Valorization of Slags Produced by Smelting of Metallurgical Dusts and Lateritic Ore Fines in Manufacturing of Slag Cements
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Tests in Raw Materials
2.2. Tests in Cement Production
3. Results and Discussion
3.1. Slags Characterization
3.1.1. Chemical Composition
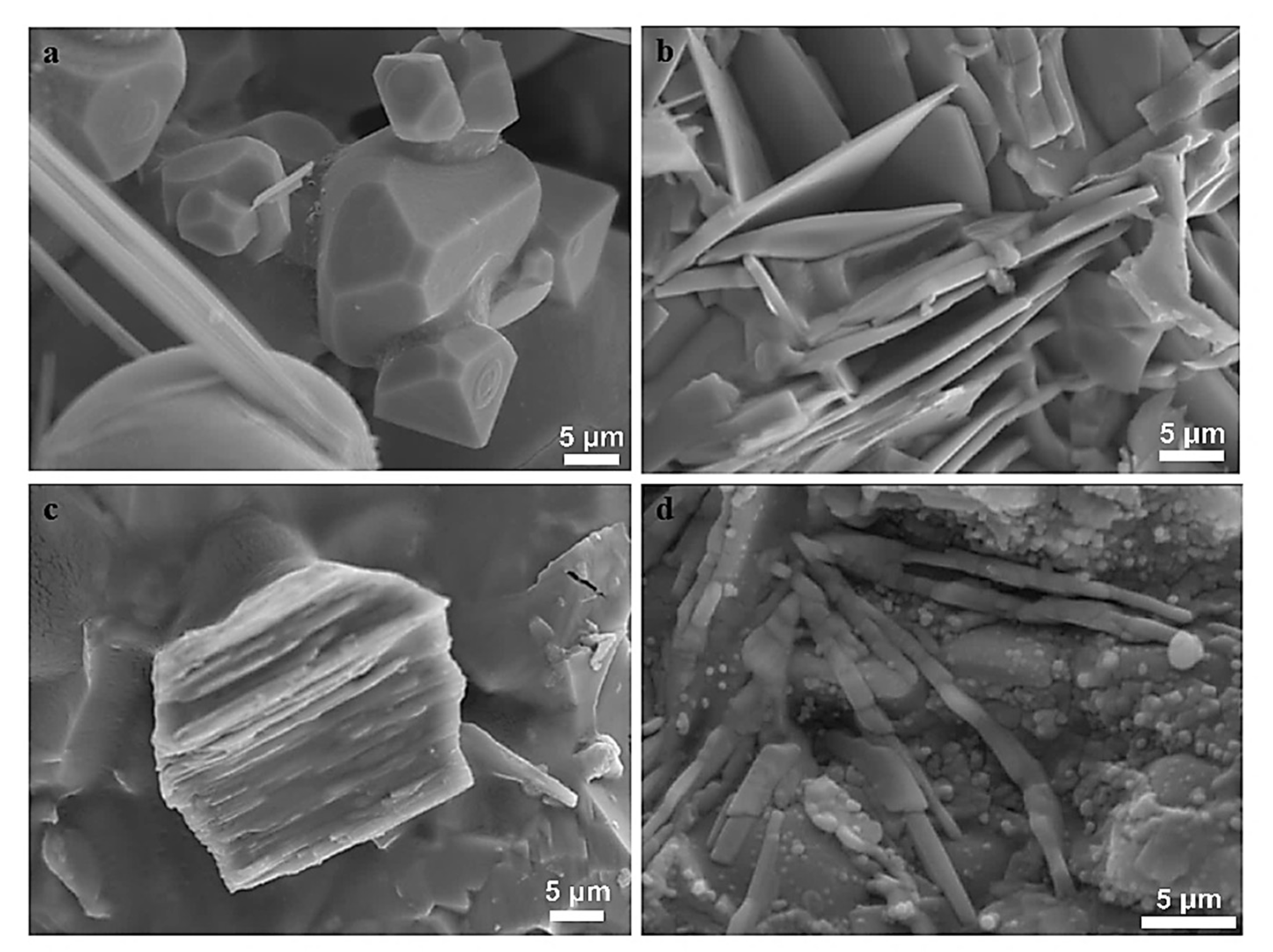
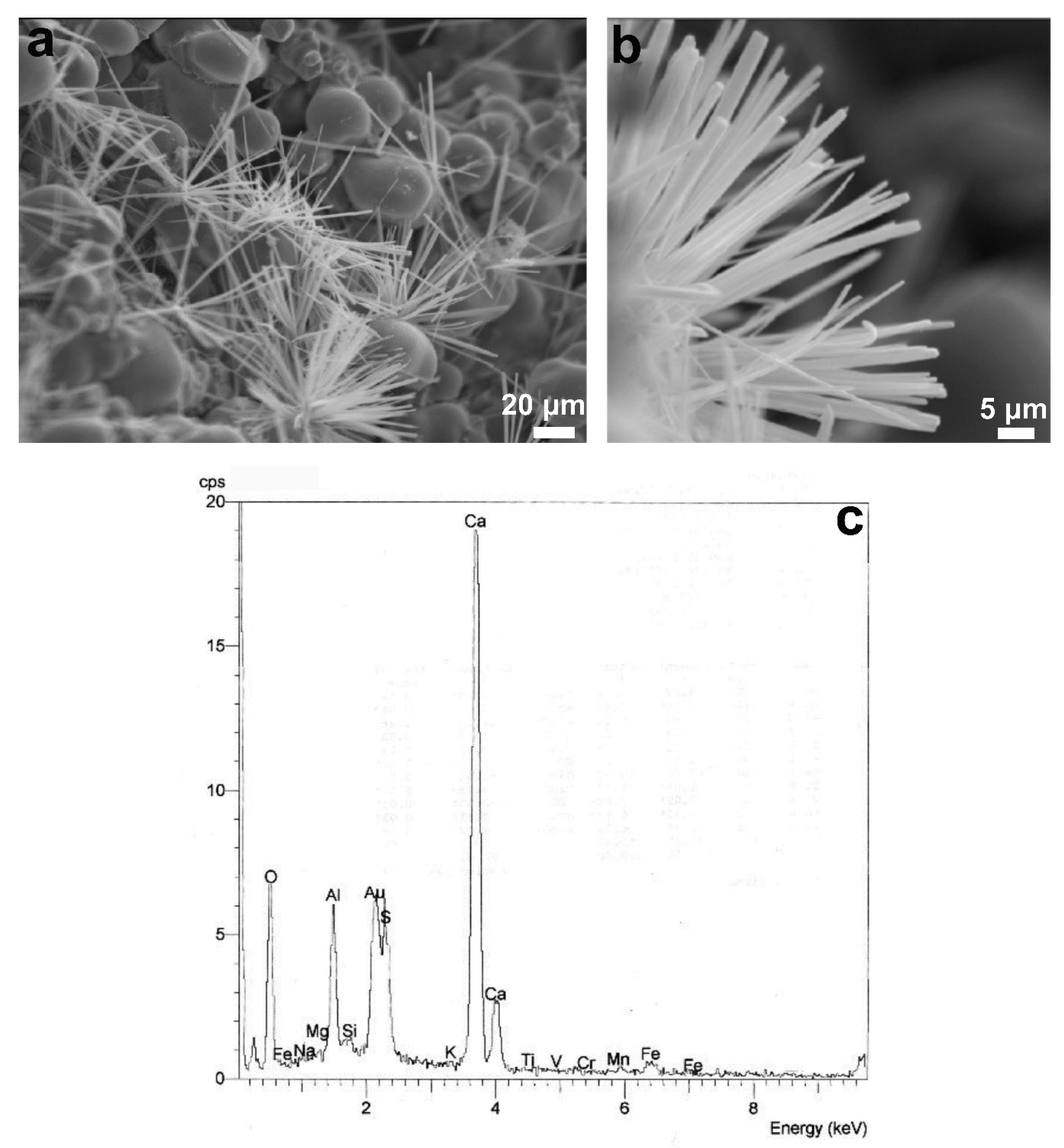
3.1.2. Mineralogical Characterization and Microstructure
3.2. Properties of Composite Portland Cements (CPCs) Manufactured by 20% Slag
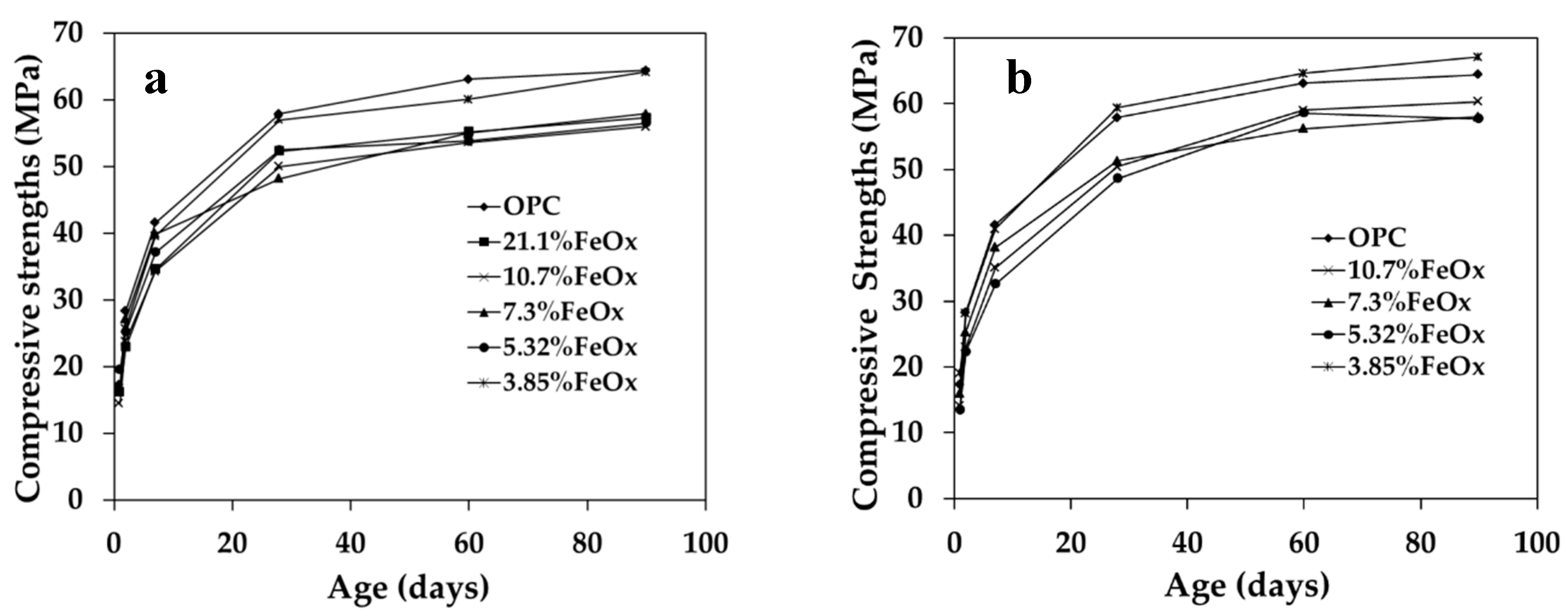
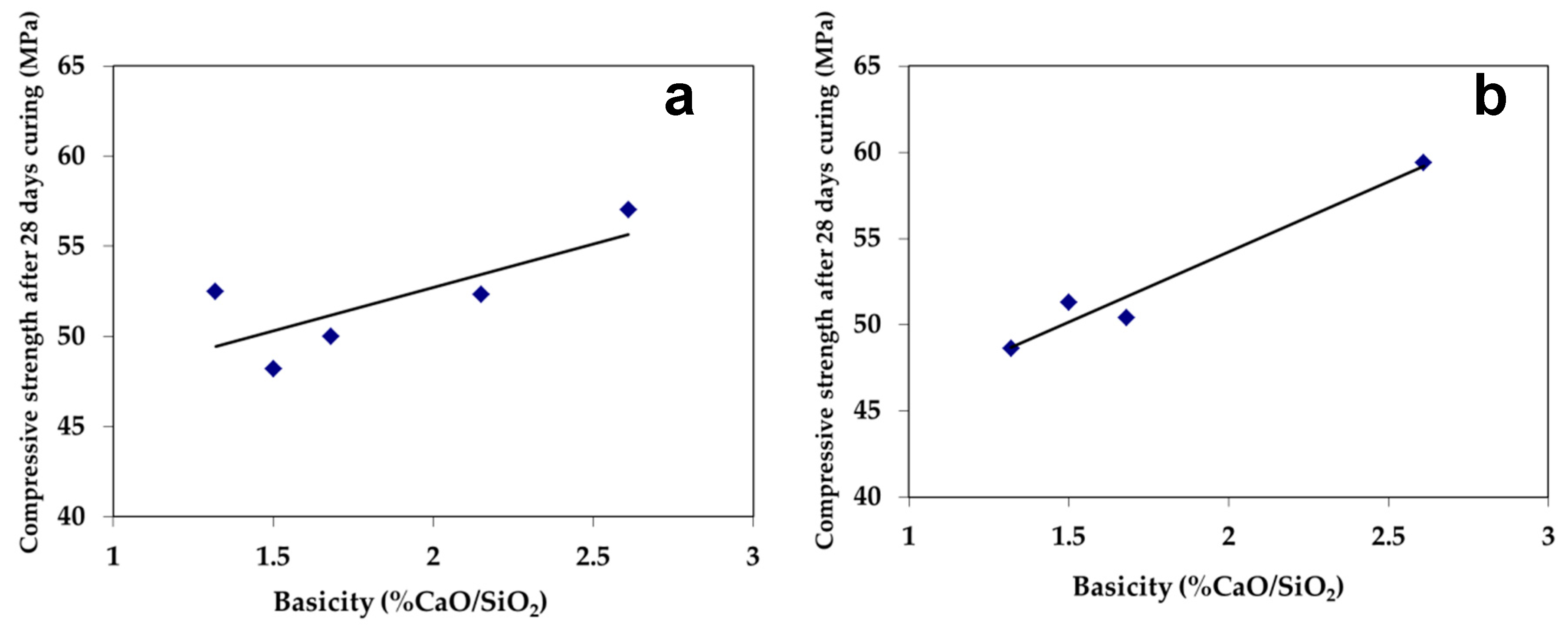
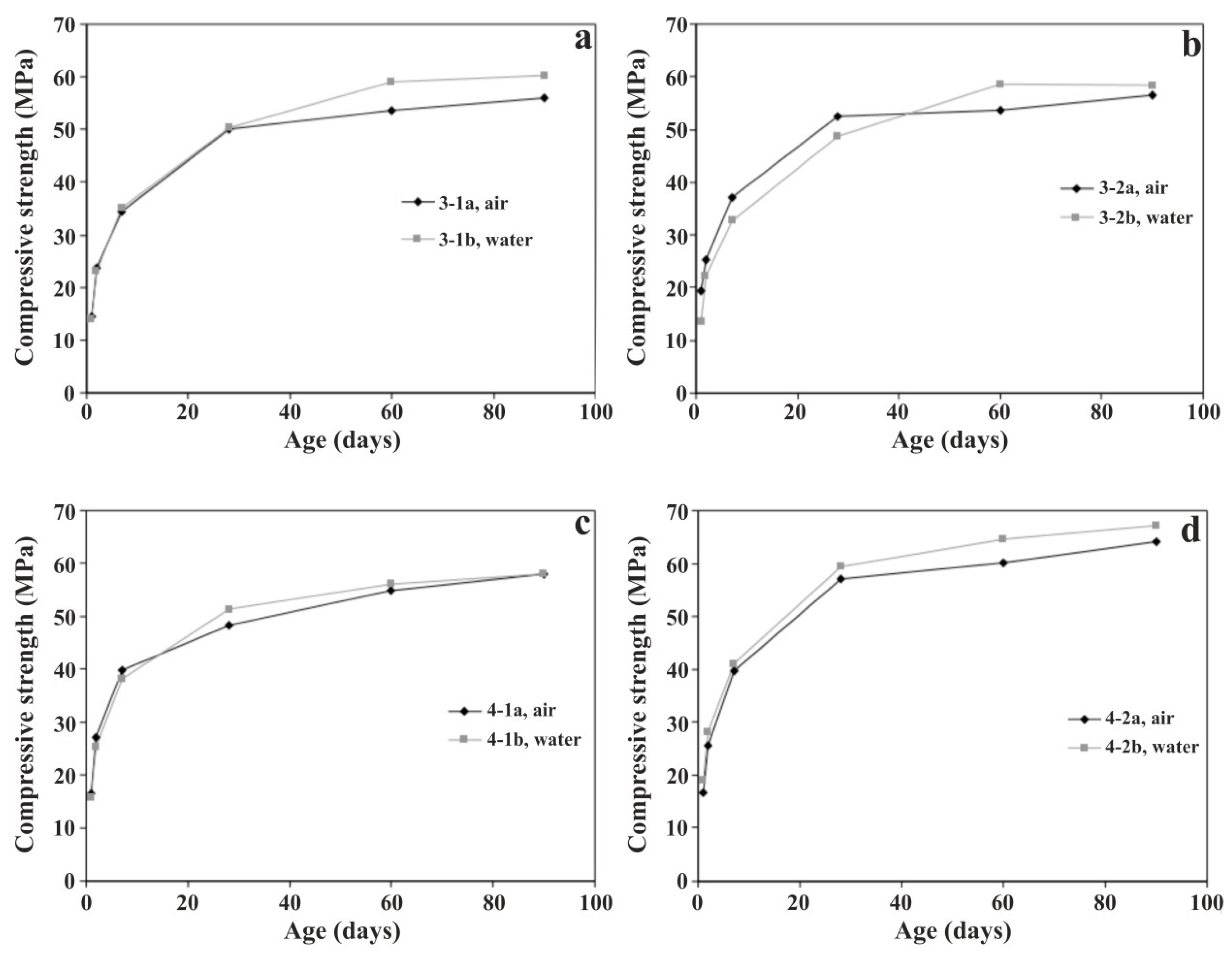
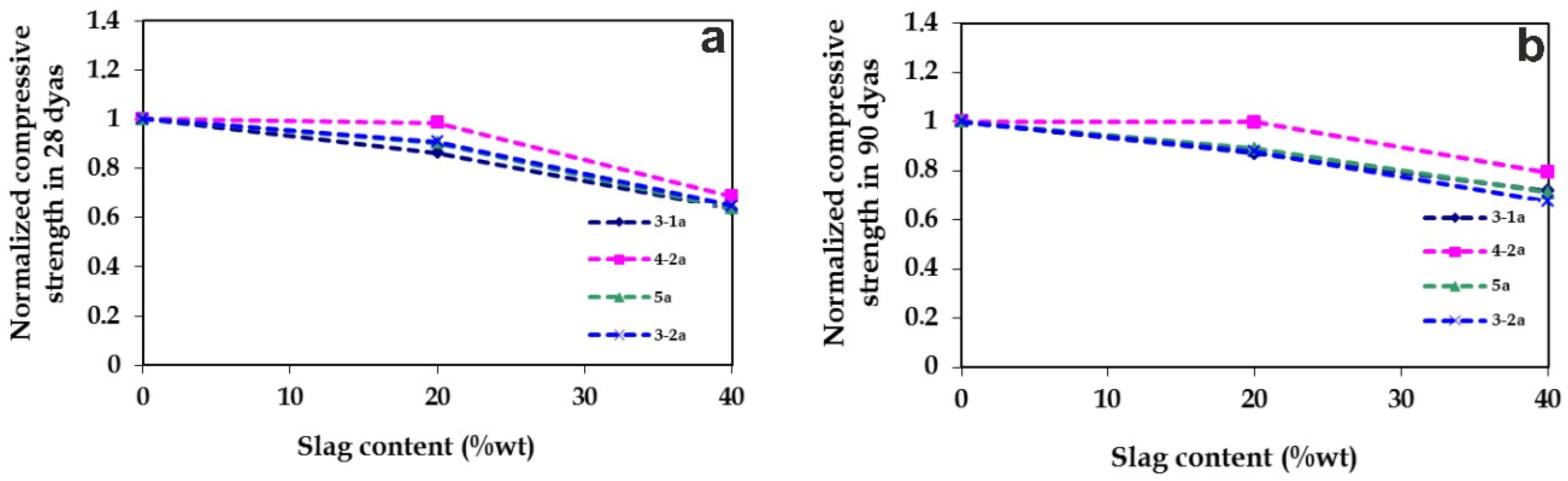
3.2.1. Compressive Strength
3.2.2. Setting Time
3.2.3. Soundness (Expansion)
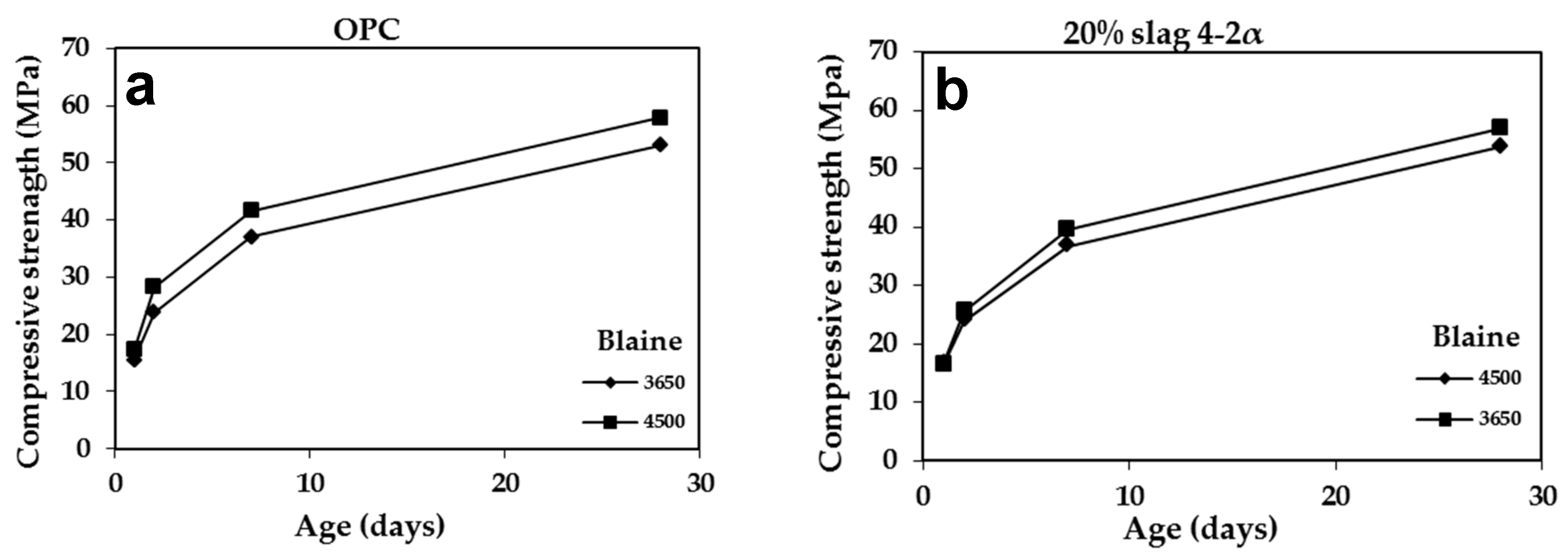
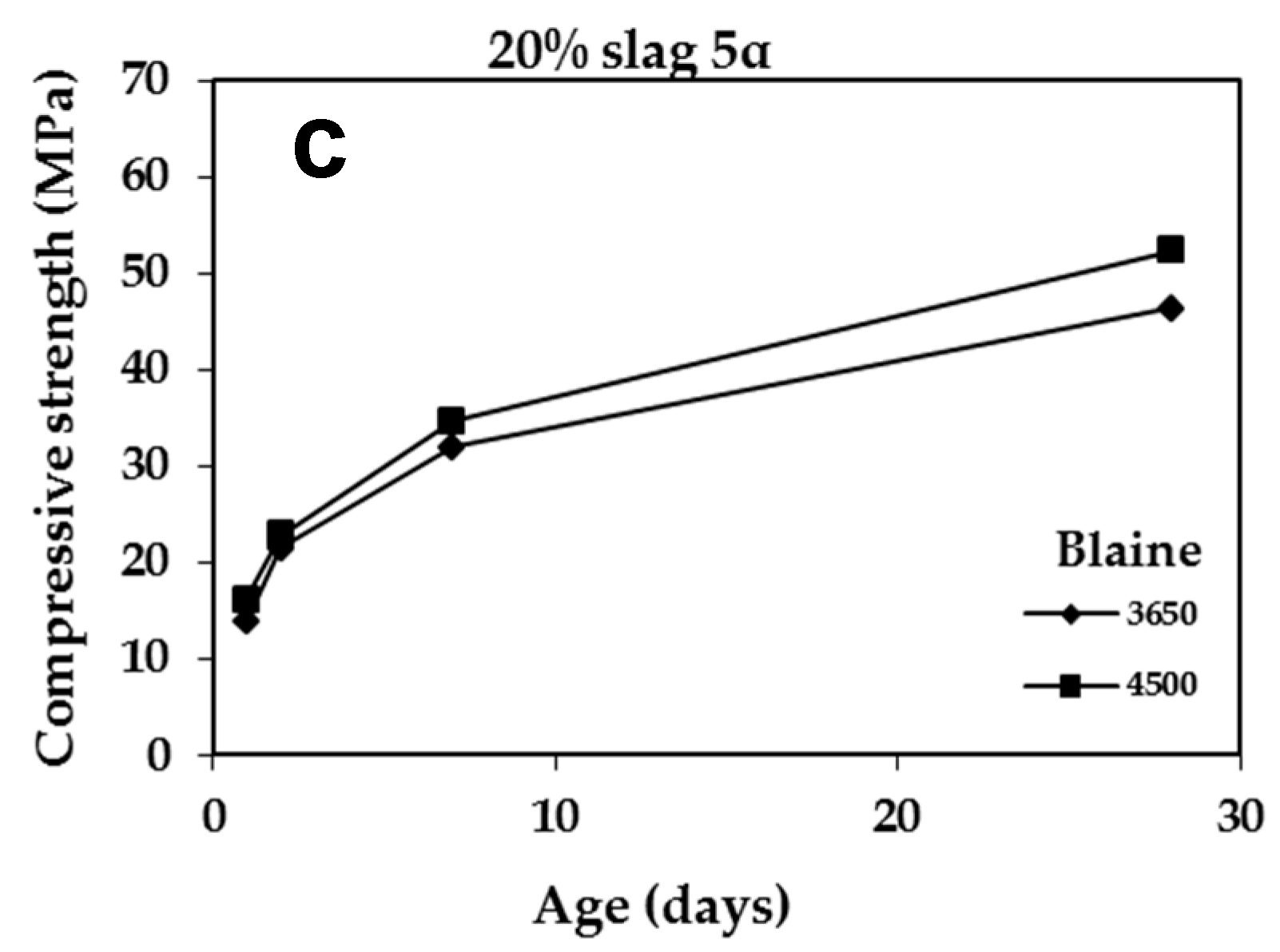
3.2.4. Effect of Blaine
3.3. Properties of Slag Cements (SCs) manufactured by 40% Slag
3.3.1. Compressive Strength
3.3.2. Setting Time
3.3.3. Soundness (Expansion)
3.3.4. Grinding Time
3.4. Suppressing CO2 Emissions by Use of Slag in Cement Manufacturing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yunos, N.F.M.; Zaharia, M.; Ismail, A.N.; Idris, M.A. Transforming Waste Materials as Resources for EAF Steel making. Int. J. Mater. Eng. 2014, 4, 167–170. [Google Scholar]
- Jones, R.T.; Hayman, D.A.; Denton, G.M. Recovery of cobalt, nickel and copper from slags using DC-arc furnace technology, International Symposium on Challenges of Process Intensification. In Proceedings of the 35th Annual Conference of Metallurgists, Montreal, QC, Canada, 24–29 August 1996. [Google Scholar]
- Burström, G.Y.; Kuhn, M.; Piret, J. Reduction of Steelmaking Slags for Recovery of Valuable Metals and Oxide Materials. In Proceedings of the 6th International Conference on Molten Slags, Fluxes and Salts, Stockholm, Sweden-Helsinki, Finland, 12–17 June 2000. [Google Scholar]
