Dental Treatment of White Spots and a Description of the Technique and Digital Quantification of the Loss of Enamel Volume
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Clinical Process to Remove White Spots Located in the Enamel
- Isolation of the operative field using anchorage clamps in the posterior regions and ligatures in the anterior regions to ensure unvarying isolation during treatment.
- Initial scan of affected teeth from which an STL file was generated. Scans were taken before and after different treatment applications to evaluate the quantity of tissue eliminated.
- Application of the HCl-based product to white spots:
- 3.1.
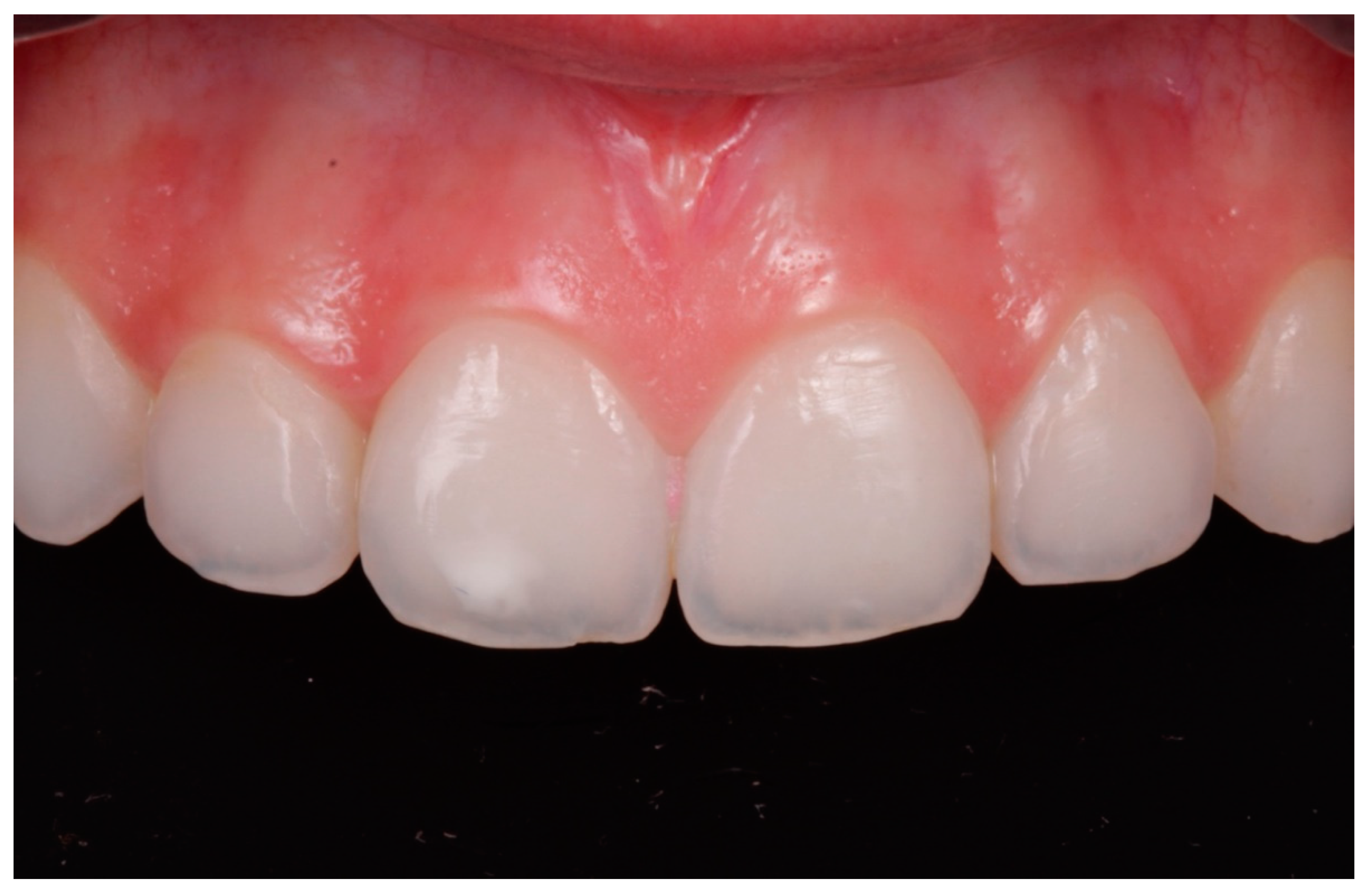
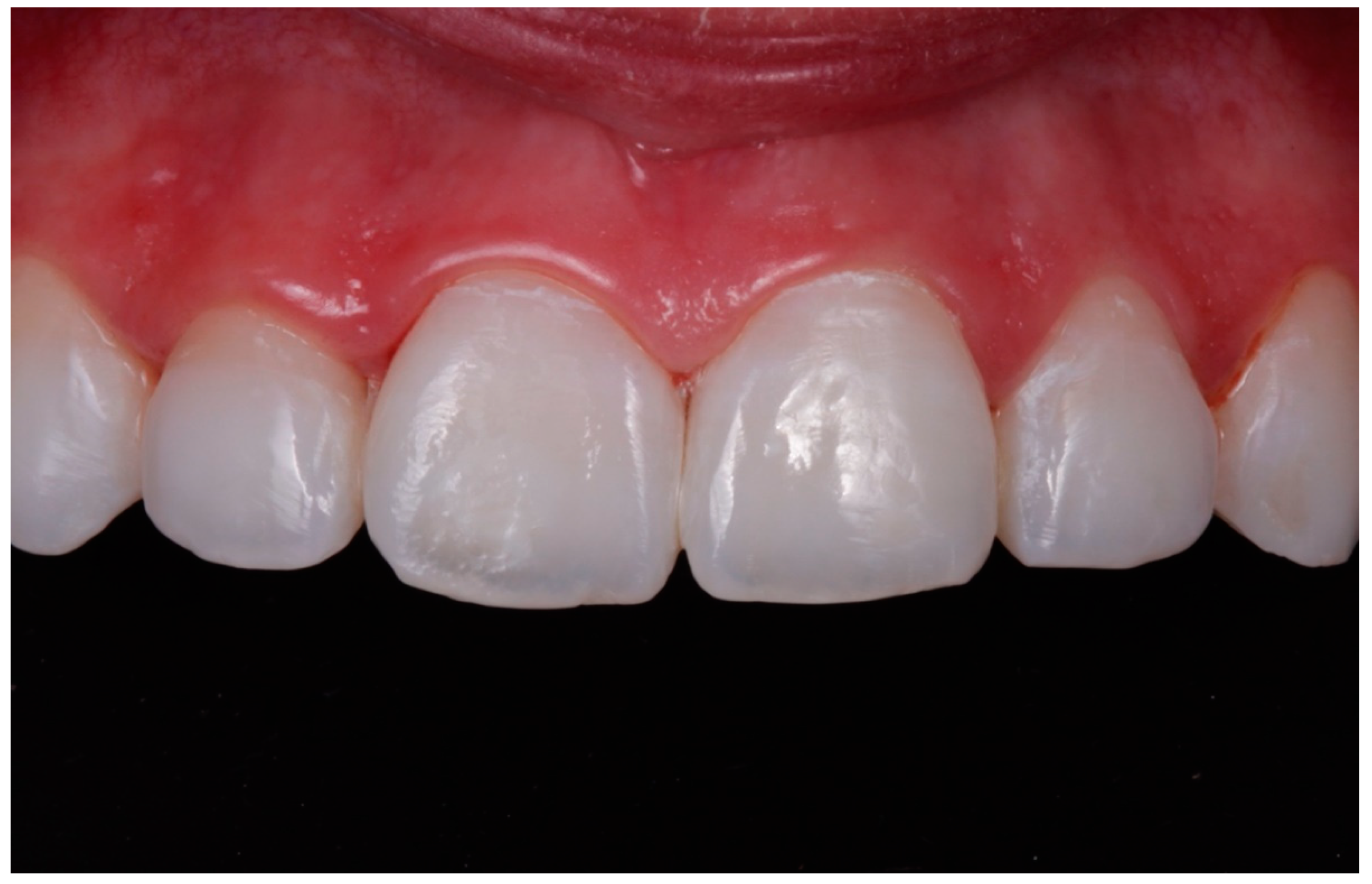
- In cases where the 15% HCl product was applied (ICON etch; DMG), each affected tooth received three applications of chemical erosion treatment for two minutes each. The product was placed with the tip of a sponge applicator on the enamel surface for 2 min. When 2 min had passed, the enamel was washed with abundant water for 30 s (Figure 1, Figure 2 and Figure 3).
