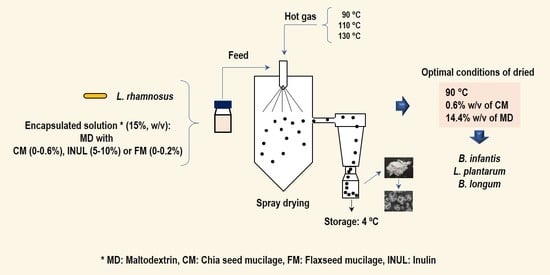
Effect of Three Polysaccharides (Inulin, and Mucilage from Chia and Flax Seeds) on the Survival of Probiotic Bacteria Encapsulated by Spray Drying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of FM and CM
2.3. Preparation of Probiotic Suspension
2.4. Effect of Inulin, or Mucilage from Chia or Flax Seeds, and Inlet Air Temperature on the Survival of L. rhamnosus after Spray Drying
2.5. Effect of Chia Seed Mucilage on the Survival After Spray Drying and Viability during Storage of L. plantarum and Strains of the Genus Bifidobacterium
2.6. Spray Drying
2.7. Analyses
2.8. Characterization of the Spray-Dried Product
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of Inulin, or Mucilage from Chia or Flax Seeds, and Inlet Air Temperature on the Survival of L. rhamnosus after Spray Drying
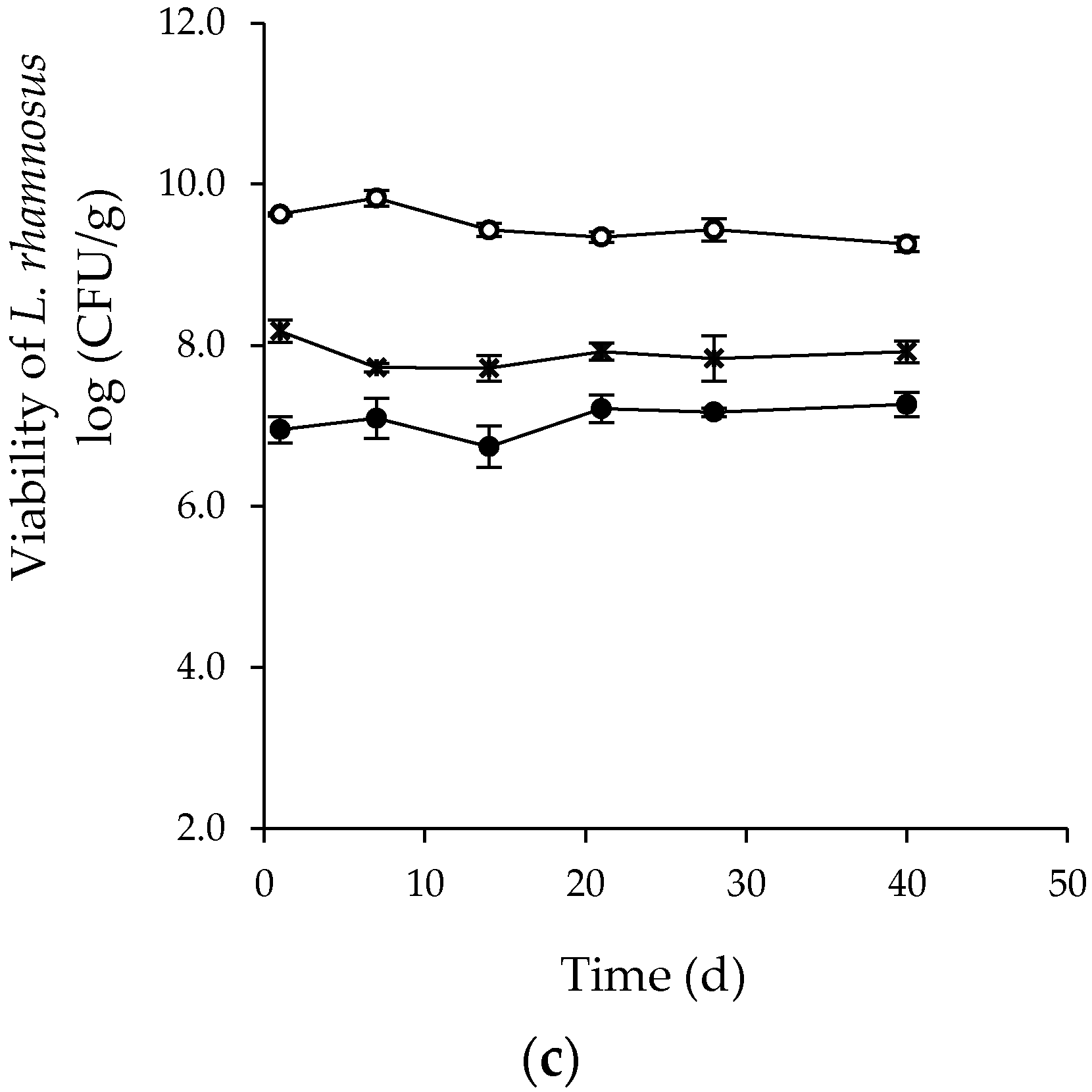
3.2. Effect of Spray Drying on the Survival of L. plantarum and Strains of the Genus Bifidobacterium and Viability during Storage
3.3. Characterization of the Spray-Dried Product
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mordor Intelligence. Probiotics Market—Growth, Trends and Forecasts (2018–2023). 2018. Available online: https://www.researchandmarkets.com/reports/4663724/probiotics-market-growth-trends-and-forecasts (accessed on 1 December 2018).
- Mordor Intelligence. Probiotics Market—Growth, Trends and Forecasts (2019–2024). 2019. Available online: https://www.researchandmarkets.com/reports/4771770/probiotics-market-growth-trends-and-forecasts (accessed on 26 September 2019).
- Bustamante, M.; Oomah, B.D.; Oliveira, W.P.; Burgos-Díaz, C.; Rubilar, M.; Shene, C. Probiotics and prebiotics potential for the care of skin, female urogenital tract, and respiratory tract. Folia Microbiol. 2019, 65, 245–264. [Google Scholar] [CrossRef] [Green Version]
- Nagpal, R.; Kumar, A.; Kumar, M.; Behare, P.V.; Jain, S.; Yadav, H. Probiotics, their health benefits and applications for developing healthier foods: A review. FEMS Microbiol. Lett. 2012, 334, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyetayo, V.O. Phenotypic characterization and assessment of the inhibitory potential of lactobacillus isolates from different sources. Afr. J. Biotechnol. 2004, 3, 355–357. [Google Scholar] [CrossRef] [Green Version]
- Gu, R.X.; Yang, Z.Q.; Li, Z.H.; Chen, S.L.; Luo, Z.L. Probiotic properties of lactic acid bacteria isolated from stool samples of longevous people in regions of Hotan, Xinjiang and Bama, Guangxi, China. Anaerobe 2008, 14, 313–317. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Micr. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Forestier, C.; De Champs, C.; Vatoux, C.; Joly, B. Probiotic activities of Lactobacillus casei rhamnosus: In vitro adherence to intestinal cells and antimicrobial properties. Res. Microbiol. 2001, 152, 167–173. [Google Scholar] [CrossRef]
