Maleic Anhydride Modified Dicyclopentadiene Resin for Improving Wet Skid Resistance of Silica Filled SSBR/BR Composites
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
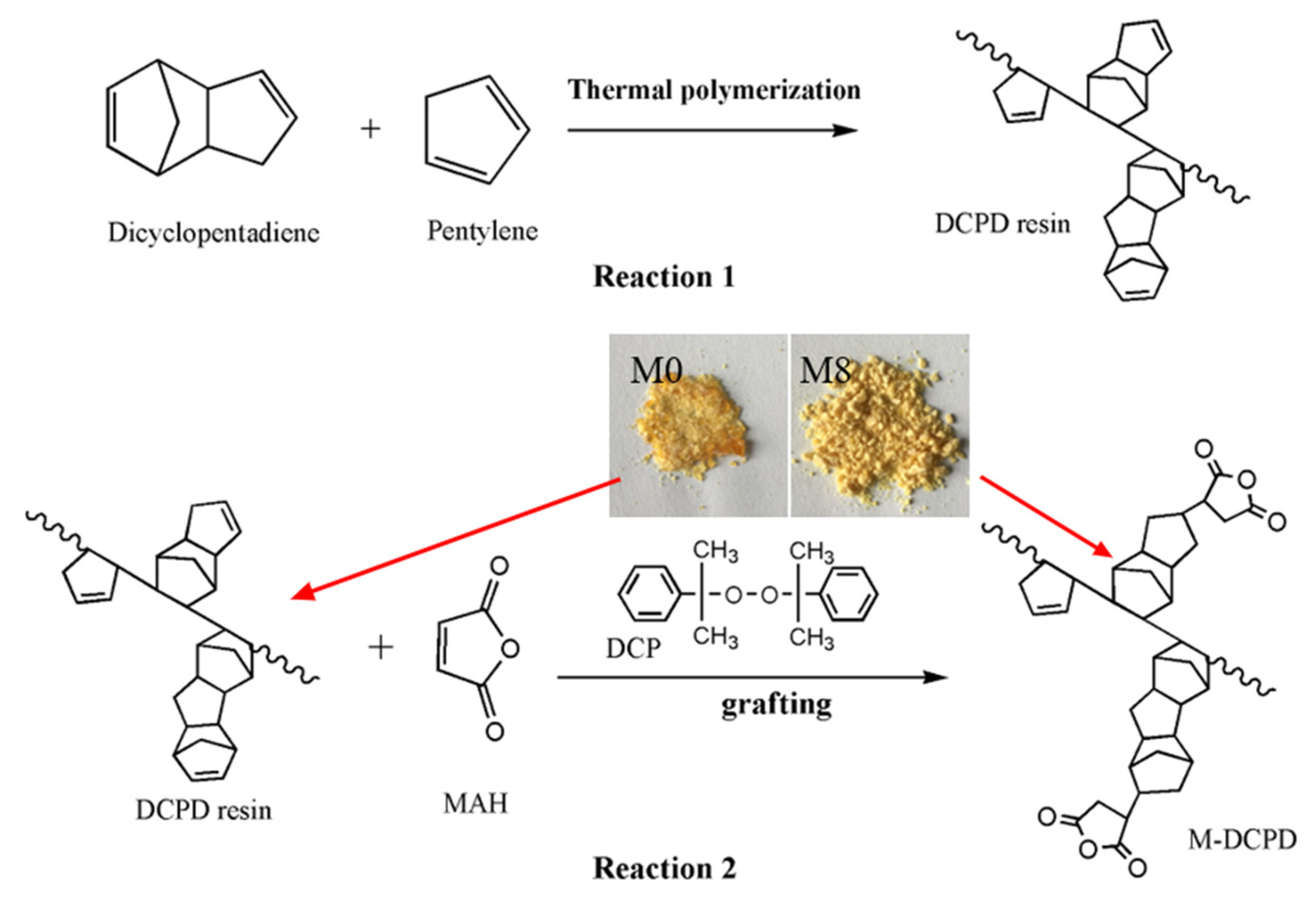
2.2. Preparation of M-DCPD Resin
2.3. Preparation of Vulcanizates
2.4. Characterization
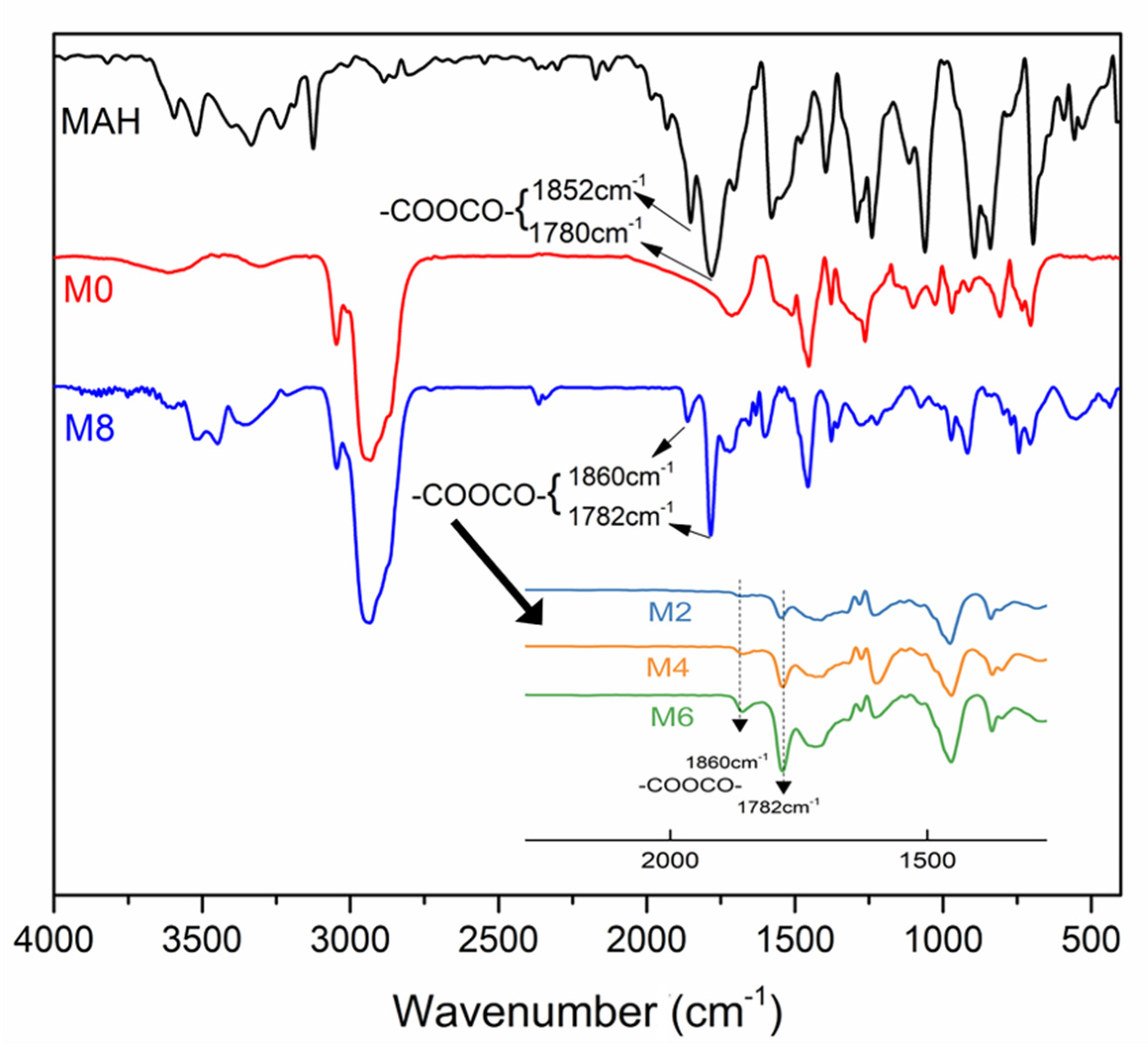
2.4.1. Characterization of the M-DCPD Resin
2.4.2. Characterization of the Rubber Vulcanizates
3. Results and Discussion
3.1. Characterization of Copolymers
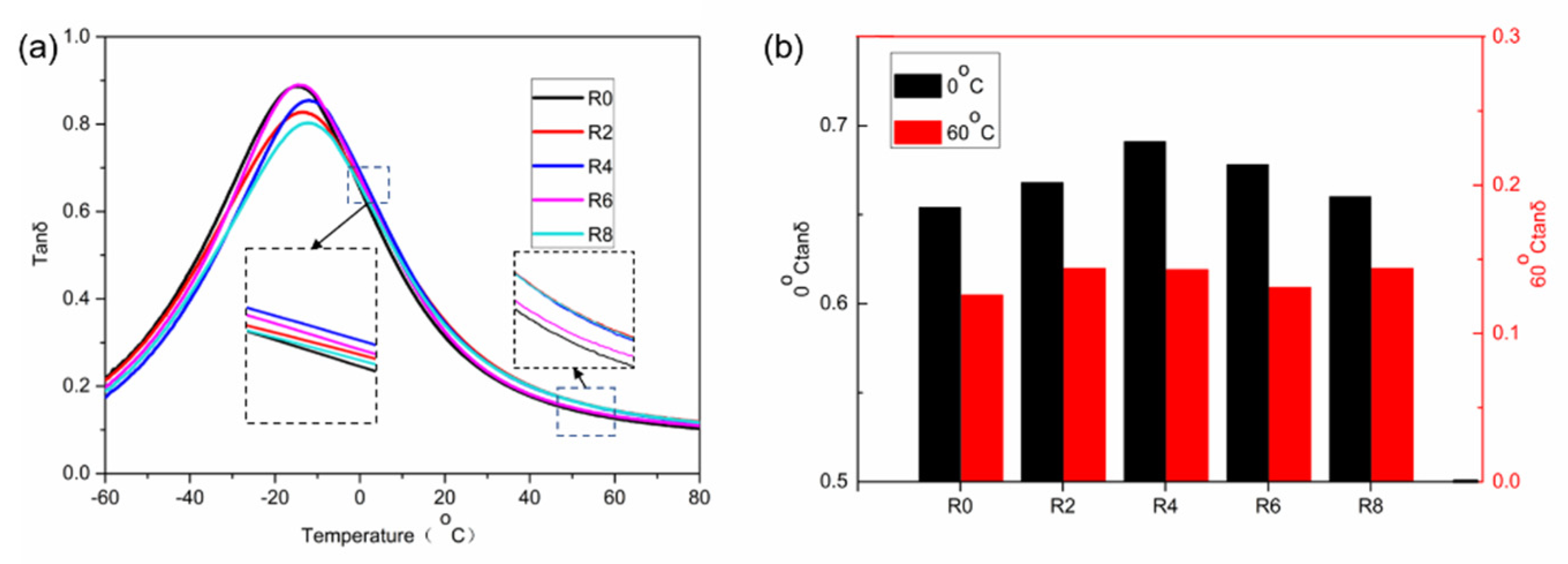
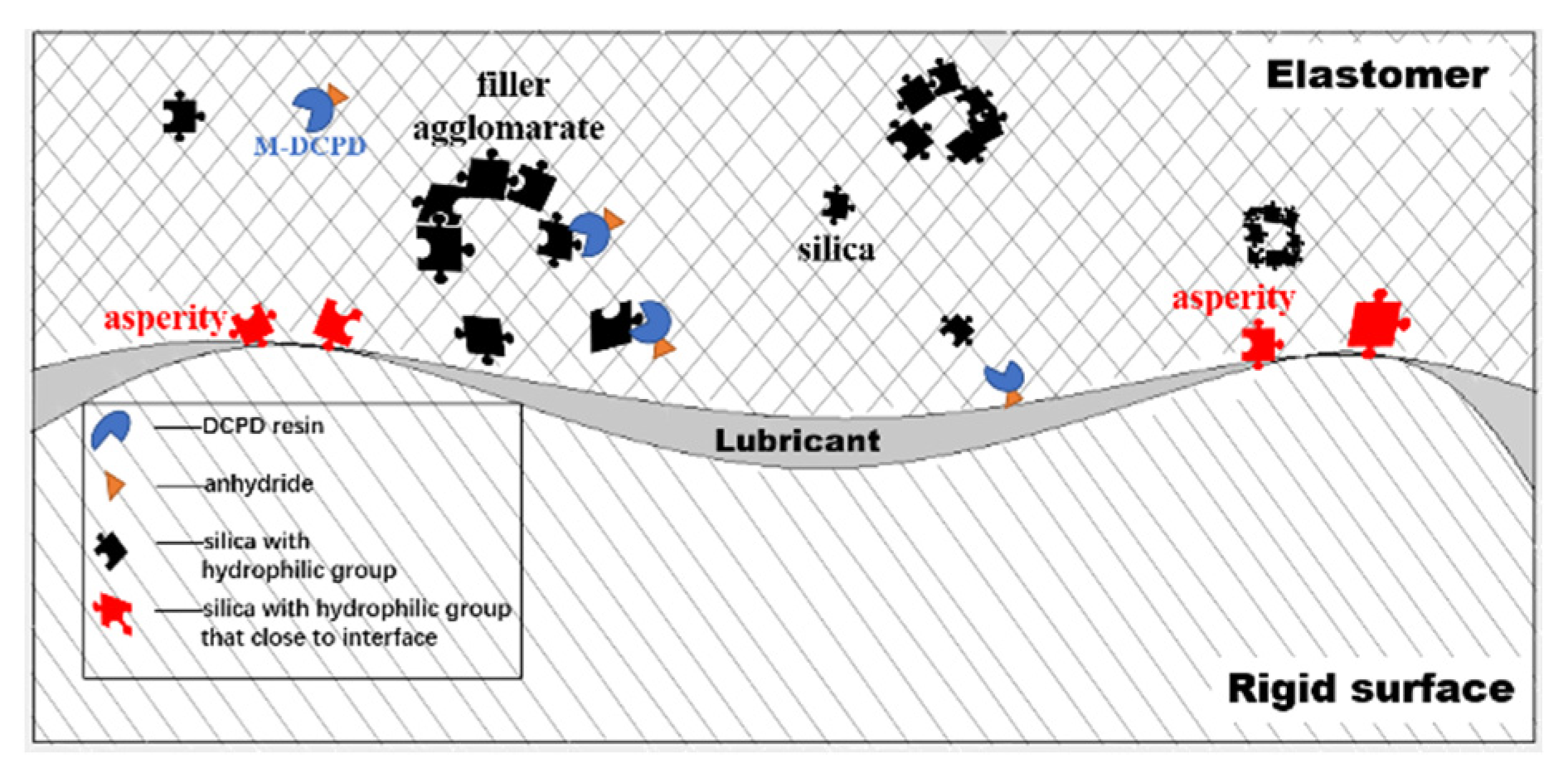
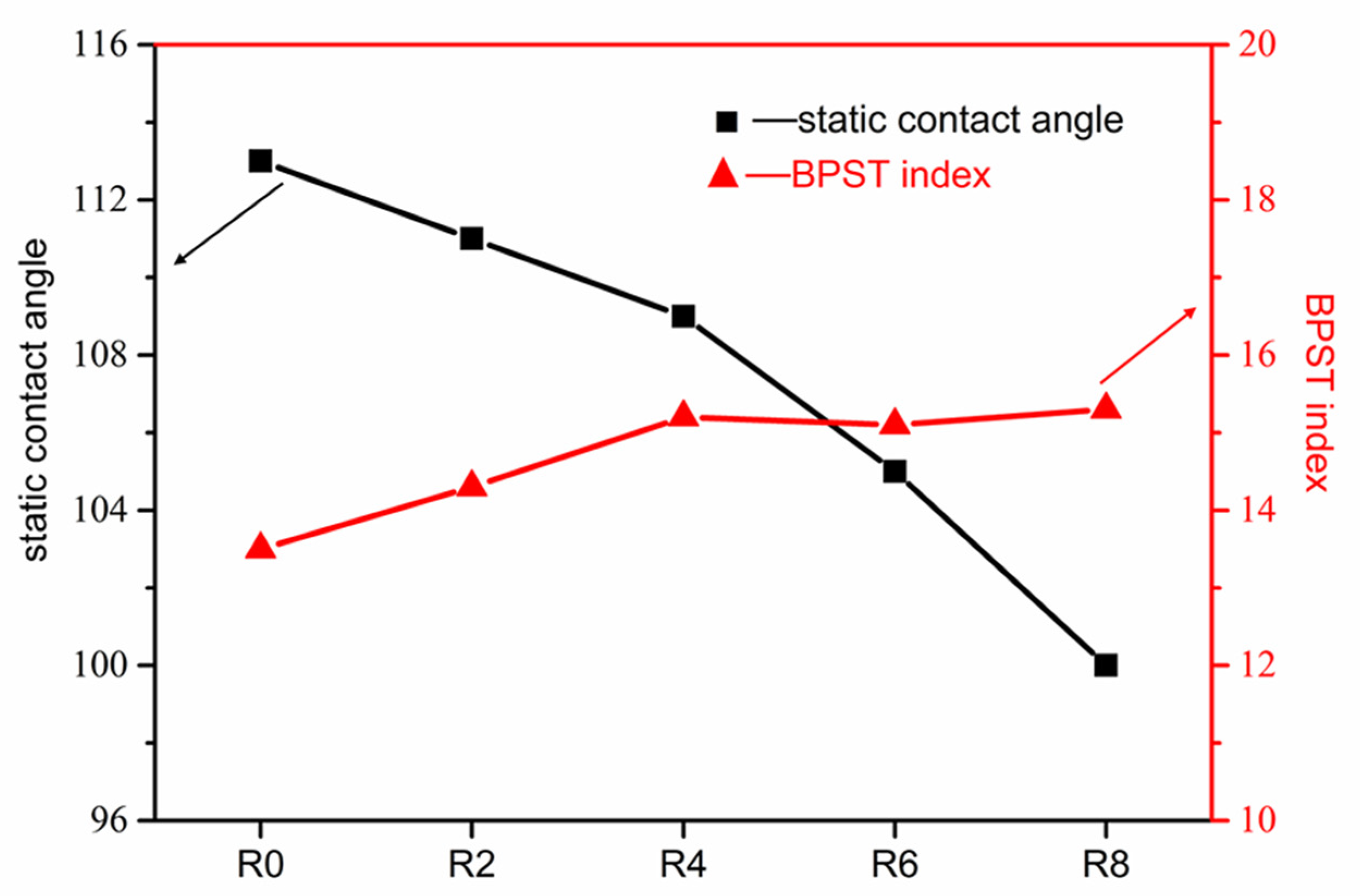
3.2. WSR of the Vulcanizates
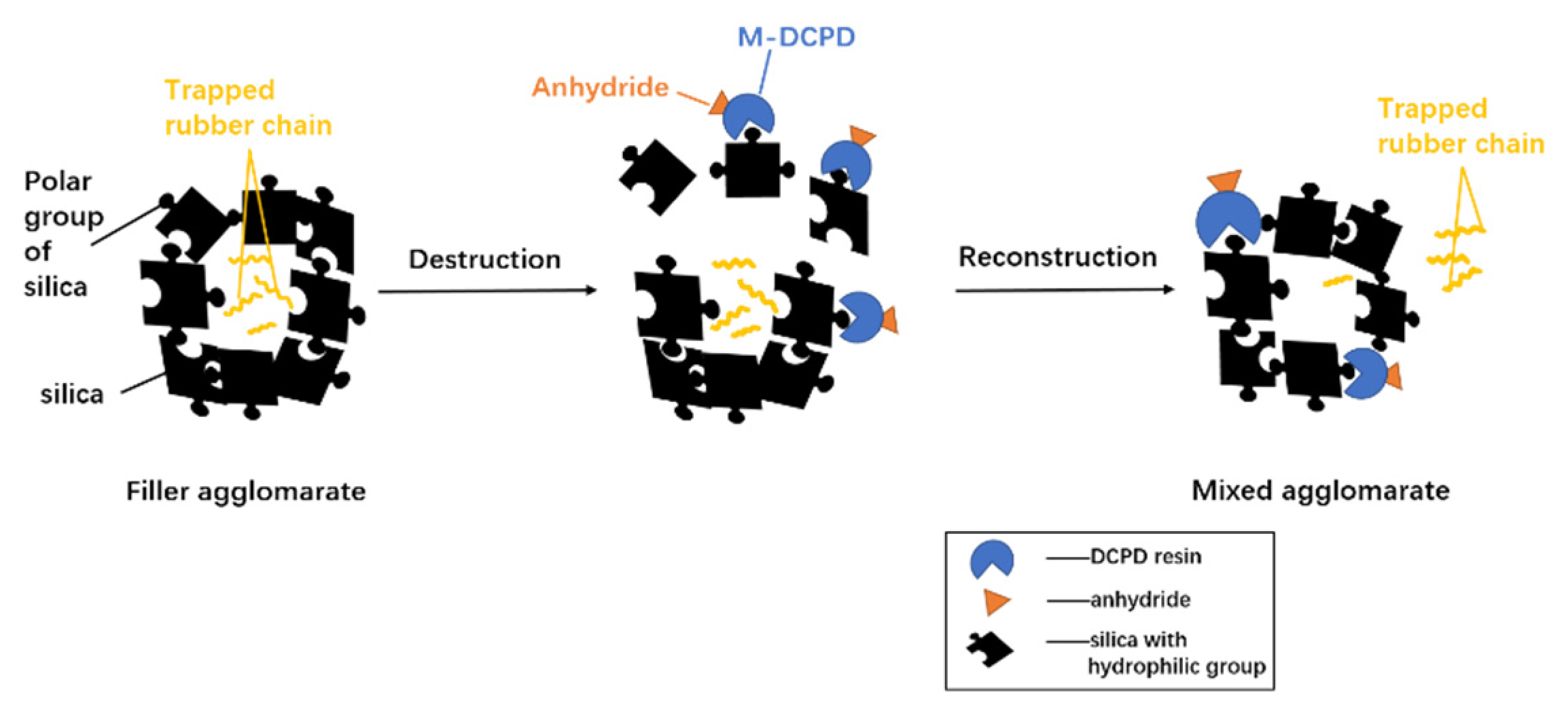
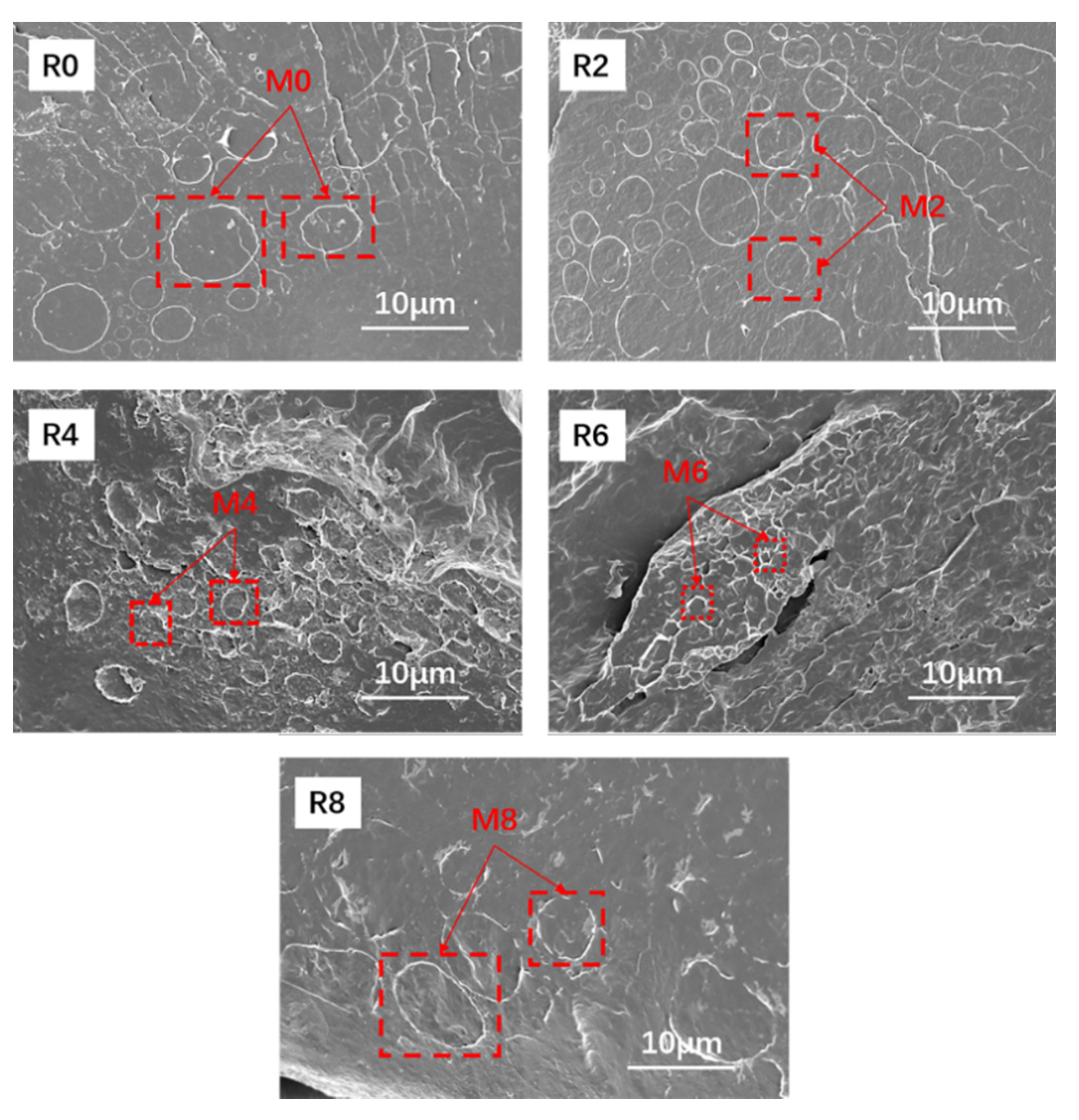
3.3. Morphologies of the Vulcanizates
3.4. Mechanical Properties of the Vulcanizates