- 3.2.
- In this study, the abrasive erosion treatment (Opalustre; Ultradent) contained a 6.6% HCl slurry with silicon carbide microparticles; this combination offered a chemical stain removal along with gentle mechanical abrasion. In cases using 6.6% HCl, teeth with white spots were treated with various applications according to the manufacturer’s instructions. The product was spread on the enamel surface in a 1 mm thick uniform layer. Afterwards, it was rubbed homogeneously for 1 min using Opal Cups (Ultradent) provided by the manufacturer. The enamel was then washed in abundant water for 30 s (Figure 4, Figure 5 and Figure 6).
- Affected teeth were then scanned with an intraoral scanner (TrueDefinition, 3M-ESPE) and the data were used to generate STL files.
- Volumetric change was analyzed by means of superimposing the STL files obtained from the scans. The STL files were imported to use the reverse engineering software Geomagic Wrap (3D system), which aligns the data in each file using the best fit algorithm. To do this, the initial file reference mesh was selected, and subsequent meshes were aligned over it as independent floating meshes. For the alignment process, the vestibular area of the teeth presenting variations from one scan to another was omitted due to HCl application.
- After the final alignment, each tooth was exported in a binary STL format to a program called GOM Inspect (Precise Industrial 3D Metrology). This program generates a gradient color map representing variations from 250 µm to −250 µm so that any point exhibiting a difference between the initial mesh and the succession of the HCl application is represented by a specific color.
- After quantifying the volume of eliminated enamel, the data were processed to calculate the depth affected by the treatment within the enamel layer. In this way, the dentist was able to know whether to continue applying HCl, to terminate treatment, or to carry out more invasive treatment.
2.2. In Vitro Volumetric Study of Application of HCl-Based Agents
2.3. Scanning Electron Microscopic (SEM) Analysis
3. Results
- In chemical erosion treatment cases, i.e., those that used 15% HCl (Icon Etch; DMG), the mean volume of enamel eliminated was −0.042 mm (Figure 9).
- In cases that used the erosive abrasion treatment, i.e., those treated with 6.6% HCl (Opalustre; Ultradent), the mean volume of enamel eliminated was −0.12 mm (Figure 10).
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nanci, A. Enamel: Composition, formation, and structure. In Ten Cate’s oral Histology Development, Structure, and Function; Nanci, A., Ed.; Mosby Elsevier: St. Louis, MO, USA, 2008; pp. 141–190. [Google Scholar]
- Freiman, A.; Borsuk, D.; Barankin, B.; Sperber, G.H.; Krafchik, B. Dental manifestations of dermatologic conditions. J. Am. Acad. Dermatol. 2009, 60, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Seow, W.K. Enamel hipoplasia in the primary dentition: A review. J. Dent. Child. 1991, 58, 441–452. [Google Scholar]
- Seow, W.K. Developmental defects of enamel and dentine: Challenges for basic science research and clinical management. Aust. Dental. J. 2014, 59, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.; Vicente, M.; García, V.; Montiel, J.M.; Paredes, V.; Almerich, J.M.; Bellot, C. Enamel remineralization therapies for treating post orthodontic white-spot lesions. J. Am. Dent. Assoc. 2018, 149, 778–786. [Google Scholar] [CrossRef]