- Bu, L.N.; Chang, M.H.; Ni, Y.H.; Chen, H.L.; Cheng, C.C. Lactobacillus casei rhamnosus Lcr35 in children with chronic constipation. Pediatr. Int. 2007, 49, 485–490. [Google Scholar] [CrossRef]
- Forestier, C.; Guelon, D.; Cluytens, V.; Gillart, T.; Sirot, J.; De Champs, C. Oral probiotic and prevention of Pseudomonas aeruginosa infections: A randomized, double-blind, placebo-controlled pilot study in intensive care unit patients. Crit. Care. 2008, 12, R69. [Google Scholar] [CrossRef] [Green Version]
- Kovachev, S.; Dobrevski-Vacheva, R. Effect of Lactobacillus casei var rhamnosus (Gynophilus) in restoring the vaginal flora by female patients with bacterial vaginosis-randomized, open clinical trial. Akush. Ginekol. (Sofiia) 2013, 52, 48–53. [Google Scholar]
- Lash, B.W.; Mysliwiec, T.H.; Gourama, H. Detection and partial characterization of a broad-range bacteriocin produced by Lactobacillus plantarum (ATCC 8014). Food Microbiol. 2005, 22, 199–204. [Google Scholar] [CrossRef]
- Vahedi Shahandashti, R.; Kasra Kermanshahi, R.; Ghadam, P. The inhibitory effect of bacteriocin produced by Lactobacillus acidophilus ATCC 4356 and Lactobacillus plantarum ATCC 8014 on planktonic cells and biofilms of Serratia marcescens. Turk. J. Med. Sci. 2016, 46, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.R.A.V.; do Carmo, M.S.; Melo, B.O.; Alves, M.S.; dos Santos, C.I.; Monteiro, S.G.; Bomfim, M.R.Q.Q.; Fernandes, E.S.; Monteiro-Neto, V. In vitro antimicrobial activity and probiotic potential of Bifidobacterium and Lactobacillus against species of Clostridium. Nutrients 2019, 11, 448. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; O’Sullivan, D.J. Genomic insights into Bifidobacteria. Microbiol. Mol. Biol. Rev. 2010, 74, 378–416. [Google Scholar] [CrossRef] [Green Version]
- Arboleya, S.; Watkins, C.; Stanton, C.; Ross, P. Gut bifidobacteria populations in human health and aging. Front. Microbiol. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yung, B.; Song, M.; Park, D.J.; Oh, S. Beneficial effect of Bifidobacterium longum ATCC 15707 on survival rate of Clostridium difficile infection in mice. Korean J. Food Sci. Anim. Resour. 2017, 37, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, F.C.; Castro, A.S.B.; Rodrigues, V.C.; Fernandes, S.A.; Fontes, E.A.F.; de Oliveira, T.T.; Martino, H.S.D.; de Luces Fortes Ferreira, C.L. Yacon flour and Bifidobacterium longum modulate bone health in rats. J. Med. Food 2012, 15, 664–670. [Google Scholar] [CrossRef]