- Tzevelekou, T.V.; Geck, H.G.; Hüllen, P.; Höfer, F.; Papamantellos, D.C. Direct Smelting of Metallurgical Dusts and Ore Fines in a 125t DC-HEP Furnace. Steel Res. Int. 2004, 75, 382–392. [Google Scholar] [CrossRef]
- Memoli, F.; Mapelli, C.; Guzzon, M. Recycling of Ladle Slag in the EAF: A Way to Improve Environmental Conditions and Reduce Variable Costs in Steel Plants. Iron Steel Technol. 2007, 4, 68–76. [Google Scholar]
- Heo, J.H.; Kim, T.S.; Sahajwalla, V.; Park, J.H. Observations of FeO Reduction in Electric Arc Furnace Slag by Aluminum Black Dross: Effect of CaO Fluxing on Slag Morphology. Metal Mater. Trans. B 2020, 51, 1201–1210. [Google Scholar] [CrossRef]
- Schliephake, H.; Algermissen, D. How far away is the steel industry from the target now waste? Proceedings EUROSLAG. In Proceedings of the 10th European Slag Conference Slag based products–best practices for Circular Economy, Thessaloniki, Greece, 8–11 October 2019. [Google Scholar]
- Alexopoulou, I.E.; Angelopoulos, G.N.; Papamantellos, D.C.; Rentizelas, G. The dust recycling problem of the FeNi production at Larymna/Greece—Part II: New perspectives and proposals. Erzmetall 1994, 47, 651–657. [Google Scholar]
- Kotze, J. Pilot plant production of ferronickel from nickel oxide ores and dusts in a DC arc furnace. Miner. Eng. 2002, 15, 1017–1022. [Google Scholar] [CrossRef]
- Osmanović, Z.; Haračić, N.; Zelić, J.; Lihić, A. Slag Cement Production in Terms of Emission Reducing; ISEM: Adiyaman, Turkey, 2014. [Google Scholar]
- Schorcht, F.; Kourti, I.; Scalet, B.M.; Roudier, S.; Delgado Sancho, L. Best Available Techniques (BAT) Reference Document for the Production of Cement, Lime and Magnesium Oxide: Industrial Emissions Directive 2010/75/EU: (Integrated Pollution Prevention and Control); Luxembourg Publications Office of the European Union: Brussels, Belgium, 2013. [Google Scholar]
- Pellegrino, C.; Faleschini, F.; Meyer, C. Developments in the Formulation and Reinforcement of Concrete, 2nd ed.; Woodhead Publishing Series in Civil and Structural Engineering: Vancouver, BC, Canada, 2019. [Google Scholar]
