Effect of Alkaline Treatment on Characteristics of Bio-Calcium and Hydroxyapatite Powders Derived from Salmon Bone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Collection and Preparation of Bone from Salmon Frame
2.3. Pre-Treatment of Bones
2.4. Preparation of Bio-Calcium and Calcined Bone
2.5. Characterization of Bio-Calcium and Calcined Bone
2.5.1. Chemical Composition
2.5.2. Thiobarbituric Acid-Reactive Substances (TBARS)
2.5.3. Color
2.5.4. Mean Particle Size
2.5.5. X-ray Diffraction Analysis
2.5.6. Scanning Electron Microscopy with Energy Dispersive X-ray Spectroscopy (SEM-EDX)
2.6. Statistical Analysis
3. Results and Discussions
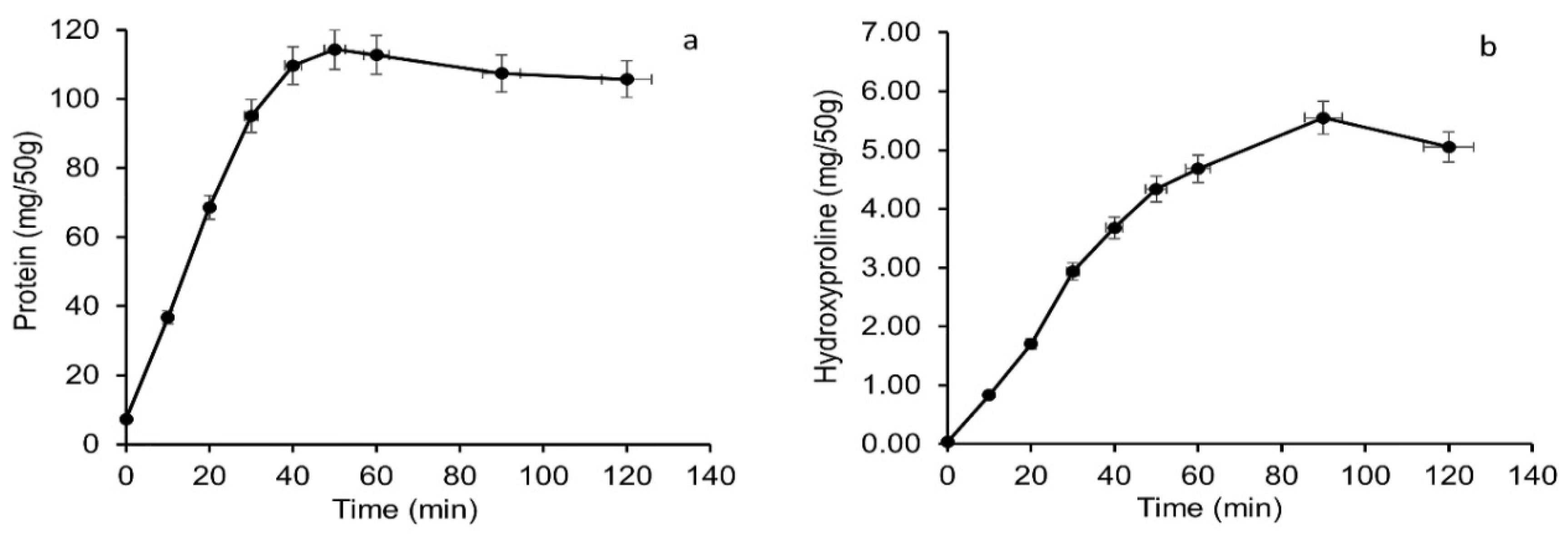
3.1. Total Soluble Protein and Hydroxyproline Content of Salmon Bone Leached out during Alkaline Treatment
3.2. Chemical Compositions of Bio-Calcium and Calcined Bone from Salmon Frame with and without Alkaline Treatment
3.3. Color of Bio-Calcium and Calcined Powders
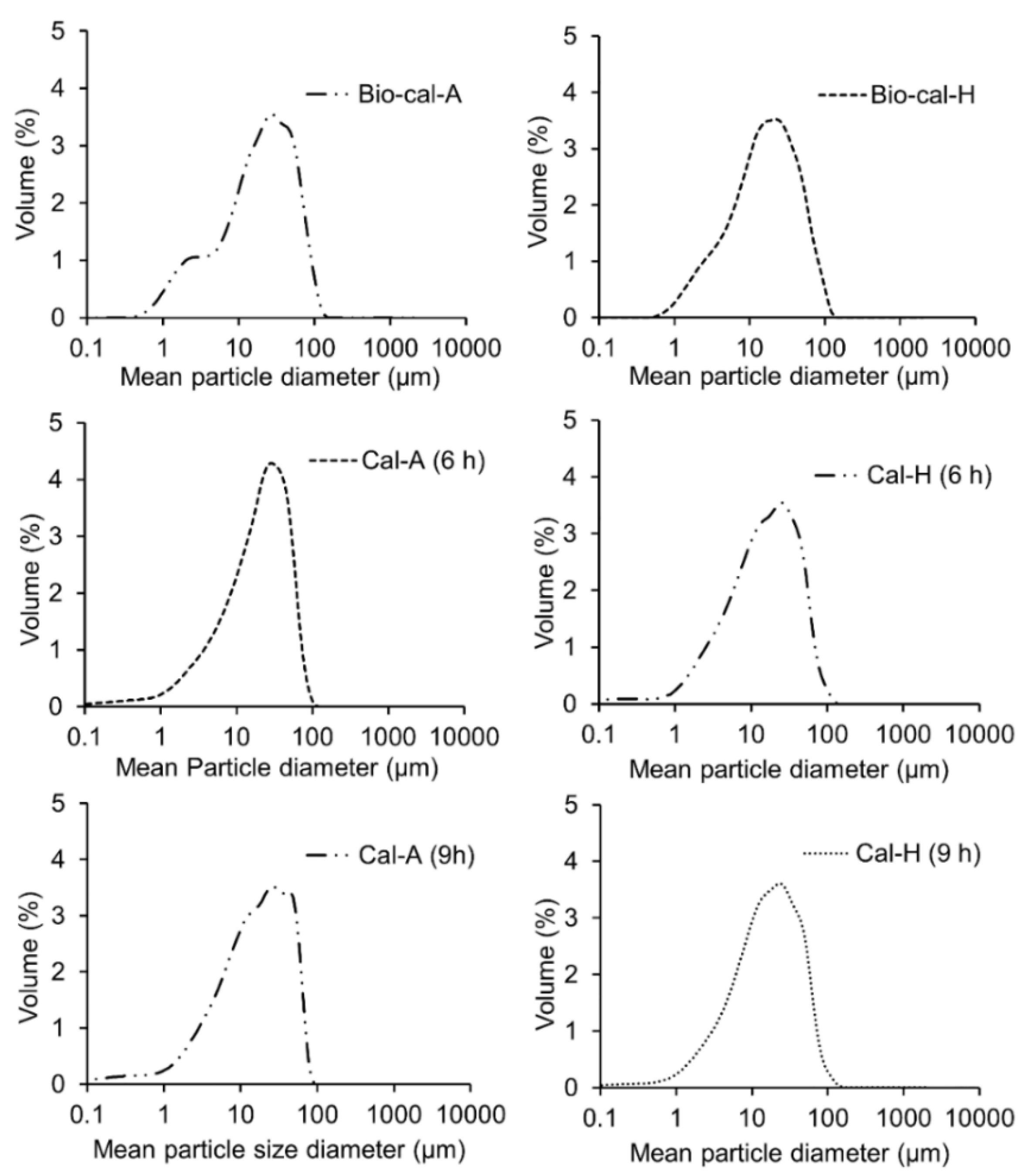
3.4. Mean Particle Size of Bio-Calcium and Calcine Bones
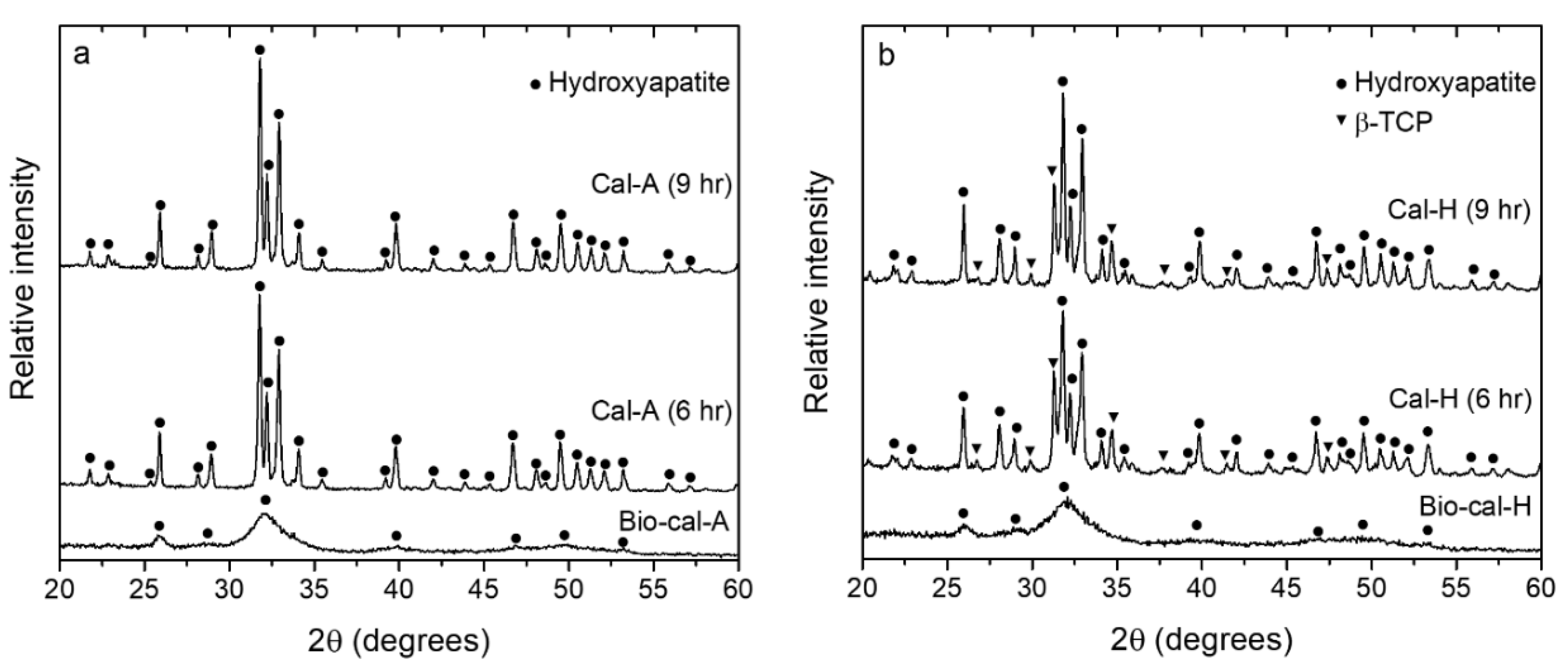
3.5. X-ray Diffraction (XRD) Patterns of Bio-Calcium and Calcine Bone Powders
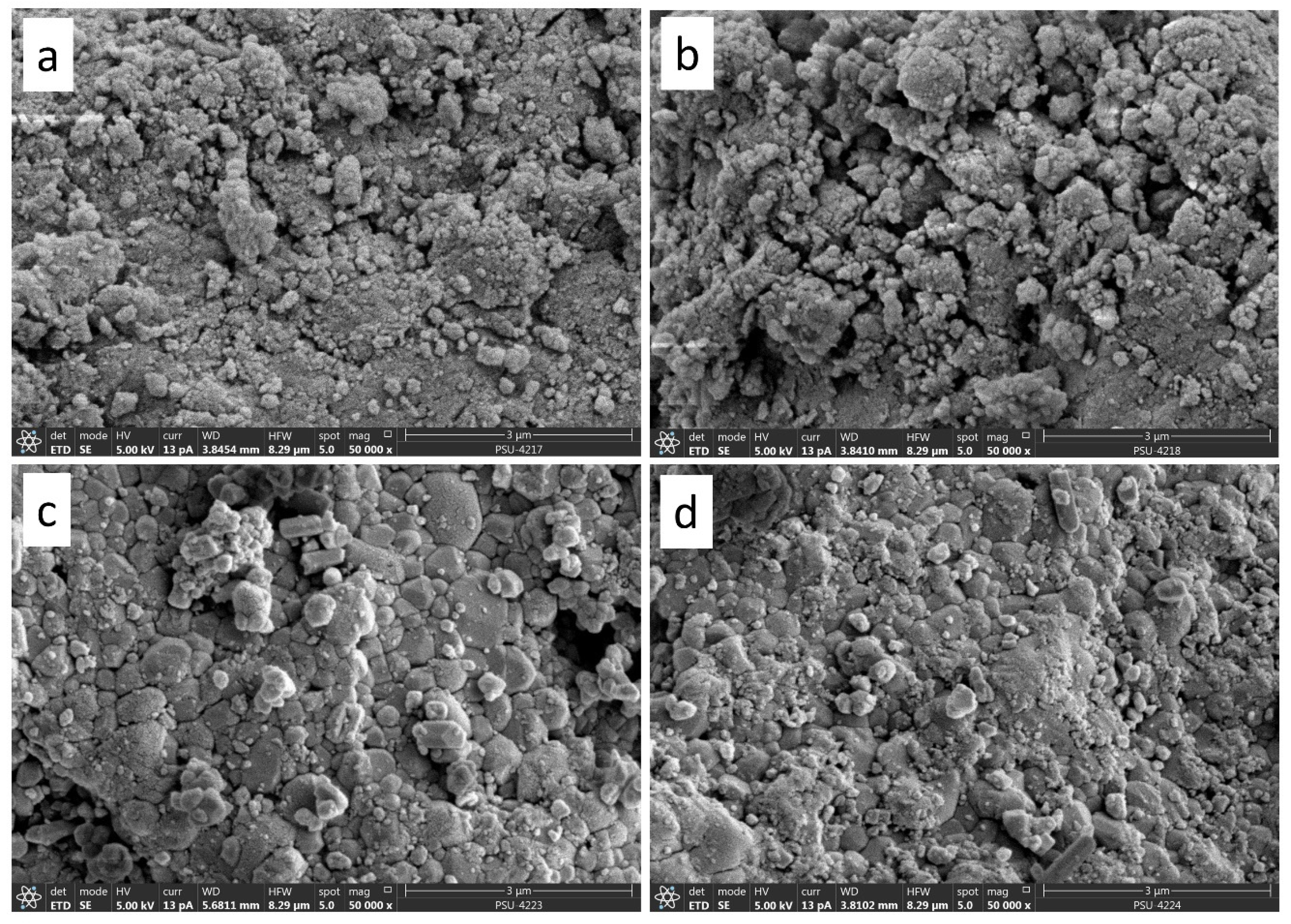
3.6. Scanning Electron Microscopy with Energy Dispersive X-ray Spectroscopy (SEM-EDX)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects: The 2017 Revision, Key Fndings and Advance Tables; Working Paper No. ESA/P/WP/248; United Nations: New York, NY, USA, 2017. [Google Scholar]