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jeon, K.; Yoo, Y.; Lee, J.; Jung, D.; Lim, S.; Park, S. Laboratory Alignment Procedure for Improving Reproducibility of Tyre Wet Grip Measurement. World Electr. Veh. J. 2015, 7, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.-D. Impact of reinforcing filler on the dynamic moduli of elastomer compounds under shear deformation in relation to wet sliding friction. Rheol. Acta 2004, 44, 379–395. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Wu, Y.-P.; Li, W.-J.; Zhang, L. Influence of filler type on wet skid resistance of SSBR/BR composites: Effects from roughness and micro-hardness of rubber surface. Appl. Surf. Sci. 2011, 257, 2058–2065. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, C.; Wang, Y.; Su, B.; Gond, B. Investigation on Wet Skid Resistance of Tread Rubber. Exp. Tech. 2018, 43, 81–89. [Google Scholar] [CrossRef]
- Choi, S.-S.; Kwon, H.-M.; Kim, Y.; Ko, E.; Lee, K.-S. Hybrid factors influencing wet grip and rolling resistance properties of solution styrene-butadiene rubber composites. Polym. Int. 2018, 67, 340–346. [Google Scholar] [CrossRef]
- Hou, G.; Tao, W.; Liu, J.; Zhang, X.; Dong, M.; Zhang, L. Effect of the structural characteristics of solution styrene–butadiene rubber on the properties of rubber composites. J. Appl. Polym. Sci. 2017, 135, 45749. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, X.; Liu, K. Experimental study on wet skid resistance at different water-film thicknesses. Ind. Lubr. Tribol. 2018, 70, 1737–1744. [Google Scholar] [CrossRef]
- Ahammed, M.A.; Tighe, S.L. Asphalt pavements surface texture and skid resistance — exploring the reality. Can. J. Civ. Eng. 2016, 39, 1–9. [Google Scholar] [CrossRef]
- Torbruegge, S.; Wies, B. Characterization of pavement texture by means of height difference correlation and relation to wet skid resistance. J. Traffic Transp. Eng. (Engl. Ed.) 2015, 2, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Milani, G.; Milani, F. Numerical