Synthesis of Multiwalled Carbon Nanotubes on Stainless Steel by Atmospheric Pressure Microwave Plasma Chemical Vapor Deposition
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. AMPCVD
2.2. Experimental Setup
2.3. Characterizations
3. Results and Discussion
3.1. Plasma Parameters
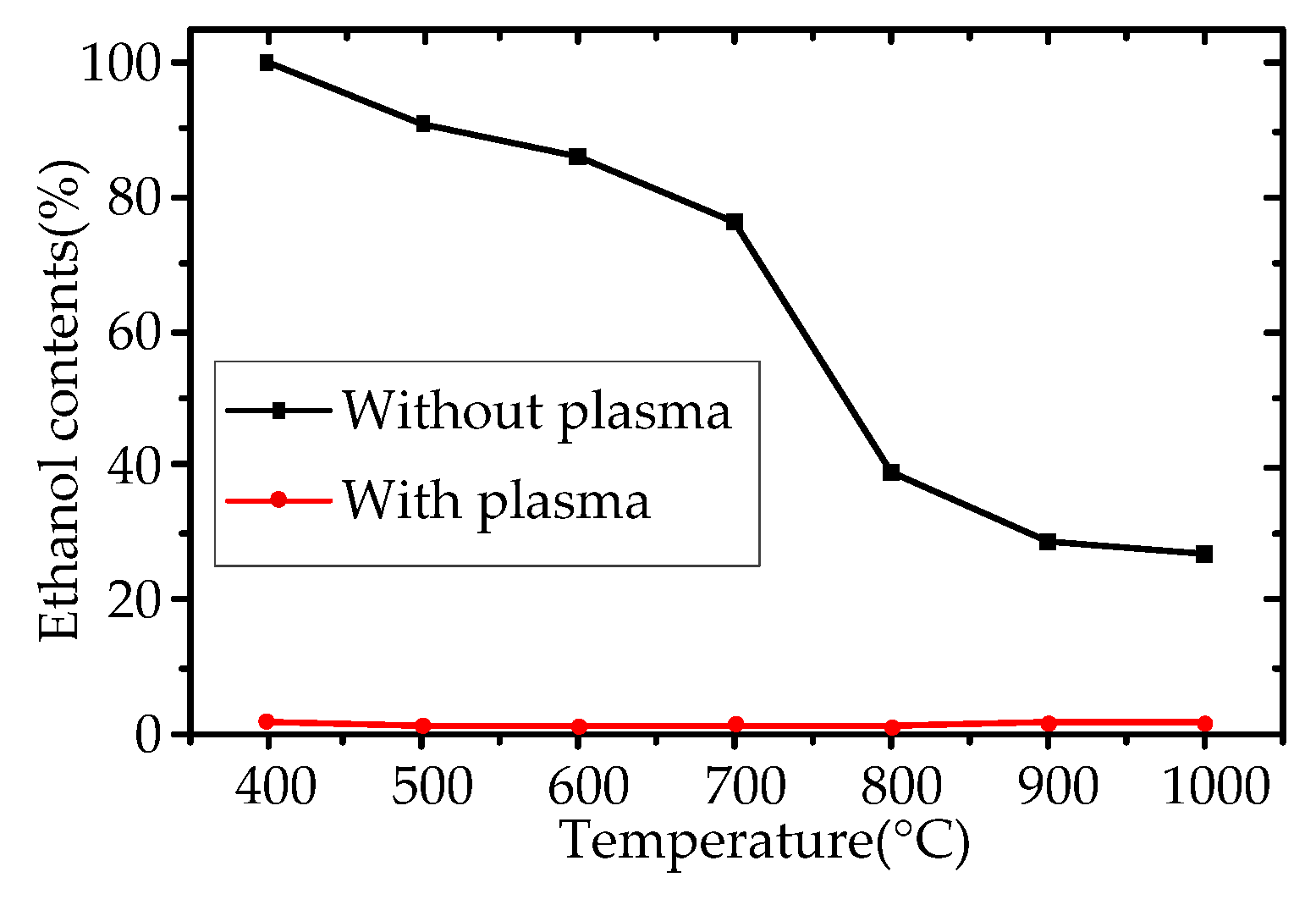
3.2. Exhaust Gas Detection
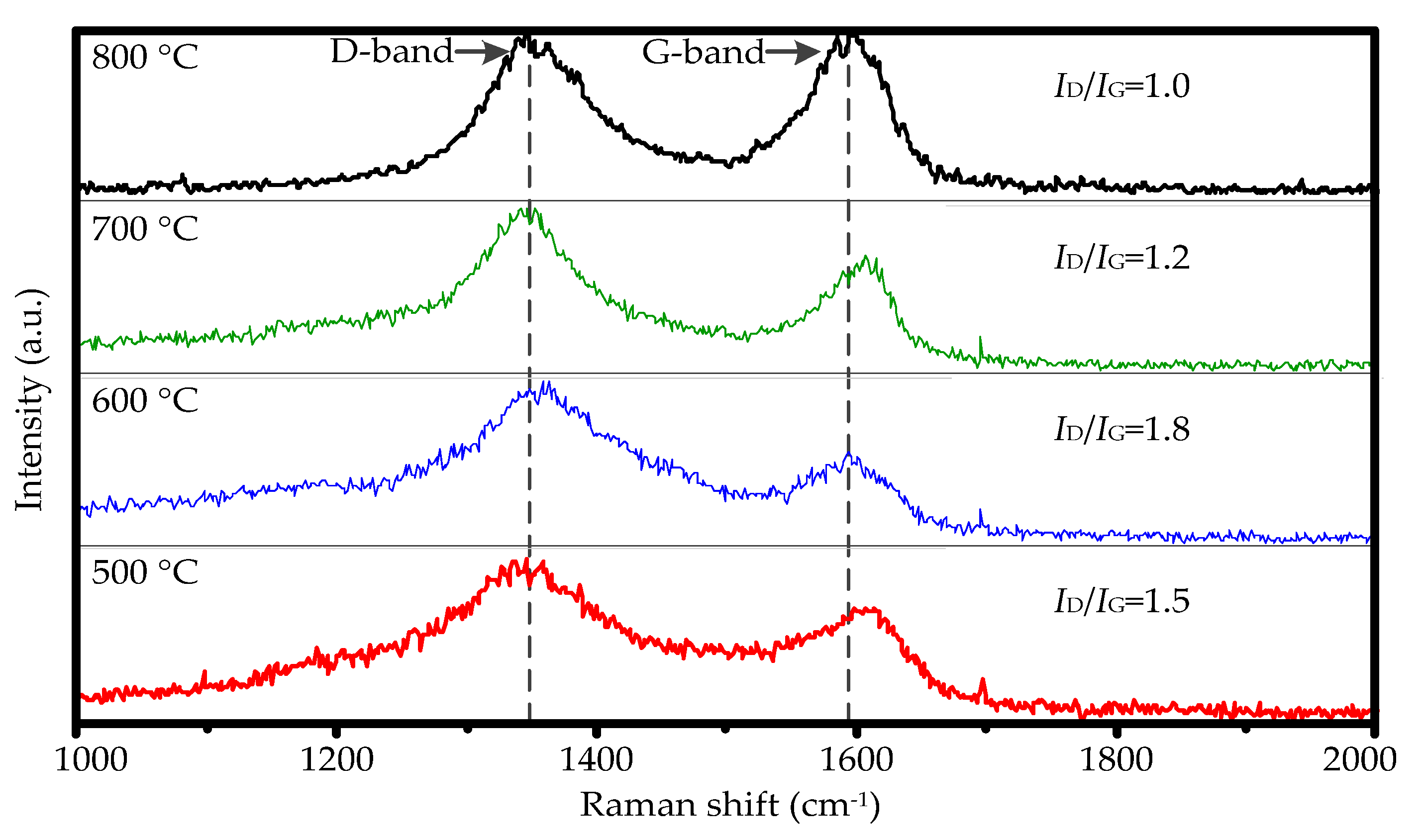
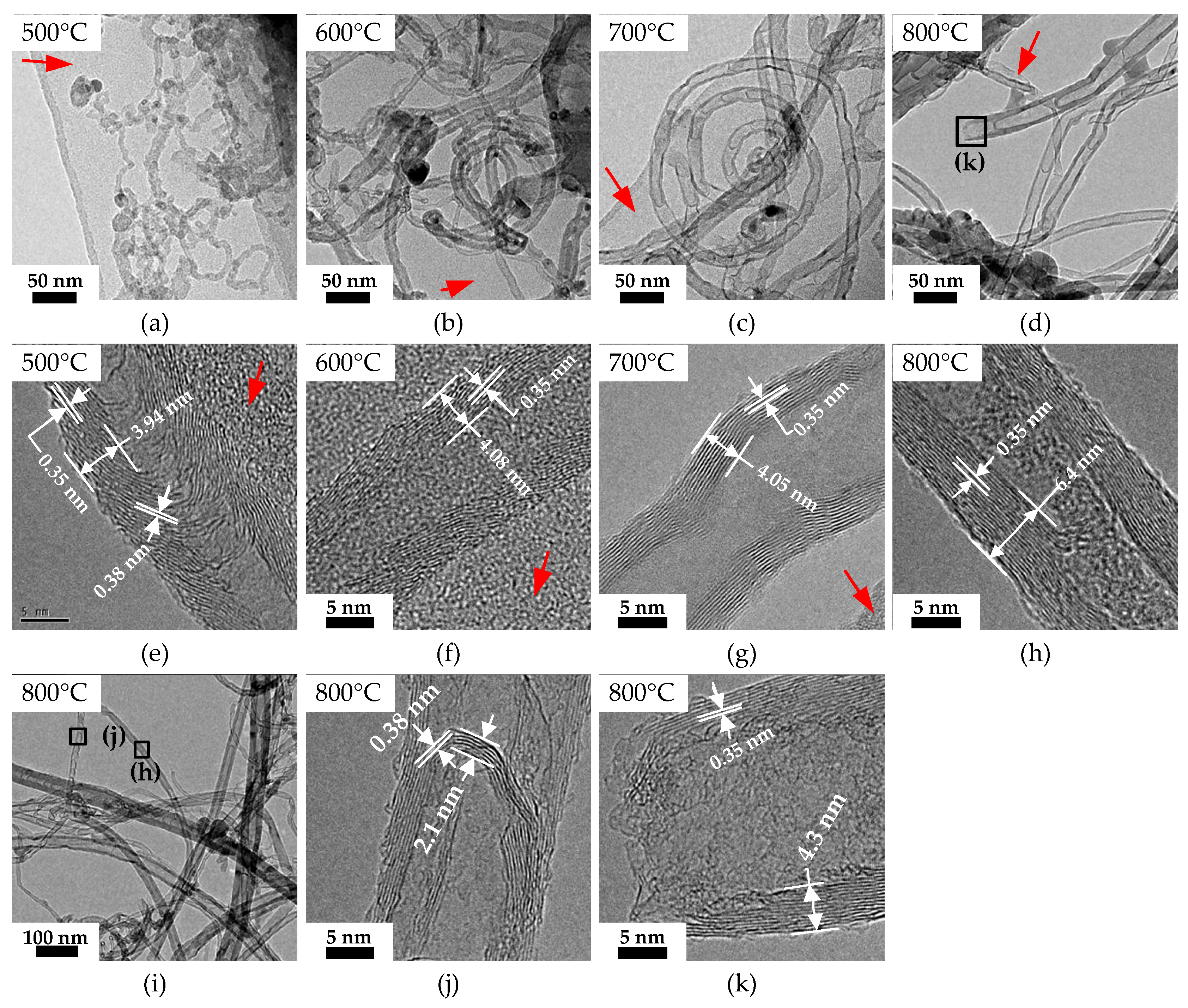
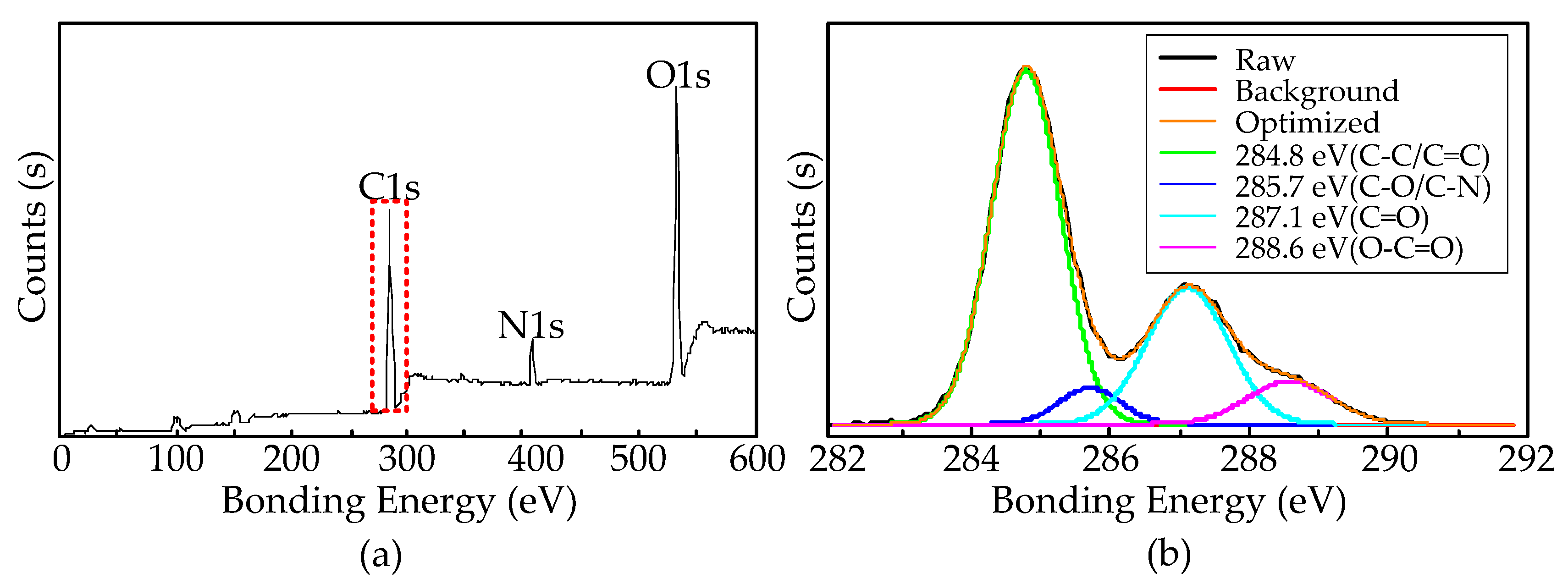
3.3. CNTs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Ren, Z.F.; Lan, Y.C.; Wang, Y. Physics, Concepts, Fabrication and Devices. In Aligned Carbon Nanotubes; Avouris, P., Bhushan, B., Bimberg, D., Klitzing, K., Sakaki, H., Weesendanger, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 67–71. [Google Scholar]
- Cheng, J.; Zou, X.P.; Yang, G.Q. Temperature Effects on Synthesis of Multi-Walled Carbon Nanotubes by Ethanol Catalyst Chemical Vapor Deposition. Adv. Mater. Res. 2010, 123, 799–802. [Google Scholar] [CrossRef]
- Li, W.Z.; Xie, S.S.; Qian, L.X.; Chang, B.H.; Zou, B.S. Large-Scale Synthesis of Aligned Carbon Nanotubes. Science 1996, 274, 1701. [Google Scholar] [CrossRef] [PubMed]