- See, S.F.; Hoo, L.; Babji, A. Optimization of enzymatic hydrolysis of salmon (Salmo salar) skin by Alcalase. Int. Food Res. J. 2011, 18, 1359–1365. [Google Scholar]
- Sinthusamran, S.; Idowu, A.T.; Benjakul, S.; Prodpran, T.; Yesilsu, A.F.; Kishimura, H. Effect of proteases and alcohols used for debittering on characteristics and antioxidative activity of protein hydrolysate from salmon frames. J. Food Sci. Technol. 2019, 57, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Mad-Ali, S.; Senphan, T.; Sookchoo, P. Biocalcium powder from precooked skipjack tuna bone: Production and its characteristics. J. Food Biochem. 2017, 41, e12412. [Google Scholar] [CrossRef]
- Cashman, K.D. Calcium intake, calcium bioavailability and bone health. Br. J. Nutr. 2002, 87, 169–177. [Google Scholar] [CrossRef]
- Watanabe, T.; Kiron, V.; Satoh, S. Trace minerals in fish nutrition. Aquaculture 1997, 151, 185–207. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Pou, J.; Comesaña, R.; Lusquiños, F.; De Carlos, A.; León, B. Biological hydroxyapatite obtained from fish bones. Mater. Sci. Eng. C 2012, 32, 478–486. [Google Scholar] [CrossRef]
- Piccirillo, C.; Silva, M.; Pullar, R.C.; Da Cruz, I.B.; Jorge, R.; Pintado, M.; Castro, P. Extraction and characterisation of apatite- and tricalcium phosphate-based materials from cod fish bones. Mater. Sci. Eng. C 2013, 33, 103–110. [Google Scholar] [CrossRef]