kinetic model with regularization for NR–PB natural and poly-butadiene rubber blends: Implementation and validation against experimental data. J. Math. Chem. 2019, 57, 1019–1034. [Google Scholar] [CrossRef]
- Wang, M.-J.; Kutsovsky, Y. Effect of Fillers on Wet Skid Resistance of Tires. Part I: Water Lubrication Vs. Filler-Elastomer Interactions. Rubber Chem. Technol. 2008, 81, 552–575. [Google Scholar] [CrossRef]
- Wang, M.-J.; Kutsovsky, Y. Effect of Fillers on Wet Skid Resistance of Tires. Part II: Experimental Observations on Effect of Filler-Elastomer Interactions on Water Lubrication. Rubber Chem. Technol. 2008, 81, 576–599. [Google Scholar] [CrossRef]
- Giles, C.G.; Lander, F.T.W. The Skid-Resisting Properties of Wet Surfaces at High Speeds: Exploratory Measurements with a Small Braking Force Trailer. J. R. Aeronaut. Soc. 1956, 60, 83–94. [Google Scholar] [CrossRef]
- Manning, D.P.; Jones, C.; Rowland, F.J.; Roff, M. The Surface Roughness of a Rubber Soling Material Determines the Coefficient of Friction on Water-Lubricated Surfaces. J. Saf. Res. 1998, 29, 275–283. [Google Scholar] [CrossRef]
- Vleugels, N.; Pille-Wolf, W.; Dierkes, W.K.; Noordermeer, J.W.M. Understanding the influence of oligomeric resins on traction and rolling resistance of silica-reinforced tire treads. Rubber Chem. Technol. 2015, 88, 65–79. [Google Scholar] [CrossRef]
- Gao, D. Development and application of Several important DCPD copolymer resin. Chem. Intermed. 2004, 8, 34–35. [Google Scholar]
- Kunhao, F. The Effect of Functional Resins on the Wet Skid Resistance Properties of SILICA Filled SSBR/BR Composites; South China University of Technology: Guangzhou, China, 2019. [Google Scholar]
- Heinrich, G. The dynamics of tire tread compounds and their relationship to wek skid behavior. Prog. Colloid Polym. Sci. 2007, 90, 16–26. [Google Scholar]
