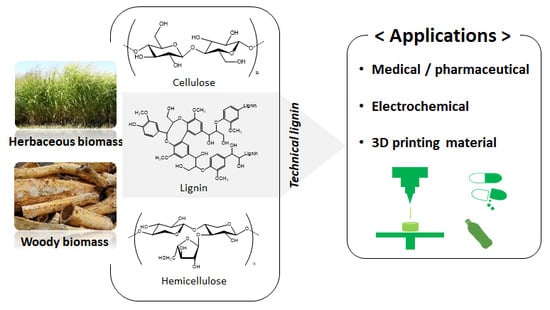
Lignin to Materials: A Focused Review on Recent Novel Lignin Applications
Abstract
:1. Introduction
2. Technical Lignins
3. Medical Applications
3.1. Wound Dressings
3.2. Pharmaceuticals
4. Electrochemical Energy Materials
5. 3D Printing Lignin−Plastic Composites
6. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. THF co-solvent enhances hydrocarbon fuel precursor yields from lignocellulosic biomass. Green Chem. 2013, 15, 3140–3145. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, J.; Wang, C.; Liu, J.; Pu, Y.; Ragauskas, A.; Li, S.; Yang, B. Lignin-derived electrochemical energy materials and systems. Biofuels Bioprod. Biorefining 2020, 14, 650–672. [Google Scholar] [CrossRef]
- Bajwa, D.; Pourhashem, G.; Ullah, A.; Bajwa, S. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crop. Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Graglia, M.; Kanna, N.; Esposito, D. Lignin refinery: Towards the preparation of renewable aromatic building blocks. ChemBioEng Rev. 2015, 2, 377–392. [Google Scholar] [CrossRef]
- Sahoo, S.; Seydibeyoğlu, M.; Mohanty, A.; Misra, M. Characterization of industrial lignins for their utilization in future value added applications. Biomass Bioenergy 2011, 35, 4230–4237. [Google Scholar] [CrossRef]
- Seydibeyoğlu, M.Ö. A novel partially biobased PAN-lignin blend as a potential carbon fiber precursor. BioMed Res. Int. 2012, 2012, 598324. [Google Scholar]
- Funaoka, M.; Matsubara, M.; Seki, N.; Fukatsu, S. Conversion of native lignin to a highly phenolic functional polymer and its separation from lignocellulosics. Biotechnol. Bioeng. 1995, 46, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Azadi, P.; Inderwildi, O.R.; Farnood, R.; King, D.A. Liquid fuels, hydrogen and chemicals from lignin: A critical review. Renew. Sustain. Energy Rev. 2013, 21, 506–523. [Google Scholar] [CrossRef]
- Al Arni, S. Extraction and isolation methods for lignin separation from sugarcane bagasse: A review. Ind. Crop. Prod. 2018, 115, 330–339. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Chen, H. Lignocellulose Biorefinery Engineering: Principles and Applications; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Sjöström, E.; Westermark, U. Chemical composition of wood and pulps: Basic constituents and their distribution. In Analytical Methods in Wood Chemistry, Pulping, and Papermaking; Springer: Heidelberg, Germany, 1999; pp. 1–19. [Google Scholar]
- Aro, T.; Fatehi, P. Production and application of lignosulfonates and sulfonated lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Dutta, T.; Ralph, J.; Mansfield, S.D.; Simmons, B.A.; Singh, S. Impact of lignin polymer backbone esters on ionic liquid pretreatment of poplar. Biotechnol. Biofuels 2017, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Eudes, A.; Jeong, K.; Yoo, C.G.; Kim, C.S.; Ragauskas, A. Integration of renewable deep eutectic solvents with engineered biomass to achieve a closed-loop biorefinery. Proc. Natl. Acad. Sci. USA 2019, 116, 13816–13824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Laftah, W.A.; Hashim, S.; Ibrahim, A.N. Polymer hydrogels: A review. Polym. Plast. Technol. Eng. 2011, 50, 1475–1486. [Google Scholar] [CrossRef]
- Yadollahi, M.; Namazi, H.; Aghazadeh, M. Antibacterial carboxylmethyl cellulose/Ag nanocomposite hydrogels cross-linked with layered double hydroxides. Int. J. Biol. Macromol. 2015, 79, 269–277. [Google Scholar] [CrossRef]