- Kim, H.S.; Kim, K.S.; Jung, S.S.; Hwang, J.I.; Choi, J.S.; Sohn, I. Valorization of electric arc furnace primary steelmaking slags for cement applications. Waste Manag. 2015, 41, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Drelich, J.W.; Neelameggham, N.R.; Guillen, D.P.; Haque, N.; Zhu, J.; Sun, Z.; Wang, T.; Howarter, J.A.; Tesfaye, F.; et al. (Eds.) Energy Technol 2017—Carbon Dioxide Management and Other Technologies, The Minerals, Metals & Materials Series, 1, XII, 499; Springer: Berlin, Germany, 2017. [Google Scholar] [CrossRef]
- Luckman, M.; Vitta, S.; Venkateswaran, D. Cementitious and pozzolanic behavior of electric arc furnace steel slags. Cem. Concr Res. 2009, 39, 102–109. [Google Scholar]
- Branca, T.A.; Colla, V.; Algermissen, D.; Granbom, H.; Martini, U.; Morillon, A.; Pietruck, R.; Rosendahl, S. Reuse and Recycling of By-Products in the Steel Sector: Recent Achievements Paving the Way to Circular Economy and Industrial Symbiosis in Europe. Metals 2020, 10, 345. [Google Scholar] [CrossRef] [Green Version]
- British Standard EN 197 Part 1. Cement-Composition, Specifications and Conformity Criteria for Common Cements; BSI: London, UK, 2011. [Google Scholar]
- Murphy, N.; Meadwcroft, T.R.; Barr, P.V. Enhancement of the cementitious properties of steelmaking slag. Can. Metal. Q. 1997, 36, 315–331. [Google Scholar] [CrossRef]
- Shi, C.; Qian, J. High performance cementing materials from industrial slags–a review. Resour. Conserv. Recycl. 2000, 29, 195–207. [Google Scholar] [CrossRef]
- Rojas, M.F.; de Rojas, M.I.S. Chemical assessment of the electric arc furnace slag as construction material: Expansive formations. Cem. Concr. Res. 2004, 34, 1881–1888. [Google Scholar] [CrossRef]
- Engstrom, F.; Pontikes, Y.; Geysen, D.; Jones, P.T.; Bjorkman, B.; Blanpain, B. Review: Hot stage engineering to improve slag valorisation options. In Proceedings of the 2nd International Slag Valorization Symposium, The transition of sustainable materials management, Leuven, Belgium, 18–20 April 2011. [Google Scholar]