- Pan, X.-D. Significance of Tuning Bulk Viscoelasticity via Polymer Molecular Design on Wet Sliding Friction of Elastomer Compounds. Tribol. Lett. 2005, 20, 209–219. [Google Scholar] [CrossRef]
- Takino, H.; Nakayama, R.; Yamada, Y.; Kohjiya, S.; Matsuo, T. Viscoelastic Properties of Elastomers and Tire Wet Skid Resistance. Rubber Chem. Technol. 1997, 70, 584–594. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, J.; Su, K.; Wang, N.; Han, B. Bio-based β-myrcene-modified solution-polymerized styrene–butadiene rubber for improving carbon black dispersion and wet skid resistance. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef]
- Pan, X.-D. Wet sliding friction of elastomer compounds on a rough surface under varied lubrication conditions. Wear 2007, 262, 707–717. [Google Scholar] [CrossRef]
- Mark, T.A. Using Impera performance resins to expand the “magic triangle” for tire tread compounds. Rubber World. 2019, 260, 24–30. [Google Scholar]


| Sample | M0 | M2 | M4 | M6 | M8 |
|---|---|---|---|---|---|
| DCPD(g) | 100 | 100 | 100 | 100 | 100 |
| DCP(g) | 2 | 2 | 2 | 2 | 2 |
| MAH(g) | 0 | 2 | 4 | 6 | 8 |
| AR *(%) | 0 | 2.4 | 4.8 | 6.8 | 8.8 |
| Control Group (phr) | Test Group (phr) | |
|---|---|---|
| SSBR (oil filled 37.5) | 96.25 | 96.25 |
| BR | 30 | 30 |
| TESPT | 5.6 | 5.6 |
| Silica | 70 | 70 |
| Stearic acid | 2 | 2 |
| ZnO | 5 | 5 |
| 6PPD | 1.5 | 1.5 |
| RW287 | 1.5 | 1.5 |
| RD | 1.5 | 1.5 |
| NS | 1.2 | 1.2 |
| Sulfur | 1.8 | 1.8 |
| D | 1.8 | 1.8 |
| M0 | 10 | 0 |
| M-DCPD resin | 0 | 10 |
| Sample | Hardness (shore A) | Cross-Linking Density (%) | 100% Modulus (MPa) | 300% Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) | Tear Strength (kN/m) | Resilience (%) |
|---|---|---|---|---|---|---|---|---|
| R0 | 54 | 20.8 | 1.6 | 10.6 | 17.6 | 414 | 33.1 | 40 |
| R2 | 53 | 22.0 | 1.6 | 11.2 | 17.7 | 404 | 32.1 | 41 |