- Sheng, K.; He, S.; Sun, M.; Zhang, G.; Kong, X.W.; Wang, J.; Wang, Y. Synbiotic supplementation containing Bifidobacterium infantis and xylooligosaccharide alleviates dextran sulfate sodium-induced ulcerative colitis. Food Funct. 2020, 11, 3964–3974. [Google Scholar] [CrossRef]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food. Food and Agriculture Organization of the United Nations/World Health Organization. London, Ontario, Canada. 2002. Available online: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 16 May 2019).
- Desmond, C.; Ross, R.P.; O’Callaghan, E.; Fitzgerald, G.; Stanton, C. Improved survival of Lactobacillus paracasei NFBC 338 in spray-drying powders containing gum acacia. J. Appl. Microbiol. 2002, 9, 1003–1011. [Google Scholar] [CrossRef] [Green Version]
- Bustamante, M.; Oomah, B.D.; Rubilar, M.; Shene, C. Effective Lactobacillus plantarum and Bifidobacterium infantis encapsulation with chia seed (Salvia hispanica L.) and flaxseed (Linum usitatissimum L.) mucilage and soluble protein by spray drying. Food Chem. 2017, 216, 97–105. [Google Scholar] [CrossRef]
- Guerin, J.; Petit, J.; Burgain, J.; Borges, F.; Bhandari, B.; Perroud, C.; Desobry, S.; Scher, J.; Gaiani, C. Lactobacillus rhamnosus GG encapsulation by spray-drying: Milk proteins clotting control to produce innovative matrices. J. Food Eng. 2017, 193, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Favaro-Trindade, C.S.; Grosso, C.R.F. Microencapsulation of L. acidophilus (La-05) and B. lactis (Bb-12) and evaluation of their survival at the pH values of the stomach and in bile. J. Microencapsul. 2002, 19, 485–494. [Google Scholar] [CrossRef]
- Fritzen-Freire, C.B.; Prudêncio, E.S.; Amboni, R.D.M.C.; Pinto, S.S.; Negrão-Murakami, A.N.; Murakami, F.S. Microencapsulation of bifidobacteria by spray drying in the presence of prebiotics. Food Res. Int. 2012, 45, 306–312. [Google Scholar] [CrossRef]
- Corcoran, B.M.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Comparative survival of probiotic lactobacilli spray-dried in the presence of prebiotic substances. J. Appl. Microbiol. 2004, 96, 1024–1039. [Google Scholar] [CrossRef]
- Sunny-Roberts, E.O.; Knorr, D. Cellular injuries on spray-dried Lactobacillus rhamnosus GG and its stability during food storage. Nutr. Food Sci. 2011, 41, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, P.; Castro, H.; Kirby, R. Inducible thermotolerance in Lactobacillus bulgaricus. Lett. Appl. Microbiol. 1994, 18, 218–221. [Google Scholar] [CrossRef]
- Chlebowska-Smigiel, A.; Gniewosz, M.; Kieliszek, M.; Bzducha-Wrobel, A. The effect of pullulan on the growth and acidifying activity of selected stool microflora of human. Curr. Pharm. Biotechnol. 2017, 18, 121–126. [Google Scholar] [CrossRef]
