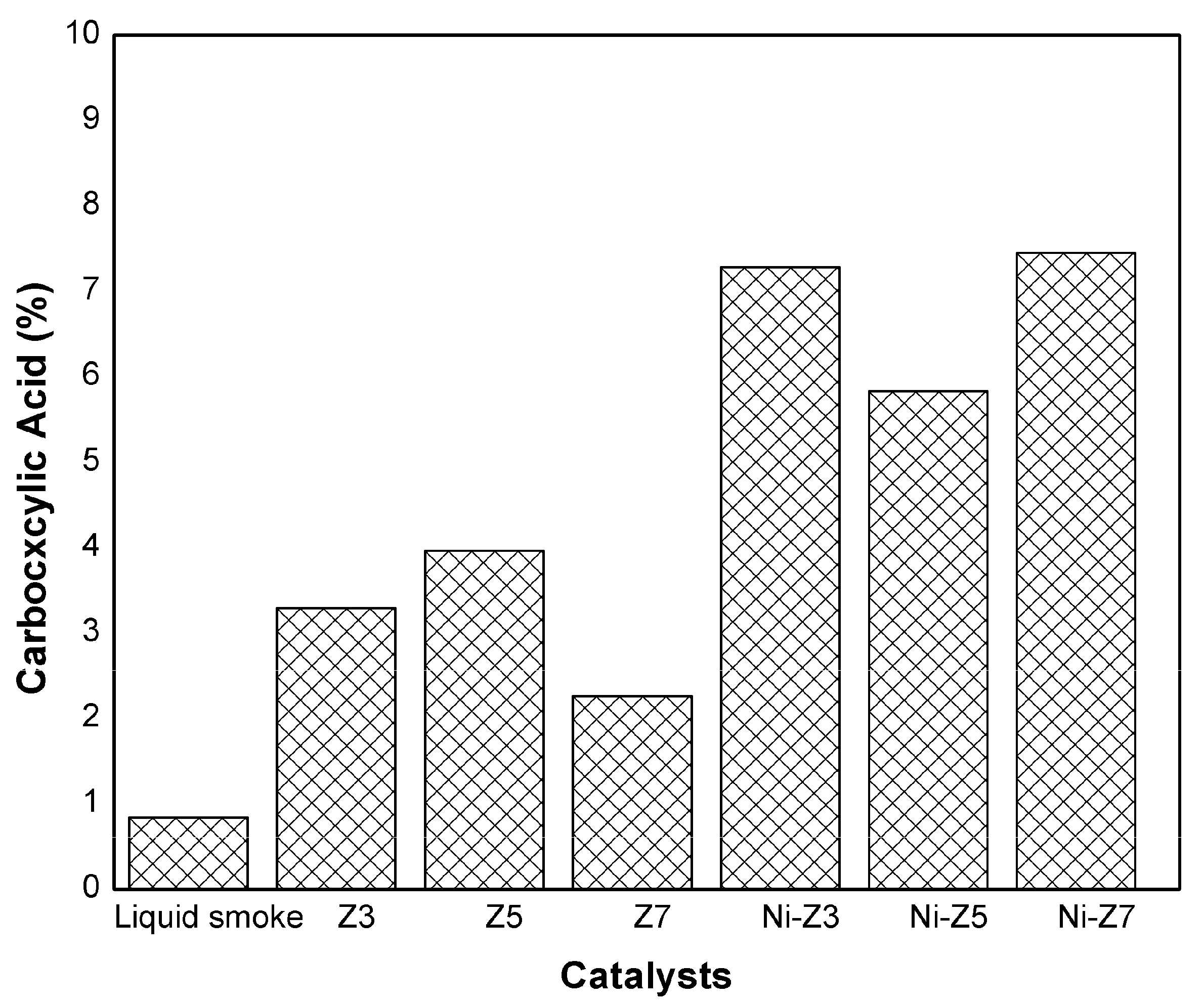
3.6. Catalyst Activity and Selectivity in Hydrodeoxygenation Reaction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hasanah, U.; Setiaji, B.; Triyono, T.; Anwar, C. The Chemical Composition and Physical Properties of the Light and Heavy Tar Resulted from Coconut Shell Pyrolysis. J. Pure Appl. Chem. Res. 2012, 1, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Hadanu, R.; Apituley, D.A.N. Volatile Compounds Detected in Coconut Shell Liquid Smoke through Pyrolysis at a Fractioning Temperature of 350–420 °C. Makara J. Sci. 2016, 20, 95–100. [Google Scholar] [CrossRef]
- Nugrahaningtyas, K.D.; Hidayat, Y.; Prayekti, P.S. Aktivitas Dan Selektivitas Katalis Mo-Co/USY Pada Reaksi Hidrodeoksigenasi Anisol. J. Penelit. Saintek 2015, 20, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Dickerson, T.; Soria, J. Catalytic Fast Pyrolysis: A Review. Energies 2013, 6, 514–538. [Google Scholar] [CrossRef] [Green Version]
- Si, Z.; Zhang, X.; Wang, C.; Ma, L.; Dong, R. An Overview on Catalytic Hydrodeoxygenation of Pyrolysis Oil and Its Model Compounds. Catalysts 2017, 7, 169. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Wei, L.; Zhao, X.; Julson, J. Application, Deactivation, and Regeneration of Heterogeneous Catalysts in Bio-Oil Upgrading. Catalysts 2016, 6, 195. [Google Scholar] [CrossRef]
- Nie, L.; De Souza, P.M.; Noronha, F.B.; An, W.; Sooknoi, T.; Resasco, D.E. Selective Conversion of M-Cresol to Toluene over Bimetallic Ni-Fe Catalysts. J. Mol. Catal. A Chem. 2014, 388–389, 47–55. [Google Scholar] [CrossRef]
- Ly, H.V.; Choi, J.H.; Woo, H.C.; Kim, S.S.; Kim, J. Upgrading Bio-Oil by Catalytic Fast Pyrolysis of Acid-Washed Saccharina Japonica Alga in a Fluidized-Bed Reactor. Renew. Energy 2019, 133, 11–22. [Google Scholar] [CrossRef]