- Figueiredo, M.M.L.; Fernando, A.; Martins, G.; Freitas, J.; Judas, F.; Figueiredo, H. Effect of the calcination temperature on the composition and microstructure of hydroxyapatite derived from human and animal bone. Ceram. Int. 2010, 36, 2383–2393. [Google Scholar] [CrossRef]
- Goto, T.; Sasaki, K. Effects of trace elements in fish bones on crystal characteristics of hydroxyapatite obtained by calcination. Ceram. Int. 2014, 40, 10777–10785. [Google Scholar] [CrossRef]
- Cheng, L.; Ye, F.; Yang, R.; Lu, X.; Shi, Y.; Li, L.; Fan, H.; Bu, H. Osteoinduction of hydroxyapatite/β-tricalcium phosphate bioceramics in mice with a fractured fibula. Acta Biomater. 2010, 6, 1569–1574. [Google Scholar] [CrossRef]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar]
- Bergman, I.; Loxley, R. Two Improved and Simplified Methods for the Spectrophotometric Determination of Hydroxyproline. Anal. Chem. 1963, 35, 1961–1965. [Google Scholar] [CrossRef]
- Benjakul, S.; Mad-Ali, S.; Senphan, T.; Sookchoo, P. Characteristics of Biocalcium from Pre-cooked Skipjack Tuna Bone as Affected by Different Treatments. Waste Biomass-Valorization 2017, 9, 1369–1377. [Google Scholar] [CrossRef]
- Ensminger, L.G. The Association of Official Analytical Chemists. Clin. Toxicol. 1976, 9, 471. [Google Scholar] [CrossRef]