- Çabuk, B.; Harsa, Ş. Whey protein-pullulan (WP/Pullulan) polymer blend for preservation of viability of Lactobacillus acidophilus. Dry. Technol. 2015, 33, 1223–1233. [Google Scholar] [CrossRef] [Green Version]
- Anekella, K.; Orsat, V. Optimization of microencapsulation of probiotics in raspberry juice by spray drying. LWT Food Sci. Technol. 2013, 50, 17–24. [Google Scholar] [CrossRef]
- Paim, D.R.S.F.; Costa, S.D.O.; Walter, E.H.M.; Tonon, R.V. Microencapsulation of probiotic jussara (Euterpe edulis M.) juice by spray drying. LWT Food Sci. Technol. 2016, 74, 21–25. [Google Scholar] [CrossRef]
- Rodríguez-Huezo, M.E.; Durán-Lugo, R.; Prado-Barragán, L.A.; Cruz-Sosa, F.; Lobato-Calleros, C.; Alvarez-Ramírez, J.; Vernon-Carter, E.J. Pre-selection of protective colloids for enhanced viability of Bifidobacterium bifidum following spray-drying and storage, and evaluation of aguamiel as thermoprotective prebiotic. Food Res. Int. 2007, 40, 1299–1306. [Google Scholar] [CrossRef]
- Rajam, R.; Anandharamakrishnan, C. Spray freeze drying method for microencapsulation of Lactobacillus plantarum. J. Food Eng. 2015, 166, 95–103. [Google Scholar] [CrossRef]
- Ross, R.P.; Desmond, C.; Fitzgerald, G.F.; Stanton, C. A Review. Overcoming the technological hurdles in the development of probiotics foods. J. Appl. Microbiol. 2005, 98, 1410–1417. [Google Scholar] [CrossRef]
- Sosa, N.; Gerbino, E.; Golowczyc, M.A.; Schebor, C.; Gómez-Zavaglia, A.; Tymczyszyn, E.E. Effect of galacto-oligosaccharides: Maltodextrin matrices on the recovery of Lactobacillus plantarum after spary-drying. Front. Microbiol. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Apolinário, A.C.; de Lima Damasceno, B.P.G.; de Macêdo Beltrão, N.E.; Pessoa, A.; Converti, A.; da Silva, J.A. Inulin-type fructans: A review on different aspects of biochemical and pharmaceutical technology. Carbohydr. Polym. 2014, 101, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.Y.; Gui, B.; Arnison, P.G.; Wang, Y.; Reaney, M.J.T. Flaxseed (Linum usitatissimum L.) bioactive compounds and peptide nomemclature: A review. Trends Food Sci. Tech. 2014, 38, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Mazza, G.; Biliaderis, C.G. Functional properties of flax seed mucilage. J. Food Sci. 1989, 5, 1302–1305. [Google Scholar] [CrossRef]
- Naran, R.; Chen, G.; Carpita, N.C. Novel Rhamnogalacturonan I and Arabinoxylan polysaccharides of flax seed mucilage. Plant. Physiol. 2008, 148, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Wanasundara, P.K.J.P.D.; Shahidi, F. Removal of flaxseed mucilage by chemical and enzymatic treatments. Food Chem. 1997, 59, 47–55. [Google Scholar] [CrossRef]
- Warrand, J.; Michaud, P.; Picton, L.; Muller, G.; Courtis, B.; Ralainirina, R.; Courtois, J. Structural investigations of the neutral polysaccharide of Linum usitatissimum L. seeds mucilage. Int. J. Biol. Macromol. 2005, 35, 121–125. [Google Scholar] [CrossRef]