- Asina, F.; Brzonova, I.; Kozliak, E.; Kubatova, A.; Ji, Y. Microbial treatment of industrial lignin: Successes, problems and challenges. Renew. Sustain. Energy Rev. 2017, 77, 1179–1205. [Google Scholar] [CrossRef]
- Zhanga, Y.; Jiangb, M.; Zhanga, Y.; Caoa, Q.; Wanga, X.; Hana, Y.; Suna, G.; Lia, Y.; Zhoua, J. Novel lignin–chitosan–PVA composite hydrogel for wound dressing. Mater. Sci. Eng. C 2019, 104, 110002. [Google Scholar] [CrossRef] [PubMed]
- Spasojević, D.; Zmejkoski, D.; Glamočlija, J.; Nikolić, M.; Soković, M.; Milošević, V.; Jarić, I.; Stojanović, M.; Marinković, E.; Barisani-Asenbauer, T.; et al. Lignin model compound in alginate hydrogel: A strong antimicrobial agent with high potential in wound treatment. Int. J. Antimicrob. Agents 2016, 48, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Lia, M.; Jianga, X.; Wangb, D.; Xua, Z.; Yanga, M. In situ reduction of silver nanoparticles in the lignin based hydrogel for enhanced antibacterial application. Colloids Surf. B Biointerfaces 2019, 177, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Robles, J.; Martin, N.K.; Fong, M.L.; Stewart, S.A.; Irwin, N.J.; Rial-Hermida, M.I.; Donnelly, R.F.; Larrañeta, E. Antioxidant PLA composites containing lignin for 3d printing applications: A potential material for healthcare applications. Pharmaceutics 2019, 11, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Chávez, J.; Gurikov, P.; Hu, X.; Meyer, R.; Reynolds, W.; Smirnova, I. Application of novel and technical lignins in food and pharmaceutical industries: Structure-function relationship and current challenges. Biomass Convers. Biorefinery 2019, 1–17. [Google Scholar] [CrossRef]
- Roopan, S.M. An overview of natural renewable bio-polymer lignin towards nano and biotechnological applications. Int. J. Biol. Macromol. 2017, 103, 508–514. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Kadota, Y.; Hasegawa, C.; Kawaminami, S. Lignosulfonic acid-induced inhibition of intestinal glucose absorption. J. Nutr. Sci. Vitaminol. 2015, 61, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Gordts, S.C.; Ferir, G.; D’huys, T.; Petrova, M.I.; Lebeer, S.; Snoeck, R.; Andrei, G.; Schols, D. The low-cost compound lignosulfonic acid (LA) exhibits broad-spectrum anti-HIV and anti-HSV activity and has potential for microbicidal applications. PLoS ONE 2015, 10, e0131219. [Google Scholar] [CrossRef]
- Norikura, T.; Mukai, Y.; Fujita, S.; Mikame, K.; Funaoka, M.; Sato, S. Lignophenols Decrease Oleate-Induced Apolipoprotein-B Secretion in HepG2 Cells. Basic Clin. Pharmacol. Toxicol. 2010, 107, 813–817. [Google Scholar] [CrossRef]
- Elangovan, S.; Afanasenko, A.; Haupenthal, J.r.; Sun, Z.; Liu, Y.; Hirsch, A.K.; Barta, K. From wood to tetrahydro-2-benzazepines in three waste-free steps: Modular synthesis of biologically active lignin-derived scaffolds. ACS Cent. Sci. 2019, 5, 1707–1716. [Google Scholar] [CrossRef] [Green Version]
- Wahba, S.M.; Darwish, A.S.; Shehata, I.H.; Elhalem, S.S.A. Sugarcane bagasse lignin, and silica gel and magneto-silica as drug vehicles for development of innocuous methotrexate drug against rheumatoid arthritis disease in albino rats. Mater. Sci. Eng. C 2015, 48, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.; Bauleth-Ramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B. In vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef]