- Eres, G.; Puretzky, A.A.; Geohegan, D.B. In situ control of the catalyst efficiency in chemical vapor deposition of vertically aligned carbon nanotubes on predeposited metal catalyst film. Appl. Phys. Lett. 2004, 84, 1759–1761. [Google Scholar] [CrossRef]
- Suman, N.; Mauricio, L.; Melissa, C. Synthesis and field emission properties of vertically aligned carbon nanotube arrays on copper. Carbon 2012, 50, 2641–2650. [Google Scholar]
- Wang, Y.H.; Lin, J.; Huan, C.H. Synthesis of large area aligned carbon nanotube arrays from C2H2-H2 mixture by rf plasma-enhanced chemical vapor deposition. Appl. Phys. Lett. 2001, 79, 680–682. [Google Scholar] [CrossRef]
- Caughman, J.B.O.; Baylor, L.R.; Guillorn, M.A.; Merkulov, V.I.; Lowndes, D.H. Growth of vertically aligned carbon nanofibers by low-pressure inductively coupled plasma-enhanced chemical vapor deposition. Appl. Phys. Lett. 2003, 83, 1207. [Google Scholar] [CrossRef]
- Okai, M.; Muneyoshi, T.; Yaguchi, T.; Sasaki, S. Structure of carbon nanotubes grown by microwave-plasma-enhanced chemical vapor deposition. Appl. Phys. Lett. 2000, 77, 3468. [Google Scholar] [CrossRef]