- Chen, H.; Xu, S.; Wang, Z. Gelation properties of flaxseed gum. J. Food Eng. 2006, 77, 295–303. [Google Scholar] [CrossRef]
- Thakur, G.; Mitra, A.; Pal, K.; Rousseau, D. Effect of flaxseed gum on reduction of blood glucose and cholesterol in type 2 diabetic patients. Int. J. Food Sci. Nutr. 2009, 60, 126–136. [Google Scholar] [CrossRef]
- Brahe, L.K.; Le Chatelier, E.; Prifti, E.; Pons, N.; Kennedy, S.; Blædel, T.; Håkansson, J.; Dalsgaard, T.K.; Hansen, T.; Pedersen, O.; et al. Dietary modulation of the gut microbiota-a randomised controlled trial in obese postmenopausal women. Br. J. Nutr. 2015, 114, 406–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, M.; Savorani, F.; Christensen, S.; Engelsen, S.B.; Bugel, S.; Toubro, S.; Tetens, I.; Astrup, A. Flaxseed dietary fibers suppress postprandial lipemia and apetite sensation in young men. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 136–143. [Google Scholar] [CrossRef]
- Ullah, R.; Nadeem, M.; Khalique, A.; Imran, M.; Mehmood, S.; Javid, A.; Hussain, J. Nutritional and therapeutic perspectives of Chia (Salvia hispanica L.): A review. J. Food Sci. Technol. 2016, 53, 1750–1758. [Google Scholar] [CrossRef] [Green Version]
- Commission Implementing Regulation (EU) 2017/2470. Establishing the Union List of Novel Foods in accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. 2017. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R2470 (accessed on 1 April 2020).
- Lin, K.Y.; Daniel, J.R.; Whistler, R.L. Structure of chia seed polysaccharide exudate. Carbohydr. Polym. 1994, 23, 13–18. [Google Scholar] [CrossRef]
- BeMiller, J.N.; Whistler, R.L.; Barkalow, D.G.; Chen, C.C. Chapter 9—Aloe, chia, flaxseed, okra, psyllium seed, quince seed, and tamarind gums. In Industrial Gums: Polysaccharides and Their Derivatives, 3rd ed.; Whistler, R.L., BeMiller, J.N., Eds.; Academic Press. Inc.: San Diego, CA, USA, 1993; pp. 227–256. [Google Scholar] [CrossRef]
- Bustamante, M.; Villarroel, M.; Rubilar, M.; Shene, C. Lactobacillus acidophilus La-05 encapsulated by spray drying: Effect of mucilage and protein from flaxseed (Linum usitatissimum L.). LWT Food Sci. Technol. 2015, 62, 1162–1168. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; AOAC Inc.: Gaithersburg, MD, USA, 1995. [Google Scholar]
- Simpson, P.J.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Intrinsic tolerance of Bifidobacterium species to heat and oxygen and survival following spray drying and storage. J. Appl. Microbiol. 2005, 99, 493–501. [Google Scholar] [CrossRef] [PubMed]