- Pontikes, Y.; Malfliet, A. Slag Valorisation as a Contribution to Zero-Waste Metallurgy. J. Sustain. Metall. 2016, 2, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Gang, D.; Guo, Z. Convert hot slag into value-added materials by modification methods. In Proceedings of the 5th International Slag Valorization Symposium, Leuven, Belgium, 3–5 April 2017. [Google Scholar]
- Mudersbach, D.; Kühn, M.; Geisler, J.; Koch, K. Chrome Immobilisation in EAF-Slags from High-Alloy Steelmaking: Tests at FEhS Institute and Development of an Operational Slag Treatment Process. In Proceedings of the First International Slag Valorisation Symposium, Leuven, Belgium, 6–7 April 2009. [Google Scholar]
- Primavera, A.; Pontoni, L.; Mombelli, D.; Barella, S.; Mapelli, C. EAF Slag Treatment for Inert Materials’ Production. J. Sustain. Metall. 2016, 2, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Tzevelekou, T.; Stivanakis, V.; Papamantellos, D.C.; Hoefer, F.; Haniotakis, E.; Fragoulis, D. Properties of slags and Portland-slag-cements produced by smelting of metallurgical dusts and ore fines in steelmaking DC-HEP furnace. In Proceedings of the Advances in Mineral Resources Management and Environmental Geotechnology, Chania, Greece, 7–9 June 2004. [Google Scholar]
- Tzevelekou, T.V.; Geck, H.G.; Hüllen, P.V.; Höfer, F.; Poulakis, S.; Papamantellos, D.C. Recycling of nickel ferrous rotary kiln dust by injection through hollow electrode in a 125t DC-HEP furnace. In Proceedings of the International Symposium on Metals and Energy Recovery Proc, Skellefteå, Sweden, 25–26 June 2003. [Google Scholar]
- Tzevelekou, T.V. Development of a Process for Recycling and Producing New Materials by Reduction Smelting of Das Cleaning Systems Dusts from the Ferronickel Industry. Ph. D Thesis, Department of Chemical Engineering, University of Patras, Patras, Greece, 2004. [Google Scholar]