- Freitas, M.C.C.A.; Nunes, L.V.; Comar, L.P.; Rios, D.; Magalhaes, A.C.; Honório, H.M.; Wang, L. In vitro effect of a resin infiltrant on different artificial caries-like enamel. Arch. Oral Biol. 2018, 95, 118–124. [Google Scholar] [CrossRef]
- Denis, M.; Atlan, A.; Vennat, E.; Tirlet, G.; Attal, J.P. White defects on enamel: Diagnosis and anatomopathology: Two essential factors for proper treatment (part 1). Int. Orthod. 2013, 11, 139–165. [Google Scholar] [CrossRef]
- Borges, A.B.; Caneppele, T.M.F.; Materson, D.; Maia, L.C. Is resin infiltration an effective esthetic treatment for enamel development defects and White spot lesions? A systematic review. J. Dent. 2017, 56, 11–18. [Google Scholar] [CrossRef]
- Mazur, M.; Westland, S.; Guerra, F.; Corridore, D.; Vichi, M.; Maurotti, A. Objective and subjective aesthetic performance of Icon treatment for enamel hypomineralization lesions in young adolescents: A retrospective single center study. J. Dent. 2018, 68, 104–108. [Google Scholar] [CrossRef]
- Castiblanco, G.A.; Martignon, S.; Castellanos, J.E.; Mejía, W.A. Pathogenesis of dental fluorosis: Biochemical and cellular. Rev. Fac. Odontol. Univ. Antioq. 2017, 28, 408–421. [Google Scholar] [CrossRef][Green Version]
- Weerheijm, K.L.; Jälevikb, B.; Alaluusua, S. Molar incisor-hypomineralisation. Caries. Res. 2001, 35, 390–391. [Google Scholar] [CrossRef]
- Gorelick, L.; Geiger, A.M.; Gwinett, A.J. Incidence of White spot formation after bonding and banding. Am. J. Orthod. 1982, 81, 93–98. [Google Scholar] [CrossRef]
- Hadler-Olsen, S.; Sandvik, K.; El-Agroudi, M.A.; Øgaard, B. The incidence of caries and white spot lesions in orthodontically treated adolescents with a comprehensive caries prophylactic regimen—A prospective study. Eur. J. Orthod. 2011, 34, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Boersma, J.G.; Van der Veen, M.H.; Lagerweij, M.D.; Bokhout, B.; Prahl-Andresen, B. Caries prevalence measured with QLF after treatment with fixed orthodontic appliances: Influencing factors. Caries Res. 2005, 39, 41–47. [Google Scholar] [CrossRef]
- Eltayeb, M.K.; Ibrahim, I.E.; El Karim, I.A.; Sanhouri, N.M. Distribution of white spot lesions among orthodontic patients attending teaching institutes in Khartoum. BMC Oral. Health. 2017, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Paula, A.B.; Fernandes, A.R.; Coelho, A.S.; Marto, C.M.; Ferreira, M.M.; Caramelo, F.; do Vale, F.; Carrilho, E. Therapies for White Spot Lesions—A Systematic Review. J. Evid. Based Dent. Pract. 2017, 17, 23–38. [Google Scholar] [CrossRef]
- Román-Rodríguez, J.L.; Agustín-Panadero, R.; Roig-Vanaclocha, A.; Amengual-Lorenzo, J. A tooth whitening and chemical abrasive protocol for the treatment of developmental enamel defects. J. Prosthet. Dent. 2020, 123, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Knösel, M.; Attin, R.; Beckers, K.; Attin, T. A randomized CIE L*a*b* evaluation of external bleaching therapy effects on fluorotic enamel stains. Quint. Int. 2008, 39, 391–399. [Google Scholar]
- Bailey, D.L.; Adams, G.G.; Tsao, C.E.; Hyslop, A.; Escobar, K.; Manton, D.J. Regression of post-orthodontic lesions by a remineralizing cream. J. Dent. Res. 2009, 88, 1148–1153. [Google Scholar] [CrossRef]
- Willmot, D.R. White lesions after orthodontic treatment: Does low fluoride make a difference? J. Orthod. 2004, 31, 235–242. [Google Scholar] [CrossRef]
- Cate, J.M.; Arends, J. Remineralization of artificial enamel lesions in vitro. Caries Res. 1977, 12, 277–286. [Google Scholar] [CrossRef]