- IDF. Dried milk and dried cream: Determination of water content. International Dairy Federation standard 26A. In International Dairy Federation; IDF: Brussels, Belgium, 1993. [Google Scholar]
- Timilsena, Y.P.; Adhikari, R.; Kasapis, S.; Adhikari, B. Molecular and functional characteristics of purified gum from Australian chia seeds. Carbohydr. Polym. 2016, 136, 128–136. [Google Scholar] [CrossRef]
- Maskan, M.; Gogus, F. Effect of sugar on the rheological properties of sunflower oil-water emulsions. J. Food Eng. 2000, 43, 173–177. [Google Scholar] [CrossRef]
- Hernández-López, Z.; Rangel-Vargas, E.; Castro-Rosas, J.; Gómez-Aldapa, C.A.; Cadena-Ramírez, A.; Acevedo-Sandoval, O.A.; Gordillo-Martínez, A.J.; Falfán-Cortés, R.N. Optimization of a spray-drying process for the production of maximally viable microencapsulated Lactobacillus pentosus using a mixture of starch-pulque as wall material. LWT Food Sci. Technol. 2018, 95, 216–222. [Google Scholar] [CrossRef]
- De Lara Pedroso, D.; Thomazini, M.; Heinemann, R.J.B.; Favaro-Trindade, C.S. Protection of Bifidobacterium lactis and Lactobacillus acidophilus by microencapsulation using spray-chilling. Int. Dairy J. 2012, 26, 127–132. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Felisberto, M.H.F.; Wahanik, A.L.; Gomes-Ruffi, C.R.; Clerici, M.T.P.S.; Chang, Y.K.; Steel, C.J. Use of chia (Salvia hispanica L.) mucilage gel to reduce fat in pound cakes. LWT Food Sci. Technol. 2015, 63, 1049–1055. [Google Scholar] [CrossRef] [Green Version]
- Sandoval-Oliveros, M.R.; Paredes-López, O. Isolation and characterization of proteins from chia seeds (Salvia hispanica L.). J. Agric. Food Chem. 2013, 61, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Segura-Campos, M.R.; Ciau-Solís, N.; Rosado-Rubio, G.; Chel-Guerrero, L.; Betancur-Ancona, D. Chemical and functional properties of chia seed (Salvia hispanica L.) gum. Int. J. Food Sci. 2014, 2014, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Li-Chan, E.C.Y.; Ma, C.Y. Thermal analysis of flaxseed (Linum usitatissimum) proteins by differential scanning calorimetry. Food Chem. 2002, 77, 495–502. [Google Scholar] [CrossRef]
- Jung, H.; Lee, Y.; Yoon, W. Effect of moisture content on the grinding process and powder properties in food: A review. Processes 2018, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, P.; Castro, H.; Kirby, R. Spray drying as a method for preparing concentrated cultures of Lactobacillus bulgaricus. J. Appl. Bacteriol. 1995, 78, 456–462. [Google Scholar] [CrossRef]
- Lian, W.C.; Hsiao, H.C.; Chou, C.C. Survival of bifidobacterial after spray-drying. Int. J. Food Microbiol. 2002, 74, 79–86. [Google Scholar] [CrossRef]
- Shah, N.P. Functional cultures and health benefits. Int. Dairy J. 2007, 17, 1262–1277. [Google Scholar] [CrossRef]