- Papamantellos, D.C. UntersuchungenzurEntchromung von Roheisen, Doktor-Arbeit, FakultätfürBergbau und Hüttenwesen der RWTH; Aachen University: Aachen Germany, 1965. [Google Scholar]
- Hong, H.; Fu, Z.; Min, X. Effect of cooling performance on the mineralogical character of Portland cement clinker. Cem. Concr. Res. 2001, 31, 287–290. [Google Scholar] [CrossRef]
- Moseley, D.; Glasser, F.P. Identity, Composition and Stability of bredigite-structure phase T. Cem. Concr. Res. 1981, 11, 559–565. [Google Scholar] [CrossRef]
- Luxán, M.P.; Sotolongo, R.; Dorrego, F.; Herrero, E. Characteristics of the slags produced in the fusion of scrap steel by electric arc furnace. Cem. Concr. Res. 2000, 30, 517–519. [Google Scholar] [CrossRef]
- Bai, J.; Chaipanich, A.; Kinuthia, J.M.; O’Farrell, M.; Sabir, B.B.; Wild, S.; Lewis, M.H. Compressive strength and hydration of wastepaper sludge ash-ground granulated blast furnace slag blended pastes. Cem. Concr. Res. 2003, 33, 1189–1202. [Google Scholar] [CrossRef]
- Hofmänner, F. Microstructure of Portland Cement Clinker; «Holder bank» Management & Consulting Ltd.: Holderbank, Switzerland, 1975. [Google Scholar]
- Qian, G.R.; Sun, D.D.; Tay, J.H.; Lai, Z.Y. Hydrothermal reaction and autoclave stability of Mg bearing RO phase in steel slag. Br. Ceram. Trans. 2002, 101, 159–164. [Google Scholar] [CrossRef]
- Engström, F. Mineralogical Influence on Leaching Behaviour of Steelmaking Slags. Ph.D. Thesis, Department of Chemical Engineering and Geoscience, Division of Minerals and Metallurgical Engineering, University of Technology, Luleå, Sweden, 2010. [Google Scholar]
- Stark, J.; Bollmann, K. Delayed Ettringite Formation in Concrete Nordic, Concrete Research (NCR), Bauhaus-University Weimar Publication No.23, Germany. Available online: https://www.imxtechnologies.com/storage/app/media/uploaded-files/ettringite.pdf (accessed on 2 July 2020).