- Schmitt, C.C.; Reolon, M.B.G.; Zimmermann, M.; Raffelt, K.; Grunwaldt, J.D.; Dahmen, N. Synthesis and Regeneration of Nickel-Based Catalysts for Hydrodeoxygenation of Beech Wood Fast Pyrolysis Bio-Oil. Catalysts 2018, 8, 449. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kim, H.; Yu, M.J.; Ko, C.H.; Jeon, J.K.; Jae, J.; Park, S.H.; Jung, S.C.; Park, Y.K. Catalytic Hydrodeoxygenation of Bio-Oil Model Compounds over Pt/HY Catalyst. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Shamanaev, I.V.; Deliy, I.V.; Gerasimov, E.Y.; Pakharukova, V.P.; Bukhtiyarova, G.A. Enhancement of HDO Activity of MoP/SiO2 Catalyst in Physical Mixture with Alumina or Zeolites. Catalysts 2019, 10, 45. [Google Scholar] [CrossRef] [Green Version]
- Ameen, M.; Azizan, M.T.; Yusup, S.; Ramli, A.; Yasir, M.; Kaur, H.; Wai, C.K. H-Y Zeolite as Hydrodeoxygenation Catalyst for Diesel Range Hydrocarbon Production from Rubber Seed Oil. In Materials Today: Proceedings; Elsevier Ltd.: Langkawi, Malaysia, 2019; Volume 16, pp. 1742–1749. [Google Scholar] [CrossRef]
- Feng, J.; Hse, C.-Y.; Yang, Z.; Wang, K.; Jiang, J.; Xu, J. Liquid Phase in Situ Hydrodeoxygenation of Biomass-Derived Phenolic Compounds to Hydrocarbons over Bifunctional Catalysts. Appl. Catal. A Gen. 2017, 542, 163–173. [Google Scholar] [CrossRef]
- Ayodele, O.B.; Farouk, H.U.; Mohammed, J.; Uemura, Y.; Daud, W.M.A.W. Hydrodeoxygenation of Oleic Acid into N- and Iso-Paraffin Biofuel Using Zeolite Supported Fluoro-Oxalate Modified Molybdenum Catalyst: Kinetics Study. J. Taiwan Inst. Chem. Eng. 2015, 50, 142–152. [Google Scholar] [CrossRef]
- Sihombing, J.L.; Gea, S.; Wirjosentono, B.; Agusnar, H.; Pulungan, A.N.; Herlinawati, H.; Yusuf, M.; Hutapea, Y.A. Characteristic and Catalytic Performance of Co and Co-Mo Metal Impregnated in Sarulla Natural Zeolite Catalyst for Hydrocracking of MEFA Rubber Seed Oil into Biogasoline Fraction. Catalysts 2020, 10, 121. [Google Scholar] [CrossRef] [Green Version]
- Sriningsih, W.; Saerodji, M.G.; Trisunaryanti, W.; Triyono; Armunanto, R.; Falah, I.I. Fuel Production from LDPE Plastic Waste over Natural Zeolite Supported Ni, Ni-Mo, Co and Co-Mo Metals. Procedia Environ. Sci. 2014, 20, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Rahayu, F.L.; Nuryanto, R.; Suyati, L. Pengaruh Diameter Kanal Pelet Katalis Zeolit Aktif Dan Ni-Zeolit Terhadap Pirolisis Limbah Batang Pohon Sagu (Metroxylonsp.). J. Kim. Sains Dan Apl. 2013, 16, 33. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, E.; Wu, K.; Wang, J.; Yang, M.; Wu, Y. Recent Progress on Upgrading of Bio-Oil to Hydrocarbons over Metal/Zeolite Bifunctional Catalysts. Catal. Sci. Technol. 2017, 7, 2385–2415. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.; Sun, Z.; Li, X.; Wang, A.; Camaioni, D.M.; Lercher, J.A. Ni3P as a High-Performance Catalytic Phase for the Hydrodeoxygenation of Phenolic Compounds. Green Chem. 2018, 20, 609–619. [Google Scholar] [CrossRef]
- Prihatini, D.; Ulfa, S.M.; Iftitah, E.D. Uji Aktivitas Katalis Ni/ZrO2-SiO2 Untuk Reaksi Hidrodeoksigenasi Campuran Senyawa Furfurilidena Aseton (FAc) Dan Difurfurilidena Aseton (F2Ac). Natural 2016, 3, 253–259. [Google Scholar]
- Sihombing, J.L.; Gea, S.; Pulungan, A.N.; Agusnar, H.; Wirjosentono, B.; Hutapea, Y.A. The Characterization of Sarulla Natural Zeolite Crystal and Its Morphological Structure. In AIP Conference Proceedings; American Institute of Physics Inc.: Surabaya, Indonesia, 2018; Volume 2049, p. 020062. [Google Scholar] [CrossRef]
- Sari, R.M.; Gea, S.; Wirjosentono, B.; Hendrana, S.; Hutapea, Y.A. Improving Quality and Yield Productionof Coconut Shell Charcoal Through a Modified Reactor with Tar Scrubberto Reduce Smoke Pollution. Polish J. Environ. Stud. 2020, 29, 1815–1824. [Google Scholar] [CrossRef]
- Prasetyo, A.; Nafsiati, R.; Kholifah, S.N.; Botianovi, A. Analisis Permukaan Zeolit Alam Malang Yang Mengalami Modifikasi Pori Dengan Uji SEM-EDS. SAINTIS 2012, 1, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Sentosa, L.; Subagio, B.S.; Rahman, H.; Yamin, R.A. Aktivasi Zeolit Alam Asal Bayah Dengan Asam Dan Basa Sebagai Aditif Campuran Beraspal Hangat (Warm Mixed Asphalt (WMA)). J. Tek. Sipil 2018, 25, 203. [Google Scholar] [CrossRef] [Green Version]