- Ávila-Reyes, S.V.; Garcia-Suarez, F.J.; Jiménez, M.T.; San Martín-Gonzalez, M.F.; Bello-Perez, L.A. Protection of L. rhamnosus by spray drying using two prebiotics colloids to enhance the viability. Carbohydr. Polym. 2014, 102, 423–430. [Google Scholar] [CrossRef]
- Saénz, C.; Tapia, S.; Chávez, J.; Robert, P. Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica). Food Chem. 2009, 114, 616–622. [Google Scholar] [CrossRef]




| Treatment | Encapsulating Solution | T (°C) | ||
|---|---|---|---|---|
| EAC † (%, w/v) | MD † (%, w/v) | |||
| 1 | INL | 0.0 | 15.0 | 90 |
| 2 | 110 | |||
| 3 | 130 | |||
| 4 | 5.0 | 10.0 | 90 | |
| 5 | 110 | |||
| 6 | 130 | |||
| 7 | 10.0 | 5.0 | 90 | |
| 8 | 110 | |||
| 9 | 130 | |||
| 10 | CM | 0.0 | 15.0 | 90 |
| 11 | 110 | |||
| 12 | 130 | |||
| 13 | 0.3 | 14.7 | 90 | |
| 14 | 110 | |||
| 15 | 130 | |||
| 16 | 0.6 | 14.4 | 90 | |
| 17 | 110 | |||
| 18 | 130 | |||
| 19 | FM | 0.0 | 15.0 | 90 |
| 20 | 110 | |||
| 21 | 130 | |||
| 22 | 0.1 | 14.9 | 90 | |
| 23 | 110 | |||
| 24 | 130 | |||
| 25 | 0.2 | 14.8 | 90 | |
| 26 | 110 | |||
| 27 | 130 | |||
| Treatment | µ † (mPa s) | Moisture † (%) | Predicted | Experimental Survival †† (%) | Student Residual |
|---|---|---|---|---|---|
| survival (%) | |||||
| 1 | 3.21 ± 0.02 de | 5.68 ± 0.27 bc | 80.89 | 79.54 ± 0.25 | −1.18 |
| 2 | 4.01 ± 0.24 ef | 67.48 | 68.72 ± 1.20 | 0.71 | |
| 3 | 3.48 ± 0.16 gl | 61.46 | 61.57 ± 0.74 | 0.09 | |
| 4 | 2.14 ± 0.09 e | 6.53 ± 0.28 a | 75.24 | 78.47 ± 0.74 | 1.83 |
| 5 | 3.96 ± 0.24 ef | 59.33 | 58.14 ± 1.52 | −1.08 | |
| 6 | 3.98 ± 0.19 ef | 50.81 | 51.14 ± 2.18 | 0.18 | |
| 7 | 2.06 ± 0.07 e | 5.14 ± 0.16 cd | 83.02 | 81.14 ± 0.63 | −1.64 |
| 8 | 3.66 ± 0.20 ef | 64.60 | 66.91 ± 2.09 | 1.31 | |
| 9 | 3.61 ± 0.14 ef | 53.58 | 53.16 ± 0.29 | −0.37 | |
| 10 | 3.21 ± 0.11 de | 5.68 ± 0.27 bc | 81.79 | 83.45 ± 3.56 | 0.92 |
| 11 | 4.01 ± 0.24 ef | 70.16 | 68.72 ± 1.20 | −0.71 | |
| 12 | 3.48 ± 0.16 gl | 58.53 | 61.57 ± 0.74 | 1.69 | |
| 13 | 18.30 ± 0.79 b | 4.04 ± 0.18 j | 84.43 | 83.04 ± 0.59 | −0.68 |
| 14 | 4.01 ± 0.31 ef | 72.80 | 71.67 ± 1.98 | 0.43 | |
| 15 | 2.99 ± 0.15 g | 61.18 | 59.44 ± 1.84 | −0.85 | |
| 16 | 37.78 ± 1.54 a | 4.72 ± 0.21 d | 87.07 | 89.98 ± 0.90 | 1.62 |
| 17 | 3.73 ± 0.17 ef | 75.44 | 73.91 ± 0.73 | −0.75 | |
| 18 | 3.71 ± 0.17 ef | 63.82 | 65.70 ± 0.63 | 1.05 | |
| 19 | 3.21 ± 0.01 de | 6.23 ± 0.26 ab | 80.73 | 79.54 ± 0.25 | −0.41 |
| 20 | 4.01 ± 0.24 ef | 70.37 | 68.72 ± 1.20 | −0.49 | |
| 21 | 3.48 ± 0.16 gl | 60.00 | 61.57 ± 0.74 | 0.54 | |
| 22 | 4.79 ± 0.10 cd | 6.20 ± 0.31 ab | 83.46 | 80.81 ± 0.07 | −0.79 |
| 23 | 5.14 ± 0.12 cd | 73.09 | 76.34 ± 2.07 | 0.84 | |
| 24 | 3.54 ± 0.17 ef | 62.72 | 58.14 ± 0.29 | −1.37 | |