- Farhat, W.; Venditti, R.; Mignard, N.; Taha, M.; Becquart, F.; Ayoub, A. Polysaccharides and lignin based hydrogels with potential pharmaceutical use as a drug delivery system produced by a reactive extrusion process. Int. J. Biol. Macromol. 2017, 104, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Żółtowska-Aksamitowska, S.; Bartczak, P.; Zembrzuska, J.; Jesionowski, T. Removal of hazardous non-steroidal anti-inflammatory drugs from aqueous solutions by biosorbent based on chitin and lignin. Sci. Total Environ. 2018, 612, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Avilés, A.; Peñas-Garzón, M.; Bedia, J.; Rodriguez, J.; Belver, C. C-modified TiO2 using lignin as carbon precursor for the solar photocatalytic degradation of acetaminophen. Chem. Eng. J. 2019, 358, 1574–1582. [Google Scholar] [CrossRef]
- Camiré, A.; Espinasse, J.; Chabot, B.; Lajeunesse, A. Development of electrospun lignin nanofibers for the adsorption of pharmaceutical contaminants in wastewater. Environ. Sci. Pollut. Res. 2020, 27, 3560–3573. [Google Scholar] [CrossRef]
- Zhu, J.; Yan, C.; Zhang, X.; Yang, C.; Jiang, M.; Zhang, X. A sustainable platform of lignin: From bioresources to materials and their applications in rechargeable batteries and supercapacitors. Prog. Energy Combust. Sci. 2020, 76, 100788. [Google Scholar] [CrossRef]
- Temerov, F.; Belyaev, A.; Ankudze, B.; Pakkanen, T.T. Preparation and photoluminescence properties of graphene quantum dots by decomposition of graphene-encapsulated metal nanoparticles derived from Kraft lignin and transition metal salts. J. Lumin. 2019, 206, 403–411. [Google Scholar] [CrossRef]
- Tang, P.-D.; Du, Q.-S.; Li, D.-P.; Dai, J.; Li, Y.-M.; Du, F.-L.; Long, S.-Y.; Xie, N.-Z.; Wang, Q.-Y.; Huang, R.-B. Fabrication and characterization of graphene microcrystal prepared from lignin refined from sugarcane bagasse. Nanomaterials 2018, 8, 565. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Li, F.; Wen, J.; Wang, X.; Sun, R. Gram-scale synthesis of single-crystalline graphene quantum dots derived from lignin biomass. Green Chem. 2018, 20, 1383–1390. [Google Scholar] [CrossRef]
- Lai, C.; Zhou, Z.; Zhang, L.; Wang, X.; Zhou, Q.; Zhao, Y.; Wang, Y.; Wu, X.-F.; Zhu, Z.; Fong, H. Free-standing and mechanically flexible mats consisting of electrospun carbon nanofibers made from a natural product of alkali lignin as binder-free electrodes for high-performance supercapacitors. J. Power Sour. 2014, 247, 134–141. [Google Scholar] [CrossRef]
- Ago, M.; Borghei, M.; Haataja, J.S.; Rojas, O.J. Mesoporous carbon soft-templated from lignin nanofiber networks: Microphase separation boosts supercapacitance in conductive electrodes. Rsc Adv. 2016, 6, 85802–85810. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhao, M.; Liu, R.; Wang, X.; Lin, H. Hierarchical porous carbon derived from lignin for high performance supercapacitor. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 518–527. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Opra, D.P.; Sinebryukhov, S.L.; Tsvetnikov, A.K.; Ustinov, A.Y.; Sergienko, V.I. Hydrolysis lignin: Electrochemical properties of the organic cathode material for primary lithium battery. J. Ind. Eng. Chem. 2014, 20, 903–910. [Google Scholar] [CrossRef]
- Chang, Z.-Z.; Yu, B.-J.; Wang, C.-Y. Influence of H2 reduction on lignin-based hard carbon performance in lithium ion batteries. Electrochim. Acta 2015, 176, 1352–1357. [Google Scholar] [CrossRef]