- Kuttel, O.M.; Groening, O.; Emmenegger, C.; Schlapbach, L. Electron field emission from phase pure nanotube films grown in a methane/hydrogen plasma. Appl. Phys. Lett. 1998, 73, 2113. [Google Scholar] [CrossRef]
- Bower, C.; Zhu, W.; Jin, S.H.; Zhou, O. Plasma-induced alignment of carbon nanotubes. Appl. Phys. Lett. 2000, 77, 830. [Google Scholar] [CrossRef]
- Xiao, Y.; Ahmed, Z.; Ma, Z.C.; Zhou, C.J.; Zhang, L.N.; Chan, M. Low Temperature Synthesis of High-Density Carbon Nanotubes on Insulating Substrate. Nanomaterials 2019, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Perry, W.L.; Xu, H. Plasma torch production of macroscopic carbon nanotube structures. Carbon 2003, 41, 2555–2560. [Google Scholar] [CrossRef]
- Shin, D.H.; Hong, Y.C.; Uhm, H.S. Production of Carbon Nanotubes by Microwave Plasma-Torch at Atmospheric Pressure. Phys. Plasmas 2005, 12, 053504. [Google Scholar]
- Lenka, Z.; Marek, E.; Ondrej, J. Characterization of Carbon Nanotubes Deposited in Microwave Torch at Atmospheric Pressure. Plasma Process Polym. 2007, 4, S245–S249. [Google Scholar]