- Pertiwi, R.; Tursiloadi, S.; Adilina, I.B.; Sembiring, K.C.; Oaki, Y. Nickel Supported Natural Zeolite as a Bifunctional Catalysts for Conversion of Citronella Oil Crude to Menthols. J. Kim. Terap. Indones. 2017, 18, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Suharto, T.E.; Gustin, I.; Sudaryono, A. Pembuatan Dan Karakterisasi Katalis Bifungsional Dari Zeolit Alam. J. Gradien 2007, 3, 267–272. [Google Scholar]
- Sriatun, S.; Darmawan, A. Dealuminasi zeolit alam cipatujah melalui penambahan asam dan oksidator. J. Kim. Sains Dan Apl. 2005, 8, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Pulungan, A.N.; Sihombing, J.L.; Nasution, H.I.; Syafriani, D.; Wibowo, A.A. Study of Rubber Seed Oil into Biodiesel Fraction with Hetergen Acid Catalyst. In International Seminar on Trends in Science and Science Education; Faculty of Mathematics and Natural Sciences, Universitas Negeri Medan: Medan, Indonesia, 2015. [Google Scholar]
- Cullity, D.B. Elements of X-Ray Diffraction; Addison-Wesley: London, UK, 1959. [Google Scholar]
- Zhang, X.; Zhang, Q.; Chen, L.; Xu, Y.; Wang, T.; Ma, L. Effect of Calcination Temperature of Ni/SiO2-ZrO2 Catalyst on Its Hydrodeoxygenation of Guaiacol. Cuihua Xuebao/Chinese J. Catal. 2014, 35, 302–309. [Google Scholar] [CrossRef]
- Pardoyo, P.; Listiana, L.; Darmawan, A. Pengaruh Perlakuan HCl Pada Kristalinitas Dan Kemampuan Adsorpsi Zeolit Alam Terhadap Ion Ca2+. J. Sains dan Mat. 2009, 17, 100–104. [Google Scholar]
- Sun, J.; Karim, A.M.; Zhang, H.; Kovarik, L.; Li, X.S.; Hensley, A.J.; McEwen, J.S.; Wang, Y. Carbon-Supported Bimetallic Pd-Fe Catalysts for Vapor-Phase Hydrodeoxygenation of Guaiacol. J. Catal. 2013, 306, 47–57. [Google Scholar] [CrossRef]
- Grilc, M.; Likozar, B.; Levec, J. Hydrodeoxygenation and Hydrocracking of Solvolysed Lignocellulosic Biomass by Oxide, Reduced and Sulphide Form of NiMo, Ni, Mo and Pd Catalysts. Appl. Catal. B Environ. 2014, 150–151, 275–287. [Google Scholar] [CrossRef]
- Espro, C.; Gumina, B.; Paone, E.; Mauriello, F. Upgrading Lignocellulosic Biomasses: Hydrogenolysis of Platform Derived Molecules Promoted by Heterogeneous Pd-Fe Catalysts. Catalysts 2017, 7, 78. [Google Scholar] [CrossRef]