- Feist, B.; Mikula, B. Preconcentration of heavy metals on activated carbon and their determination in fruits by inductively coupled plasma optical emission spectrometry. Food Chem. 2014, 147, 302–306. [Google Scholar] [CrossRef]
- Mad-Ali, S.; Benjakul, S.; Prodpran, T.; Maqsood, S. Characteristics and gel properties of gelatin from goat skin as affected by spray drying. Dry. Technol. 2017, 35, 218–226. [Google Scholar] [CrossRef]
- Chuaychan, S.; Benjakul, S.; Kishimura, H. Characteristics of acid- and pepsin-soluble collagens from scale of seabass (Lates calcarifer). LWT 2015, 63, 71–76. [Google Scholar] [CrossRef]
- Kumoro, A.C.; Sofiah, S.; Aini, N.; Retnowati, D.S.; Budiyati, C.S. Effect of Temperature and Particle Size on the Alkaline Extraction of Protein From Chicken Bone Waste. Reaktor 2010, 13, 124. [Google Scholar] [CrossRef]
- Liu, D.; Wei, G.; Li, T.; Hu, J.; Lu, N.; Regenstein, J.M.; Zhou, P. Effects of alkaline pretreatments and acid extraction conditions on the acid-soluble collagen from grass carp (Ctenopharyngodon idella) skin. Food Chem. 2015, 172, 836–843. [Google Scholar] [CrossRef]
- Wu, H.-C.; Chen, H.-M.; Shiau, C.-Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 2003, 36, 949–957. [Google Scholar] [CrossRef]
- Al-Qtaitat, A.I.; Aldalaen, S.M. The Isolation and Characterization of Glycosylated Phosphoproteins From Herring Fish Bones. J. Biol. Chem. 2010, 285, 36170–36178. [Google Scholar]