- Naumova, E.A.; Niemann, N.; Aretz, L.; Arnold, W.H. Effects of different amine fluoride concentrations on enamel remineralization. J. Dent. 2012, 40, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Van der Veen, M.H.; Mattousch, T.; Boersma, J.G. Longitudinal development of caries lesions after orthodontic treatment evaluated by quantitative light-induced fluorescence. Am. J. Orthod. Dentofacial. Orthop. 2007, 131, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Ardu, S.; Castioni, N.V.; Benbachir, N.; Krehci, I. Minimally invasive treatment of white spot enamel lesions. Quint. Int. 2007, 38, 633–636. [Google Scholar]
- Øgaard, B. Incidence of filled surfaces from 10 to 18 years of age in an orthodontically treated and untreated group in Norway. Eur. J. Orthod. 1989, 11, 116–119. [Google Scholar] [CrossRef]
- Benbachir, N.; Ardu, S.; Krejci, I. Indications and limits of the microabrasion technique. Quint. Int. 2007, 38, 811–815. [Google Scholar]
- Croll, T.P. Enamel microabrasion: The technique. Quint. Int. 1989, 20, 395–400. [Google Scholar]
- Croll, T.P.; Helpin, M.L. Enamel microabrasion: A new approach. J. Esthet. Dent. 2000, 12, 64–71. [Google Scholar] [CrossRef]
- Sundfeld, R.H.; Croll, T.P.; Briso, A.L.; de Alexandre, R.S.; Sundfeld-Neto, D. Considerations about enamel microabrasion after 18 years. Am. J. Dent. 2007, 20, 67–72. [Google Scholar]
- Raper, H.R.; Manser, J.G. Removal of brown stains from fluorine mottled teeth. Dent. Digit. 1941, 9, 390–396. [Google Scholar]
- Ardu, S.; Benbachir, N.; Stavridakis, M.; Dietschi, D.; Krejci, I.; Feilzer, A. A combined chemo-mechanical approach for aesthetic management of superficial enamel defects. Br. Dent. J. 2009, 206, 205–208. [Google Scholar] [CrossRef]
- Croll, T.P. Bonded resin selant for smooth surface enamel defects: New concepts in microrestorative dentistry. Quint. Int. 1987, 18, 5–10. [Google Scholar]
- Paris, S.; Meyer-Lueckel, H.; Kielbassa, A.M. Resin infiltration of natural caries lesions. J. Dent. Res. 2007, 7, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Paris, S.; Meyer-Lueckel, H. Masking of labial enamel white spot lesions by resin infiltration- a clinical report. Quint. Int. 2009, 40, 713–718. [Google Scholar]
- Paris, S.; Dorfer, C.E.; Meyer-Lueckel, H. Surface conditioning of natural enamel caries lesions in deciduous teeth in preparation for resin infiltration. J. Dent. 2010, 38, 65–71. [Google Scholar] [CrossRef]
- Tirlet, G.; Chabouis, H.F.; Attal, J.P. Infiltration, a new therapy for masking enamel White spots: A 19-month follow-up case series. J. Esthet. Dent. 2013, 8, 180–190. [Google Scholar]
- Cazzolla, A.P.; De Franco, A.R.; Lacaita, M.; Lacarbonara, V. Efficacy of 4-year treatment of icon infiltration resinon postorthodontic white spot lesions. BMJ Case Rep. 2018. [Google Scholar] [CrossRef]
- Atsu, S.S.; Aka, P.; Cenker, H.; Atakan, C. Age-related changes in tooth enamel as measured by electron microscopy: Implications for porcelain laminate veneers. J. Prosthet. Dent. 2005, 94, 336–341. [Google Scholar] [CrossRef]
- Sanmarco, G. Combined minimally invasive treatment of White and brown fluorotic discolorations in a teenager: A case report. Int. J. Esthet. Dent. 2019, 14, 148–155. [Google Scholar]
- Burke, F.J.; Kelleher, M.G. The “daughter test” in elective esthetic dentistry. J. Esthet. Restor. Dent. 2009, 21, 143–146. [Google Scholar] [CrossRef]