- Altun, A.; Yilmaz, I. Study on steel furnace slags with high MgO as additive in Portland cement. Cem. Concr. Res. 2002, 32, 1247–1249. [Google Scholar] [CrossRef]
- Xuequan, W.; Hong, Z.; Xinkai, H.; Husen, L. Study on steel slag and fly ash composite Portland cement. Cem. Concr. Res. 1999, 29, 1103–1106. [Google Scholar] [CrossRef]
- Chatterjee, A.K. High belite cements-present status and future technological options: Part I. Cem. Concr. Res. 1996, 26, 1213–1225. [Google Scholar] [CrossRef]
- Petrounias, P.; Giannakopoulou, P.P.; Rogkala, A.; Kalpogiannaki, M.; Koutsovitis, P.; Damoulianou, M.-E.; Koukouzas, N. Petrographic Characteristics of Sandstones as a Basis to Evaluate Their Suitability in Construction and Energy Storage Applications. A Case Study from Klepa Nafpaktias (Central Western Greece). Energies 2020, 13, 1119. [Google Scholar] [CrossRef] [Green Version]
- Arvanitis, A.; Koutsovitis, P.; Koukouzas, N.; Tyrologou, P.; Karapanos, D.; Karkalis, C.; Pomonis, P. Potential Sites for Underground Energy and CO2 Storage in Greece: A Geological and Petrological Approach. Energies 2020, 13, 2707. [Google Scholar] [CrossRef]
- Parron-Rubio, M.E.; Perez-Garcia, F.; Gonzalez-Herrera, A.; Oliveira, M.J.; Rubio-Cintas, M.D. Slag Substitution as a Cementing Material in Concrete: Mechanical, Physical and Environmental Properties. Materials 2019, 12, 2845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
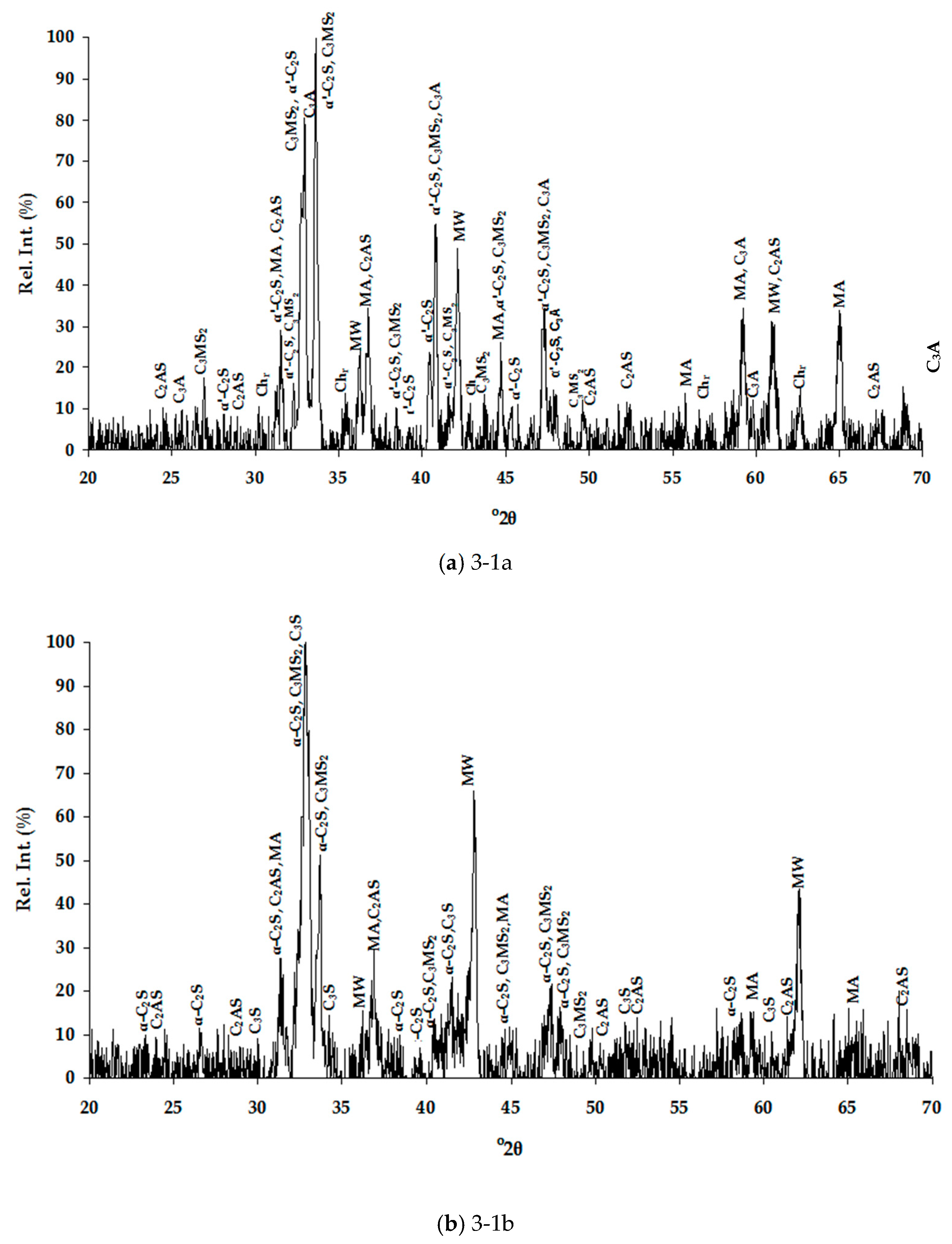
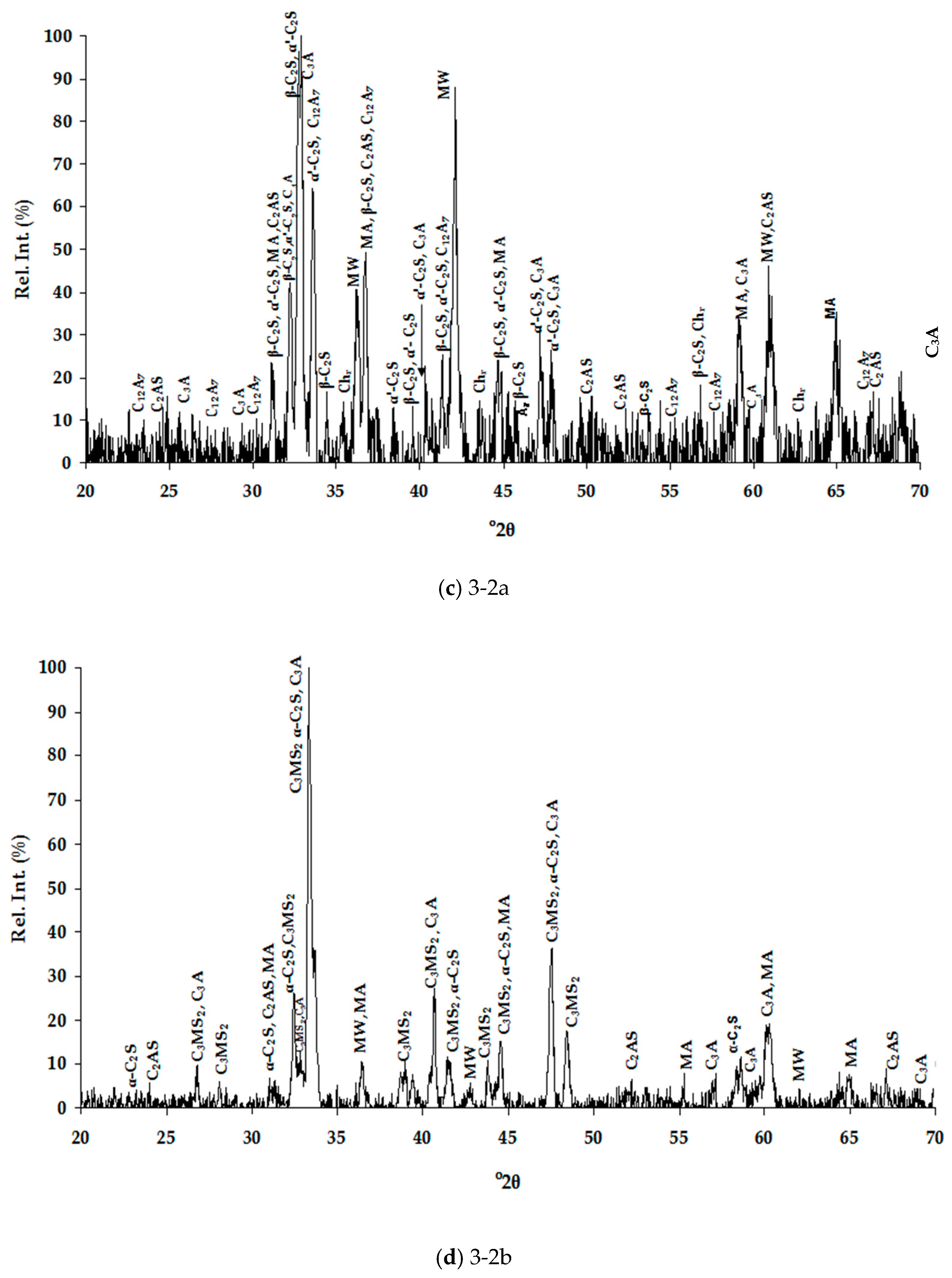
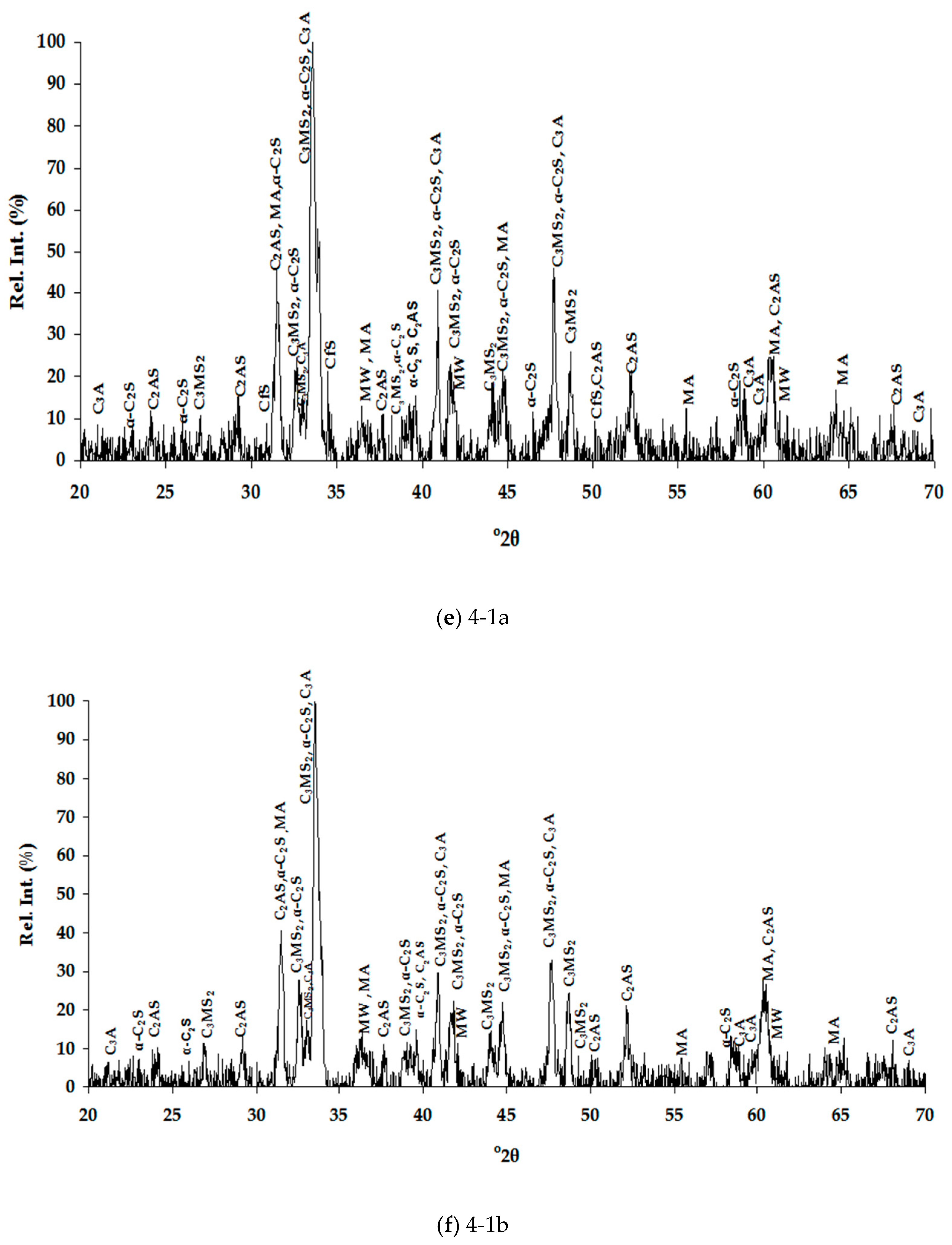
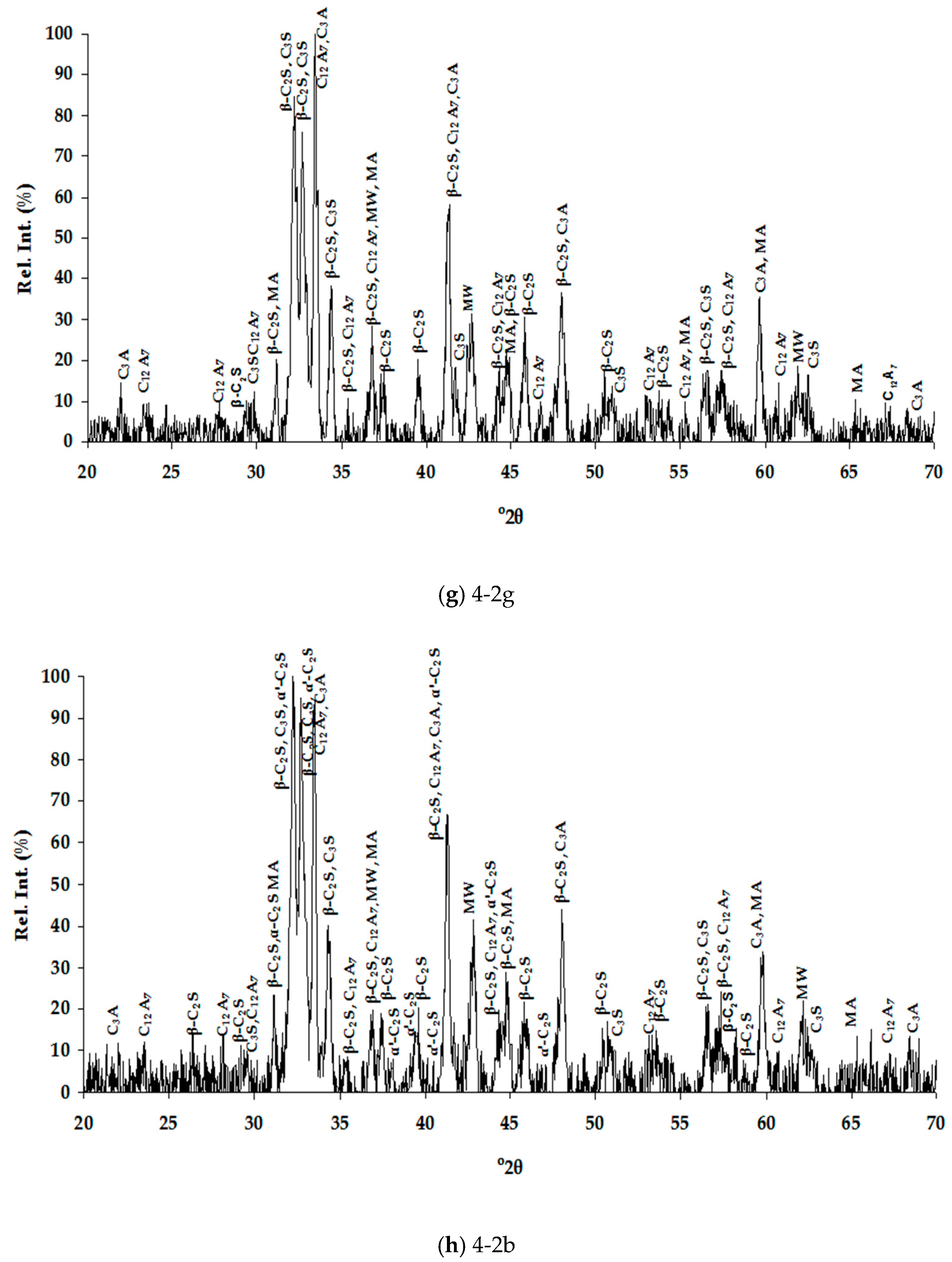




| Dust | Ore Fines | |
|---|---|---|
| FeOx | 30.44 | 36.65 |
| SiO2 | 35.00 | 34.85 |
| MnO | 0.50 | 0.87 |
| Cr2O3 | 3.00 | 5.82 |
| Al2O3 | 11.55 | 4.31 |
| CaO | 4.10 | 4.18 |
| MgO | 5.27 | 5.92 |
| NiO | 1.80 | 1.37 |
| S | 0.44 | 0.20 |
| Ctot | 3.60 | 0.94 |
| Moisture | 0.24 | 1.82 |
| No. (*) | Bas. | FeOx (wt.%) | Al2O3 (wt.%) | MgO (wt.%) | MnO (wt.%) | Crtot (wt.%) | S (wt.%) |
|---|---|---|---|---|---|---|---|
| 3-1a, b | 1.68 | 10.7 | 7.72 | 10.8 | 2.48 | 0.53 | 0.29 |
| 3-2a, b | 1.32 | 5.32 | 8.57 | 11.5 | 2.51 | 0.60 | 0.30 |