- Garner, S.; Anderson, J. Skeletal Tissues and Mineralization. Diet Nutr. Bone Health 2011, 33, 49–50. [Google Scholar]
- Sae-Leaw, T.; Benjakul, S. Physico-chemical properties and fishy odour of gelatin from seabass (Lates calcarifer) skin stored in ice. Food Biosci. 2015, 10, 59–68. [Google Scholar] [CrossRef]
- Benjakul, S.; Mad-Ali, S.; Sookchoo, P. Characteristics of Biocalcium Powders from Pre-Cooked Tongol (Thunnus tonggol) and Yellowfin (Thunnus albacores) Tuna Bones. Food Biophys. 2017, 12, 412–421. [Google Scholar] [CrossRef]
- Pısecký, J. Handbook of milk powder manufacture. Niro A/S, Copenhagen. Proc. Eng. 1997, 3, 3–9. [Google Scholar]
- Londoño-Restrepo, S.M.; Jeronimo-Cruz, R.; Millán-Malo, B.M.; Rivera-Muñoz, E.M.; Rodríguez-García, M.E. Effect of the Nano Crystal Size on the X-ray Diffraction Patterns of Biogenic Hydroxyapatite from Human, Bovine, and Porcine Bones. Sci. Rep. 2019, 9, 5915. [Google Scholar] [CrossRef]
- Meinke, D.K.; Skinner, H.C.W.; Thomson, K.S. X-ray diffraction of the calcified tissues inPolypterus. Calcif. Tissue Int. 1979, 28, 37–42. [Google Scholar] [CrossRef]
- Choël, M.; Deboudt, K.; Osán, J.; Flament, P.; Van Grieken, R. Quantitative Determination of Low-ZElements in Single Atmospheric Particles on Boron Substrates by Automated Scanning Electron Microscopy−Energy-Dispersive X-ray Spectrometry. Anal. Chem. 2005, 77, 5686–5692. [Google Scholar] [CrossRef]