- Yim, H.K.; Kwon, H.K.; Kim, B.I. Modification of surface pre-treatment for resin infiltration to mask natural white spot lesions. J. Dent. 2014, 42, 588–594. [Google Scholar] [CrossRef]
- Meyer-Lueckel, H.; Paris, S.; Kielbassa, A.M. Surface layer erosion of natural caries lesions with phosphoric and hydrochloric acid gels in preparation for resin infiltration. Caries. Res. 2007, 41, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Balmer, R.C.; Laskey, D.; Mahoney, E.; Toumba, K.J. Prevalence of enamel defects and MIH in non-fluoridates and fluoridated communities. Eur. J. Paediatr. Dent. 2005, 6, 209–212. [Google Scholar] [PubMed]
- Robles, M.J.; Ruiz, M.; Bravo-Perez, M.; González, E.; Peñalver, M.A. Prevalence of enamel defects in primary and permanent teeth in a group of school children from Granada (Spain). Med. Oral. Patol. Oral. Cir. Bucal. 2013, 18, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Paris, S.; Schwendicke, F.; Keltsch, J.; Dörfer, C.; Meyer-Lueckel, H. Masking of white spot lesions by resin infiltration in vitro. J. Dent. 2013, 41, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.K.; Min, J.H.; Kwon, H.K.; Kim, B.I. Modification of surface pretreatment of white spot lesions to improve the safety and efficacy of resin infiltration. Korean J. Orthod. 2014, 44, 195–202. [Google Scholar] [CrossRef]
- Arnold, W.H.; Haddad, B.; Schaper, K.; Hagemann, K.; Lippold, C.; Danesh, G. Enamel surface alterations after repeated conditioning with HCL. Head Face Med. 2015, 11, 32. [Google Scholar] [CrossRef]
- Fons-Badal, C.; Pérez-Barquero, J.A.; Martínez-Martínez, N.; Faus-López, J.; Fons-Font, A.; Agustín-Panadero, R. A novel, fully digital approach to quantifying volume gain after soft tissue graft surgery. A pilot study. J. Clin. Periodontol. 2019, 20, 614–620. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Wong, K.H.; Lee, W.J.; Marizan Nor, M.; Mohd Hussaini, H.; Rosli, T.I. In vitro study of white spot lesion: Maxilla and mandibular teeth. Saudi. Dent. J. 2018, 30, 142–150. [Google Scholar] [CrossRef]
















© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roig-Vanaclocha, A.; Solá-Ruiz, M.F.; Román-Rodríguez, J.L.; Amengual-Lorenzo, J.; Alonso Pérez-Barquero, J.; Agustín-Panadero, R. Dental Treatment of White Spots and a Description of the Technique and Digital Quantification of the Loss of Enamel Volume. Appl. Sci. 2020, 10, 4369. https://doi.org/10.3390/app10124369
Roig-Vanaclocha A, Solá-Ruiz MF, Román-Rodríguez JL, Amengual-Lorenzo J, Alonso Pérez-Barquero J, Agustín-Panadero R. Dental Treatment of White Spots and a Description of the Technique and Digital Quantification of the Loss of Enamel Volume. Applied Sciences. 2020; 10(12):4369. https://doi.org/10.3390/app10124369
Chicago/Turabian StyleRoig-Vanaclocha, Ana, María Fernanda Solá-Ruiz, Juan Luis Román-Rodríguez, José Amengual-Lorenzo, Jorge Alonso Pérez-Barquero, and Rubén Agustín-Panadero. 2020. "Dental Treatment of White Spots and a Description of the Technique and Digital Quantification of the Loss of Enamel Volume" Applied Sciences 10, no. 12: 4369. https://doi.org/10.3390/app10124369
APA StyleRoig-Vanaclocha, A., Solá-Ruiz, M. F., Román-Rodríguez, J. L., Amengual-Lorenzo, J., Alonso Pérez-Barquero, J., & Agustín-Panadero, R. (2020). Dental Treatment of White Spots and a Description of the Technique and Digital Quantification of the Loss of Enamel Volume. Applied Sciences, 10(12), 4369. https://doi.org/10.3390/app10124369