- Putri, I.F.; Nugrahaningtyas, K.D. Kajian Aktivitas Katalitik CoMo/Al2O3 Pada Reaksi Hidrodeoksigenasi Anisol Dan Guaiacol. J. Kim. dan Pendidik. Kim. 2016, 1, 164–173. [Google Scholar]
- Bouxin, F.P.; Zhang, X.; Kings, I.N.; Lee, A.F.; Simmons, M.J.H.; Wilson, K.; Jackson, S.D. Deactivation Study of the Hydrodeoxygenation of P-Methylguaiacol over Silica Supported Rhodium and Platinum Catalysts. Appl. Catal. A Gen. 2017, 539, 29–37. [Google Scholar] [CrossRef]
- Zhu, J.; Meng, X.; Xiao, F. Mesoporous Zeolites as Efficient Catalysts for Oil Refining and Natural Gas Conversion. Front. Chem. Sci. Eng. 2013, 7, 233–248. [Google Scholar] [CrossRef] [Green Version]
- Hao, W.; Zhang, W.; Guo, Z.; Ma, J.; Li, R. Mesoporous Beta Zeolite Catalysts for Benzylation of Naphthalene: Effect of Pore Structure and Acidity. Catalysts 2018, 8, 504. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yang, Y.; Luo, H.; Hu, T.; Liu, W. Preparation and Hydrodeoxygenation Properties of Co-Mo-O-B Amorphous Catalyst. React. Kinet. Mech. Catal. 2011, 102, 207–217. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Li, D.; Bui, P.; Oyama, S.T. Hydrodeoxygenation of Guaiacol as Model Compound for Pyrolysis Oil on Transition Metal Phosphide Hydroprocessing Catalysts. Appl. Catal. A Gen. 2011, 391, 305–310. [Google Scholar] [CrossRef]
- Ghampson, I.T.; Sepúlveda, C.; Garcia, R.; Radovic, L.R.; Fierro, J.L.G.; Desisto, W.J.; Escalona, N. Hydrodeoxygenation of Guaiacol over Carbon-Supported Molybdenum Nitride Catalysts: Effects of Nitriding Methods and Support Properties. Appl. Catal. A Gen. 2012, 439–440, 111–124. [Google Scholar] [CrossRef]
- Shetty, M.; Murugappan, K.; Green, W.H.; Román-Leshkov, Y. Structural Properties and Reactivity Trends of Molybdenum Oxide Catalysts Supported on Zirconia for the Hydrodeoxygenation of Anisole. ACS Sustain. Chem. Eng. 2017, 5, 5293–5301. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A Review of Catalytic Upgrading of Bio-Oil to Engine Fuels. Appl. Catal. A General. 2011, 1–19. [Google Scholar] [CrossRef]