| Chemical Composition | Bio-Cal-A | Bio-Cal-H | Cal-A (6 h) | Cal-H (6 h) | Cal-A (9 h) | Cal-H (9 h) |
|---|---|---|---|---|---|---|
| Moisture (%) | 4.82 ± 0.07 d | 7.81 ± 0.04 e | 0.27 ± 0.00 b | 0.45 ± 0.01 c | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Protein (%) * | 12.07 ± 0.18 b | 20.90 ± 0.06 c | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Fat (%) * | 0.33 ± 0.01 b | 1.70 ± 0.01 c | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Ash (%) * | 82.78 ± 0.25 b | 69.59 ± 0.57 a | 99.73 ± 0.05 d | 99.55 ± 0.32 c | 99.99 ± 0.00 f | 99.97 ± 0.00 e |
| Hydroxyproline (mg/g) * | 5.07 ± 0.01 b | 14.79 ± 0.02 c | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| TBARS (mg malonaldehyde/kg sample) * | 0.95 ± 0.00 b | 3.34 ± 0.01 c | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Calcium (%) * | 30.88 ± 0.33 b | 27.32 ± 0.17 a | 36.01 ± 0.26 e | 31.54 ± 0.20 c | 38.84 ± 0.55 f | 34.24 ± 0.40 d |
| Phosphorus (%) * | 14.40 ± 0.39 b | 13.22 ± 0.48 a | 16.79 ± 0.25 e | 15.16 ± 0.32 c | 18.11 ± 0.35 f | 16.27 ± 0.45 d |
| Mole ratio Ca/P | 1.66 | 1.60 | 1.66 | 1.61 | 1.66 | 1.63 |
| Parameters | Bio-Cal-A | Bio-Cal-H | Cal-A (6 h) | Cal-H (6 h) | Cal-A (9 h) | Cal-H (9 h) |
|---|---|---|---|---|---|---|
| Mean particle size (d43, µm) | 26.53 ± 3.49 f | 24.05 ± 3.14 d | 25.36 ± 2.78 e | 22.21 ± 2.84 a | 22.39 ± 2.64 b | 23.12 ± 2.94 c |
| L* | 95.29 ± 0.08 f | 94.84 ± 0.08 d | 93.61 ± 0.01 c | 69.49 ± 0.04 a | 95.01 ± 0.01 e | 80.68 ± 0.01 b |