- Culebras, M.; Geaney, H.; Beaucamp, A.; Upadhyaya, P.; Dalton, E.; Ryan, K.M.; Collins, M.N. Bio-derived Carbon Nanofibres from Lignin as High-Performance Li-Ion Anode Materials. ChemSusChem 2019, 12, 4516–4521. [Google Scholar] [CrossRef]
- Nguyen, N.A.; Bowland, C.C.; Naskar, A.K. A general method to improve 3D-printability and inter-layer adhesion in lignin-based composites. Appl. Mater. Today 2018, 12, 138–152. [Google Scholar] [CrossRef]
- Nguyen, N.A.; Barnes, S.H.; Bowland, C.C.; Meek, K.M.; Littrell, K.C.; Keum, J.K.; Naskar, A.K. A path for lignin valorization via additive manufacturing of high-performance sustainable composites with enhanced 3D printability. Sci. Adv. 2018, 4, eaat4967. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Liu, W.; Qiu, X. High performance thermoplastic elastomers with biomass lignin as plastic phase. ACS Sustain. Chem. Eng. 2019, 7, 6550–6560. [Google Scholar] [CrossRef]
- Tanase-Opedal, M.; Espinosa, E.; Rodríguez, A.; Chinga-Carrasco, G. Lignin: A biopolymer from forestry biomass for biocomposites and 3D printing. Materials 2019, 12, 3006. [Google Scholar] [CrossRef] [Green Version]
- Gordobil, O.; Egüés, I.; Llano-Ponte, R.; Labidi, J. Physicochemical properties of PLA lignin blends. Polym. Degrad. Stab. 2014, 108, 330–338. [Google Scholar] [CrossRef]
- Sutton, J.T.; Rajan, K.; Harper, D.P.; Chmely, S.C. Lignin-containing photoactive resins for 3D printing by stereolithography. ACS Appl. Mater. Interfaces 2018, 10, 36456–36463. [Google Scholar] [CrossRef]
- Vaidya, A.A.; Collet, C.; Gaugler, M.; Lloyd-Jones, G. Integrating softwood biorefinery lignin into polyhydroxybutyrate composites and application in 3D printing. Mater. Today Commun. 2019, 19, 286–296. [Google Scholar] [CrossRef]
- Abbati de Assis, C.; Greca, L.G.; Ago, M.; Balakshin, M.Y.; Jameel, H.; Gonzalez, R.; Rojas, O.J. Techno-economic assessment, scalability, and applications of aerosol lignin micro-and nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 11853–11868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Z.; Smith Jr, R.L. Production of Biofuels and Chemicals from Lignin; Springer: Singapore, 2016. [Google Scholar]
- Domínguez-Robles, J.; Larrañeta, E.; Fong, M.L.; Martin, N.K.; Irwin, N.J.; Mutjé, P.; Tarrés, Q.; Delgado-Aguilar, M. Lignin/poly (butylene succinate) composites with antioxidant and antibacterial properties for potential biomedical applications. Int. J. Biol. Macromol. 2020, 145, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Vinardell, M.P.; Mitjans, M. Lignins and their derivatives with beneficial effects on human health. Int. J. Mol. Sci. 2017, 18, 1219. [Google Scholar] [CrossRef] [Green Version]
- Witzler, M.; Alzagameem, A.; Bergs, M.; Khaldi-Hansen, B.E.; Klein, S.E.; Hielscher, D.; Kamm, B.; Kreyenschmidt, J.; Tobiasch, E.; Schulze, M. Lignin-derived biomaterials for drug release and tissue engineering.figure. Molecules 2018, 23, 1885. [Google Scholar] [CrossRef] [Green Version]
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.-A. Valorization of lignin in polymer and composite systems for advanced engineering applications–a review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef]
- MarketsandMarkets. 3D Printing Materials Market—Global Forecast to 2024. Available online: https://www.marketsandmarkets.com/Market-Reports/3d-printing-materials-market-1295.html (accessed on 4 June 2020).