- He, Z.; Wang, X. Hydrodeoxygenation of Model Compounds and Catalytic Systems for Pyrolysis Bio-Oils Upgrading. Catal. Sustain. Energy 2013, 1, 28–52. [Google Scholar] [CrossRef]

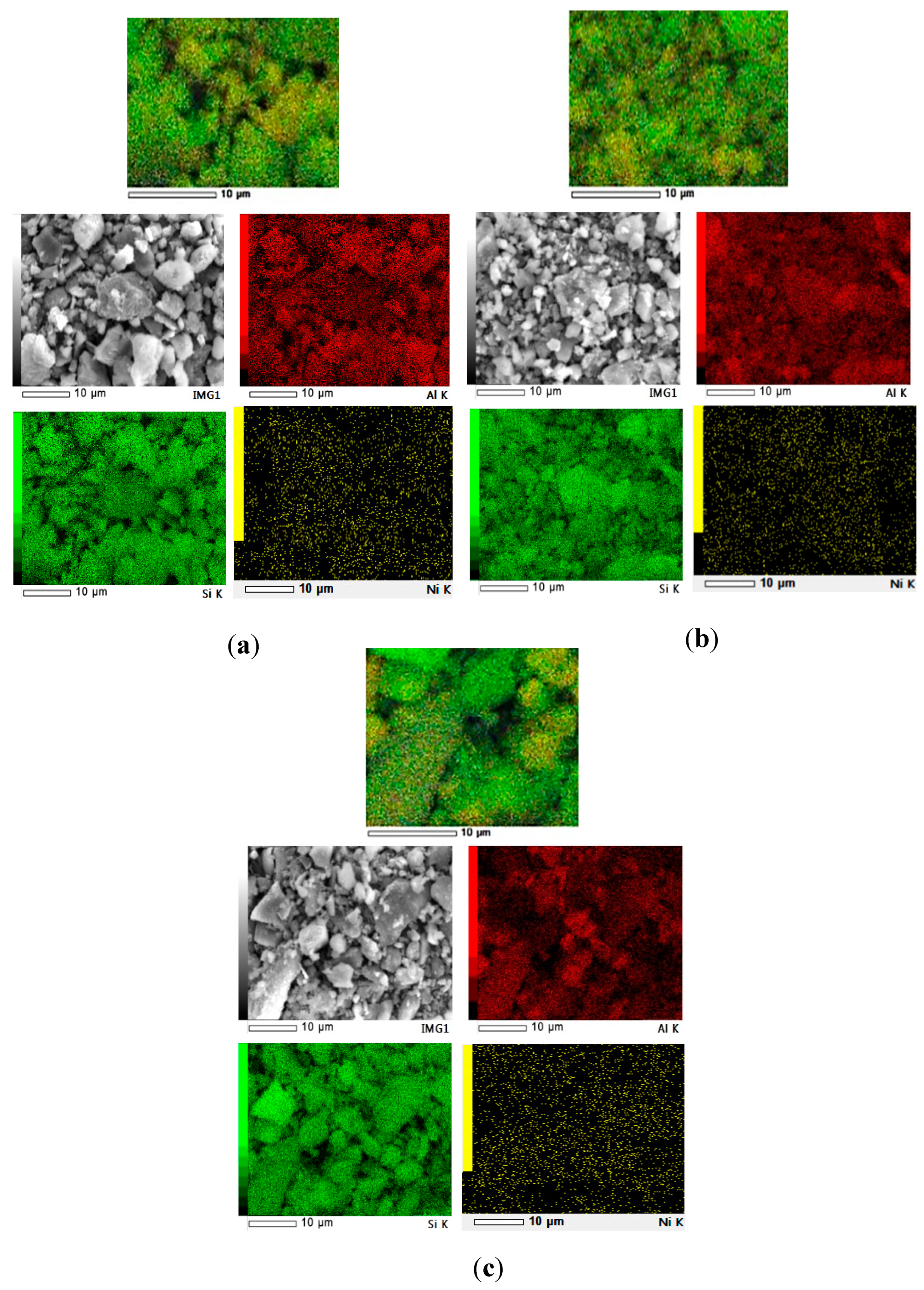


| Elements | Mass (%) | |||
|---|---|---|---|---|
| Z | Z3 | Z5 | Z7 | |
| C | - | 23.25 | 2.68 | 20.27 |
| O | 61.58 | 49.08 | 60.81 | 71.01 |
| Si | 15.73 | 23.72 | 25.35 | 23.74 |
| Al | 5.64 | 3.45 | 0.90 | 3.79 |
| K | 3.16 | - | - | 0.98 |
| Fe | 4.60 | - | - | - |
| F | - | - | 1.28 | - |
| Ca | - | 0.50 | 4.52 | - |
| Mg | 7.93 | - | 4.47 | - |
| Ti | 1.37 | - | - | - |
| Hg | - | - | - | 0.48 |
| Element | Mass (%) | ||
|---|---|---|---|
| Ni-Z3 | Ni-Z5 | Ni-Z7 | |
| C | 29.69 | 12.69 | 20.27 |
| O | 42.39 | 52.15 | 45.02 |
| Si | 18.58 | 23.80 | 27.38 |
| Al | 4.60 | 5.72 | 3.49 |
| Ni | 0.98 | 1.11 | 1.48 |
| S | 0.55 | - | 0.80 |
| K | 0.30 | 1.57 | 0.66 |
| Fe | 1.87 | 1.90 | - |
| Zn | 1.09 | - | - |
| Na | - | 0.48 | - |
| Mg | - | 0.58 | - |
| Ti | - | - | 0.90 |
| Sample | Surface Area (m2/g) | Total Pore Volume (cc/g) | Average Pore Radius (nm) |
|---|---|---|---|
| Z | 59.60 | 0.15 | 1.722 |
| Z3 | 111.66 | 0.14 | 1.597 |
| Z5 | 132.44 | 0.20 | 1.593 |
| Z7 | 132.38 | 0.28 | 2.609 |
| Ni-Z3 | 72.32 | 0.15 | 1.720 |
| Ni-Z5 | 53.96 | 0.14 | 1.600 |
| Ni-Z7 | 107.70 | 0.21 | 1.870 |
| Z | Z3 | Z5 | Z7 | ||||
|---|---|---|---|---|---|---|---|
| 2θ | Intensity | 2θ | Intensity | 2θ | Intensity | 2θ | Intensity |
| 22.03 | 83 | 21.97 | 70 | 21.88 | 98 | 22.02 | 124 |
| 23.72 | 74 | 23.66 | 77 | 23.58 | 92 | 23.71 | 121 |
| 27.93 | 196 | 27.85 | 238 | 27.71 | 215 | 27.64 | 246 |
| 30.43 | 42 | 30.37 | 131 | 30.26 | 42 | 30.39 | 59 |
| Crystallinity | 45.91% | Crystallinity | 50.35% | Crystallinity | 47.97% | Crystallinity | 47.95% |
| Z | Z3 | Z5 | Z7 | ||||
|---|---|---|---|---|---|---|---|
| 2θ (degree) | D (nm) | 2θ (degree) | D (nm) | 2θ (degree) | D (nm) | 2θ (degree) | D (nm) |