| 4-1a, b | 1.50 | 7.30 | 11.6 | 8.93 | 3.12 | 0.96 | 0.36 |
| 4-2a, b | 2.61 | 3.85 | 12.00 | 7.54 | 1.68 | 0.19 | 0.39 |
| 5a | 2.15 | 21.1 | 7.36 | 4.36 | 2.36 | 0.88 | 0.20 |
| 3-1a | 3-2a | 4-1a | 4-2a | 5a | |
|---|---|---|---|---|---|
| α-C2S | + | ||||
| α′-C2S | + | + | + | ||
| β-C2S | + | + | + | ||
| C3S | + | + | |||
| C3MS2 | + | + | |||
| C3A | + | + | + | + | + |
| C12A7 | + | + | + | ||
| C2AS | + | + | + | ||
| Mg1−xFexO | + | + | + | + | + |
| Spinel | + | + | + | + | |
| Chromite | + | + | |||
| C4AF | + | ||||
| CF2 | + | ||||
| CaFeSiO4 | + |
| 3-1b | 3-2b | 4-1b | 4-2b | |
|---|---|---|---|---|
| α-C2S | + | + | + | |
| α′-C2S | + | |||
| β-C2S | + | |||
| C3S | + | + | ||
| C3MS2 | + | + | + | |
| C3A | + | + | + | |
| C12A7 | + | |||
| C2AS | + | + | + | |
| Mg1−xFexO | + | + | + | + |
| Spinel | + | + | + | + |
| Ettringite | + | |||
| Monosulfate | + |
| Phase | C2S | MW | C4AF | C3S | C3MS2 | C3A | C12A7 | C2AS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample No/(wt.%) | 4-1a | 4-1a | 4-2a | 4-2a | 3-1b | 3-1a | 3-1b | 4-2a | 5a | 5a | 5a | 3-1a | 4-2b | 4-2b | 3-1a |
| SiO2 | 35.99 | 39.70 | 35.83 | 33.34 | 36.17 | – | 2.22 | – | 0.75 | 1.98 | 26.57 | 37.18 | – | – | 19.75 |
| Al2O3 | – | – | 1.87 | – | 1.08 | – | 1.46 | – | 0.89 | 22.59 | – | – | 41.18 | 51.97 | 24.59 |
| FeOx | – | 1.27 | – | – | 1.49 | 60.87 | 15.99 | 11.63 | 62.82 | 31.53 | – | 3.04 | – | – | 9.00 |
| MnO | – | – | – | – | 0.68 | 14.02 | 3.39 | 11.24 | 6.44 | – | – | 1.14 | – | – | 2.10 |
| MgO | 1.27 | 1.26 | 0.89 | – | 2.84 | 25.09 | 69.49 | 75.17 | 22.57 | – | – | 10.35 | – | – | 0.90 |
| CaO | 62.74 | 57.77 | 61.40 | 66.66 | 57.74 | – | 3.31 | – | 4.98 | 43.88 | 73.43 | 48.29 | 58.82 | 48.03 | 43.65 |
| CrOx | – | – | – | – | – | – | 4.15 | 1.96 | 1.55 | – | – | – | – | – | – |
| Nr of ions in | 4 O | 1 O | 10 O | 5 O | 8 O | 6 O | 33 O | 7 O | |||||||
| Si | 1.020 | 1.100 | 1.008 | 0.964 | 1.016 | – | 0.016 | – | 0.007 | 0.170 | 1.010 | 2.048 | – | – | 0.987 |
| Al | – | – | 0.064 | – | 0.036 | – | 0.013 | – | 0.010 | 2.230 | – | – | 2.142 | 14.091 | 1.442 |