| R4 | 54 | 21.2 | 1.7 | 12.6 | 16.7 | 352 | 29.9 | 39 |
| R6 | 54 | 21.4 | 2.1 | 14.4 | 16.0 | 320 | 29.7 | 40 |
| R8 | 55 | 21.4 | 1.9 | 13.3 | 16.4 | 339 | 30.6 | 39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Pan, Q.; Chen, Z.; Feng, K. Maleic Anhydride Modified Dicyclopentadiene Resin for Improving Wet Skid Resistance of Silica Filled SSBR/BR Composites. Appl. Sci. 2020, 10, 4478. https://doi.org/10.3390/app10134478
Huang R, Pan Q, Chen Z, Feng K. Maleic Anhydride Modified Dicyclopentadiene Resin for Improving Wet Skid Resistance of Silica Filled SSBR/BR Composites. Applied Sciences. 2020; 10(13):4478. https://doi.org/10.3390/app10134478
Chicago/Turabian StyleHuang, Ruoming, Qiwei Pan, Zhaohui Chen, and Kunhao Feng. 2020. "Maleic Anhydride Modified Dicyclopentadiene Resin for Improving Wet Skid Resistance of Silica Filled SSBR/BR Composites" Applied Sciences 10, no. 13: 4478. https://doi.org/10.3390/app10134478