| 22.03 | 27.45 | 21.97 | 27.85 | 21.88 | 25.49 | 22.02 | 41.05 |
| 23.72 | 27.46 | 23.66 | 30.06 | 23.58 | 25.47 | 23.71 | 40.24 |
| 27.93 | 16.71 | 27.85 | 18.97 | 27.71 | 18.10 | 27.64 | 41.51 |
| 30.43 | 19.60 | 30.37 | 31.10 | 30.26 | 20.99 | 30.39 | 35.47 |
| Ni-Z3 | Ni-Z5 | Ni-Z7 | |||
|---|---|---|---|---|---|
| 2θ (degree) | Intensity | 2θ (degree) | Intensity | 2θ (degree) | Intensity |
| 19.90 | 46 | 19.56 | 19 | 19.77 | 20 |
| 21.12 | 59 | 21.42 | 23 | 21.88 | 60 |
| 23.62 | 59 | 23.73 | 74 | 23.52 | 54 |
| 27.78 | 248 | 27.89 | 228 | 27.79 | 163 |
| 30.31 | 46 | 30.46 | 61 | 30.24 | 32 |
| Crystallinity | 46.76% | Crystallinity | 42.49% | Crystallinity | 34.15% |
| Ni-Z3 | Ni-Z5 | Ni-Z7 | |||
|---|---|---|---|---|---|
| 2θ (degree) | D (nm) | 2θ (degree) | D (nm) | 2θ (degree) | D (nm) |
| 21.12 | 22.73 | 21.42 | 40.42 | 21.88 | 19.28 |
| 23.62 | 25.39 | 23.73 | 35.55 | 23.52 | 19.50 |
| 27.78 | 16.90 | 27.89 | 26.11 | 27.19 | 13.34 |
| 30.31 | 22.61 | 30.46 | 32.93 | 30.24 | 21.26 |
| Catalysts | pH | Density (g/mL) | Water Content (%) |
|---|---|---|---|
| Commercial Liquid Smoke | 4.0 | 1.049 | 89.97 |
| Z3 | 4.0 | 1.048 | 68.71 |
| Z5 | 3.9 | 1.047 | 84.46 |
| Z7 | 3.8 | 1.047 | 82.47 |
| Ni-Z3 | 3.4 | 1.050 | 78.86 |
| Ni-Z5 | 3.6 | 1.048 | 68.06 |
| Ni-Z7 | 3.4 | 1.046 | 77.31 |
| Compound | % Area | ||||||
|---|---|---|---|---|---|---|---|
| Initial | Z3 | Z5 | Z7 | Ni-Z3 | Ni-Z5 | Ni-Z7 | |
| Phenol | 31.61 | 32.48 | 26.27 | 16.69 | 28.41 | 30.00 | 26.70 |
| Phenol,2-methyl | 8.35 | - | 1.90 | 1.60 | 1.27 | 1.87 | 1.21 |
| Phenol,4-methyl | 8.49 | - | - | - | - | - | - |
| Guaiacol | 12.37 | 1.57 | 1.41 | 0.82 | 1.56 | 1.69 | 1.61 |
| Phenol,2,6-dimethyl | 0.72 | - | - | - | - | - | - |
| Phenol, 2-ethyl | 0.77 | - | - | - | - | - | - |
| Phenol,3,5-dimethyl | 1.92 | - | - | - | - | - | - |
| Phenol,3-ethyl | 1.45 | - | - | - | - | - | - |
| 4-methylguaiacol | 7.57 | - | - | - | - | - | - |
| Phenol,2-ethyl-5-methyl | 0.69 | - | - | - | - | - | - |
| Phenol,3,4-dimethoxy | 0.15 | - | - | - | - | - | - |