| A* | −0.60 ± 0.02 c | −0.31 ± 0.07 d | −1.59 ± 0.01 b | −0.23 ± 0.04 e | −2.63 ± 0.01 a | −0.36 ± 0.03 d |
| B* | 7.13 ± 0.02 d | 6.86 ± 0.14 c | −0.19 ± 0.02 b | −0.10 ± 0.07 b | −1.67 ± 0.01 a | −0.22 ± 0.01 b |
| ΔE* | 6.94 ± 0.12 d | 6.60 ± 0.08 c | 0.87 ± 0.14 a | 24.15 ± 0.15 f | 3.00 ± 0.14 b | 12.97 ± 0.09 e |
| ΔC* | 7.15 ± 0.03 f | 6.87 ± 0.14 e | 1.60 ± 0.01 c | 0.26 ± 0.06 a | 3.11 ± 0.00 d | 0.42 ± 0.01 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idowu, A.T.; Benjakul, S.; Sinthusamran, S.; Sae-leaw, T.; Suzuki, N.; Kitani, Y.; Sookchoo, P. Effect of Alkaline Treatment on Characteristics of Bio-Calcium and Hydroxyapatite Powders Derived from Salmon Bone. Appl. Sci. 2020, 10, 4141. https://doi.org/10.3390/app10124141
Idowu AT, Benjakul S, Sinthusamran S, Sae-leaw T, Suzuki N, Kitani Y, Sookchoo P. Effect of Alkaline Treatment on Characteristics of Bio-Calcium and Hydroxyapatite Powders Derived from Salmon Bone. Applied Sciences. 2020; 10(12):4141. https://doi.org/10.3390/app10124141
Chicago/Turabian StyleIdowu, Anthony Temitope, Soottawat Benjakul, Sittichoke Sinthusamran, Thanasak Sae-leaw, Nobuo Suzuki, Yoichiro Kitani, and Pornsatit Sookchoo. 2020. "Effect of Alkaline Treatment on Characteristics of Bio-Calcium and Hydroxyapatite Powders Derived from Salmon Bone" Applied Sciences 10, no. 12: 4141. https://doi.org/10.3390/app10124141