- Tripathi, P.V.; Durbach, S.; Coville, N.J. Synthesis of Multi-Walled Carbon Nanotubes from Plastic Waste Using a Stainless-Steel CVD Reactor as Catalyst. Nanomaterials 2017, 7, 284. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.D.; Sanchez, J.C.; Berenguer, A.; Golovko, V.B. Catalytic growth of carbon nanotubes on stainless steel: Characterization and frictional properties. Diam. Relat. Mater. 2008, 17, 1853–1857. [Google Scholar] [CrossRef]
- Park, D.; Kim, Y.H.; Lee, J.K. Pretreatment of stainless steel substrate surface for the growth of carbon nanotubes by PECVD. J. Mater. Sci. 2003, 38, 4933–4939. [Google Scholar] [CrossRef]
- Park, D. Synthesis of carbon nanotubes on metallic substrates by a sequential combination of PECVD and thermal CVD. Carbon 2003, 41, 1025–1029. [Google Scholar] [CrossRef]
- Yao, B.D.; Wang, N. Carbon nanotube arrays prepared by MWCVD. J. Phys. Chem. B 2001, 105, 11395–11398. [Google Scholar] [CrossRef]
- Hashempour, M.; Vicenzo, A.; Zhao, F.; Bestetti, M. Direct growth of MWCNTs on 316 stainless steel by chemical vapor deposition: Effect of surface nano-features on CNT growth and structure. Carbon 2013, 63, 330–347. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Tong, L.; Gao, B. Synthesis of Multiwalled Carbon Nanotubes on Stainless Steel by Atmospheric Pressure Microwave Plasma Chemical Vapor Deposition. Appl. Sci. 2020, 10, 4468. https://doi.org/10.3390/app10134468
Li D, Tong L, Gao B. Synthesis of Multiwalled Carbon Nanotubes on Stainless Steel by Atmospheric Pressure Microwave Plasma Chemical Vapor Deposition. Applied Sciences. 2020; 10(13):4468. https://doi.org/10.3390/app10134468
Chicago/Turabian StyleLi, Dashuai, Ling Tong, and Bo Gao. 2020. "Synthesis of Multiwalled Carbon Nanotubes on Stainless Steel by Atmospheric Pressure Microwave Plasma Chemical Vapor Deposition" Applied Sciences 10, no. 13: 4468. https://doi.org/10.3390/app10134468
APA StyleLi, D., Tong, L., & Gao, B. (2020). Synthesis of Multiwalled Carbon Nanotubes on Stainless Steel by Atmospheric Pressure Microwave Plasma Chemical Vapor Deposition. Applied Sciences, 10(13), 4468. https://doi.org/10.3390/app10134468