| Product | Usage | Feedstock Lignin | Lignin Content (wt %) | Reaction/Process Condition | Source |
|---|---|---|---|---|---|
| Lignophenols | Potentially prevent coronary heart disease | Japanese cedar native lignin | N/A | Phase-separation with cresol and sulfuric acid. | [31] |
| Tetrahydro-2-benzazepines | Precursor to the drugs that could treat Alzheimer’s disease | Pine and poplar lignocellulose | N/A | For depolymerization step, copper-doped porous metal oxides and 40 bar H2; for synthesis of product, used choline chloride/ oxalic acid DES at 70−80 °C for 20−48 h. | [32] |
| Drug vehicles | Deliver methotrexate | Sugar cane bagasse | N/A | Lignin soaked in 100 ppm methotrexate solution with intense stirring at 40 °C for 18 h. | [33] |
| Nanoparticles | Deliver Sorafenib and Benzazulene | LignoBoost™ softwood Kraft lignin | 50% | Lignin dissolved in tetrahydrofuran and put in the dialysis bag, iron (III) isopropoxide in tetrahydrofuran for 24 h. | [34] |
| Hydrogels | Drug delivery system | Kraft lignin | Up to 80% | 20 to 200 wt % citric acid, used micro-extruder at 120 °C for 2 or 5 min of recirculation. | [35] |
| Biosorbent | Adsorb ibuprofen and acetaminophen | Kraft lignin | 50% | Kraft lignin and α-chitin powder mixed, following activation with 15% hydrogen peroxide. | [36] |
| Photocatalyst | Degrade acetaminophen | LignoTech lignin | 33% | TiO2 and NaOH, hydrothermal treatment at 130 °C for 48 h, centrifuged, dried, calcined in N2 or air. | [37] |
| Nanofibers | Adsorb fluoxetine | Alkali lignin | 30–50% | Aqueous polyvinyl alcohol and distilled water at 80 °C for 60 min, electrospinning fibers. | [38] |
| Product | Feedstock | Lignin Content (wt %) | Reaction/Process Condition | Source |
|---|---|---|---|---|
| Supercapacitor | Alkali lignin | 30–70% | Electrospinning aqueous alkali lignin and PVA, temperature stabilization in the tube furnace ramped from 25 to 220 °C, held at 220 °C for 8 h. | [43] |
| Supercapacitor | Softwood alkali lignin | 75% | Lignin, PVA, and distilled water mixed at 60 °C for 1 h and at room temperature for 4 h, followed by electrospinning. | [44] |
| Supercapacitor | Steam explosion lignin | 17–50% | Carbonization at 500 °C or 800 °C, activation with KOH, post-treatment by washing with hot water and drying at 100 °C overnight. | [45] |
| Lithium battery cathode material | Hydrolysis lignin | 76% | Ball milled to 30 microns, washed with distilled water in centrifuge for 10–12 h, dried at 60 °C for 24 h. | [46] |
| Lithium battery anode material | Acetone lignin from corn stalks | 80% | Stabilized at 300 °C for 2 h in N2 in tube furnace. | [47] |
| Lithium battery anode material | Organosolv hardwood lignin | 50–80% | Thermoplastic elastomeric polyurethane, dimethylformamide, PLA stirred at 50 °C for 5 min before electrospinning. | [48] |
| Lignin Feedstock | Polymer Feedstock | Lignin Content in 3D Printing Composite (wt %) | Source |
|---|---|---|---|
| Kraft lignin | ABS | 40% | [49] |
| Organosolv hardwood lignin | Nylon | 40–60% | [50] |
| Softwood lignin from soda cooking process | PLA | 20% and 40% | [52] |
| Alkali lignin and organosolv lignin | PLA | 0.5–20% | [53] |
| Organosolv lignin | Polymer resins | 5–15% | [54] |
| Softwood lignin from P. radiata | Polyhydroxybutyrate | 20% | [55] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, O.; Kim, K.H. Lignin to Materials: A Focused Review on Recent Novel Lignin Applications. Appl. Sci. 2020, 10, 4626. https://doi.org/10.3390/app10134626
Yu O, Kim KH. Lignin to Materials: A Focused Review on Recent Novel Lignin Applications. Applied Sciences. 2020; 10(13):4626. https://doi.org/10.3390/app10134626
Chicago/Turabian StyleYu, Osbert, and Kwang Ho Kim. 2020. "Lignin to Materials: A Focused Review on Recent Novel Lignin Applications" Applied Sciences 10, no. 13: 4626. https://doi.org/10.3390/app10134626
APA StyleYu, O., & Kim, K. H. (2020). Lignin to Materials: A Focused Review on Recent Novel Lignin Applications. Applied Sciences, 10(13), 4626. https://doi.org/10.3390/app10134626