| 1,2-benzenediol,3-methoxy | 1.45 | 1.98 | 2.28 | 1.22 | 1.67 | 1.73 | 1.79 |
| Phenol,4-ethyl-2-methoxy | 2.51 | 0.83 | 0.80 | 0.43 | 0.71 | 0.66 | 0.63 |
| 1,2-benzenediol,4-methyl | 0.87 | 4.18 | 4.86 | 2.66 | 3.39 | 3.81 | 3.58 |
| Phenol,2,6-dimethoxy | 4.13 | 7.55 | 7.78 | 4.44 | 7.67 | 7.41 | 7.63 |
| 1.3-benzenediol,4-ethyl | 0.59 | 1.70 | 2.00 | 1.10 | 1.43 | 1.55 | - |
| Phenol,3-methyl | - | 2.51 | 2.24 | 1.41 | 2.75 | 2.53 | 2.56 |
| Catechol | - | 15.67 | 16.49 | 9.13 | 14.60 | 14.69 | 16.34 |
| 1,2-benzenediol,3-methyl | - | 2.19 | 2.45 | 1.39 | 2.16 | 2.13 | 2.25 |
| Hydroquinone | - | 2.74 | 3.14 | 1.67 | 2.93 | 2.66 | 2.91 |
| 1,4-benzenediol,2-methyl | - | 0.92 | 1.04 | 0.56 | 0.94 | 0.82 | 0.97 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gea, S.; Haryono, A.; Andriayani, A.; Sihombing, J.L.; Pulungan, A.N.; Nasution, T.; Rahayu, R.; Hutapea, Y.A. The Stabilization of Liquid Smoke through Hydrodeoxygenation Over Nickel Catalyst Loaded on Sarulla Natural Zeolite. Appl. Sci. 2020, 10, 4126. https://doi.org/10.3390/app10124126
Gea S, Haryono A, Andriayani A, Sihombing JL, Pulungan AN, Nasution T, Rahayu R, Hutapea YA. The Stabilization of Liquid Smoke through Hydrodeoxygenation Over Nickel Catalyst Loaded on Sarulla Natural Zeolite. Applied Sciences. 2020; 10(12):4126. https://doi.org/10.3390/app10124126
Chicago/Turabian StyleGea, Saharman, Agus Haryono, Andriayani Andriayani, Junifa Layla Sihombing, Ahmad Nasir Pulungan, Tiamina Nasution, Rahayu Rahayu, and Yasir Arafat Hutapea. 2020. "The Stabilization of Liquid Smoke through Hydrodeoxygenation Over Nickel Catalyst Loaded on Sarulla Natural Zeolite" Applied Sciences 10, no. 12: 4126. https://doi.org/10.3390/app10124126
APA StyleGea, S., Haryono, A., Andriayani, A., Sihombing, J. L., Pulungan, A. N., Nasution, T., Rahayu, R., & Hutapea, Y. A. (2020). The Stabilization of Liquid Smoke through Hydrodeoxygenation Over Nickel Catalyst Loaded on Sarulla Natural Zeolite. Applied Sciences, 10(12), 4126. https://doi.org/10.3390/app10124126