| 25 | 6.22 ± 0.27 c | 5.61 ± 0.24 c | 86.18 | 83.46 ± 0.15 | −0.93 |
| 26 | 2.20 ± 0.12 h | 75.81 | 80.83 ± 0.11 | 1.50 | |
| 27 | 2.38 ± 0.09 h | 65.45 | 61.89 ± 1.13 | −1.21 |
| Factors | DF a | Mean Square | Fexp b | p > F |
|---|---|---|---|---|
| INL | 1 | 12.40 | 1.95 | 0.2211 |
| T | 1 | 895.19 | 140.99 | <0.0001 |
| INL2 | 1 | 114.07 | 17.97 | 0.0082 |
| T2 | 1 | 34.70 | 5.46 | 0.0666 |
| INL × T | 1 | 25.07 | 3.95 | 0.1037 |
| CM | 1 | 39.49 | 6.78 | 0.0314 |
| T | 1 | 811.03 | 139.28 | <0.0001 |
| FM | 1 | 44.55 | 2.97 | 0.1233 |
| T | 1 | 645.01 | 42.95 | 0.0002 |
| T (°C) | Composition ES (% w/v) | Viability (log CFU/g) | ||
|---|---|---|---|---|
| MD † | CM † | Day 80 | Day 250 | |
| 90 | 15 | 0 | 7.82 ± 0.25 c | 7.77 ± 0.11 b |
| 14.7 | 0.3 | 8.76 ± 0.05 ab | 8.72 ± 0.04 a | |
| 14.4 | 0.6 | 9.12 ± 0.11 a | 9.07 ± 0.12 a | |
| 110 | 15 | 0 | 7.63 ± 0.33 c | 7.43 ± 0.28 b |
| 14.7 | 0.3 | 7.51 ± 0.33 c | 7.29 ± 0.26 b | |
| 14.4 | 0.6 | 8.02 ± 0.24 bc | 7.67 ± 0.04 b | |
| 130 | 15 | 0 | 6.39 ± 0.14 e | 6.33 ± 0.17 c |
| 14.7 | 0.3 | 6.48 ± 0.24 de | 6.60 ± 0.12 c | |
| 14.4 | 0.6 | 7.29 ± 0.16 cd | 6.30 ± 0.10 c | |
| Probiotic Strain | Survival † (%) | Moisture †† (%) |
|---|---|---|
| L. rhanmosus | 91.23 ± 2.88 a | 2.95 ± 0.17 |
| L. plantarum ATCC 8014 | 94.77 ± 1.95 a | 3.52 ± 0.18 |
| B. infantis ATCC 15679 | 97.24 ± 2.32 a | 5.77 ± 0.20 |
| B. longum ATCC 15707 | 96.07 ± 0.07 a | 4.70 ± 0.41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustamante, M.; Laurie-Martínez, L.; Vergara, D.; Campos-Vega, R.; Rubilar, M.; Shene, C. Effect of Three Polysaccharides (Inulin, and Mucilage from Chia and Flax Seeds) on the Survival of Probiotic Bacteria Encapsulated by Spray Drying. Appl. Sci. 2020, 10, 4623. https://doi.org/10.3390/app10134623
Bustamante M, Laurie-Martínez L, Vergara D, Campos-Vega R, Rubilar M, Shene C. Effect of Three Polysaccharides (Inulin, and Mucilage from Chia and Flax Seeds) on the Survival of Probiotic Bacteria Encapsulated by Spray Drying. Applied Sciences. 2020; 10(13):4623. https://doi.org/10.3390/app10134623
Chicago/Turabian StyleBustamante, Mariela, Loreto Laurie-Martínez, Daniela Vergara, Rocio Campos-Vega, Mónica Rubilar, and Carolina Shene. 2020. "Effect of Three Polysaccharides (Inulin, and Mucilage from Chia and Flax Seeds) on the Survival of Probiotic Bacteria Encapsulated by Spray Drying" Applied Sciences 10, no. 13: 4623. https://doi.org/10.3390/app10134623
APA StyleBustamante, M., Laurie-Martínez, L., Vergara, D., Campos-Vega, R., Rubilar, M., & Shene, C. (2020). Effect of Three Polysaccharides (Inulin, and Mucilage from Chia and Flax Seeds) on the Survival of Probiotic Bacteria Encapsulated by Spray Drying. Applied Sciences, 10(13), 4623. https://doi.org/10.3390/app10134623