| Fe | – | 0.028 | – | – | 0.036 | 0.508 | 0.099 | 0.073 | 0.516 | 2.070 | – | 0.144 | – | – | 0.378 |
| Mn | – | – | – | – | 0.016 | 0.119 | 0.021 | 0.071 | 0.054 | – | – | 0.056 | – | – | 0.091 |
| Mg | 0.052 | 0.052 | 0.036 | – | 0.12 | 0.373 | 0.766 | 0.839 | 0.330 | – | – | 0.848 | – | – | 0.070 |
| Ca | 1.904 | 1.716 | 1.852 | 2.068 | 1.740 | – | 0.026 | – | 0.052 | 4.100 | 2.985 | 2.856 | 2.784 | 11.847 | 2.331 |
| Cr | – | – | – | – | – | – | 0.024 | 0.012 | 0.012 | – | – | – | – | – | – |
| Slag | Grinding Time (min) | Compressive Strength (MPa) | |||||
|---|---|---|---|---|---|---|---|
| 1 Day | 2 Days | 7 Days | 28 Days | 60 Days | 90 Days | ||
| – | 75 | 17.3 | 28.3 | 41.6 | 57.9 | 63.1 | 64.4 |
| 3-1a | 82 | 14.5 | 23.8 | 34.3 | 50.0 | 53.6 | 56.0 |
| 3-2a | 111 | 19.5 | 25.2 | 37.1 | 52.5 | 53.8 | 56.5 |
| 4-1a | 81 | 16.6 | 27.1 | 39.9 | 48.2 | 55.0 | 57.9 |
| 4-2a | 106 | 16.7 | 25.7 | 39.6 | 57.0 | 60.1 | 64.2 |
| 5a | 85 | 16.2 | 22.9 | 34.6 | 52.3 | 55.2 | 57.3 |
| 3-1b | 89 | 14.1 | 23.0 | 35.1 | 50.4 | 59.0 | 60.3 |
| 3-2b | 95 | 13.5 | 22.3 | 32.6 | 48.6 | 58.6 | 58.5 |
| 4-1b | 90 | 15.9 | 25.3 | 38.2 | 51.3 | 56.2 | 58.0 |
| 4-2b | 100 | 19.1 | 28.1 | 41.0 | 59.4 | 64.6 | 67.1 |
| Slag | Initial Setting Time (min) | Final Setting Time (min) |
|---|---|---|
| – | 120 | 170 |
| 3-1a | 140 | 190 |
| 3-2a | 120 | 165 |
| 4-1a | 110 | 160 |
| 4-2a | 115 | 145 |
| 5a | 100 | 150 |
| 3-1b | 165 | 230 |
| 3-2b | 145 | 185 |
| 4-1b | 140 | 200 |
| 4-2b | 120 | 155 |
| Slag | Expansion (mm) |
|---|---|
| – | 0.0 |
| 3-1a | 0.5 |
| 3-2a | 0.5 |
| 4-1a | 0.5 |
| 4-2a | 0.5 |
| 5a | 0.5 |
| 3-1b | 0.5 |
| 3-2b | 0.5 |
| 4-1b | 1.5 |
| 4-2b | 1.5 |
| Slag | Grinding Time | Compressive Strength (MPa) | |||
|---|---|---|---|---|---|
| 1 Day | 2 Days | 7 Days | 28 Days | ||
| – | 55 | 15.60 | 23.80 | 37.10 | 53.00 |
| 4-2a | 69 | 16.50 | 24.00 | 36.80 | 53.75 |
| 5a | 60 | 13.80 | 21.50 | 32.00 | 46.30 |
| Slag | Grinding Time (min) | Compressive Strength MPa | |||||
|---|---|---|---|---|---|---|---|
| 1 Day | 2 Days | 7 Days | 28 Days | 60 Days | 90 Days | ||
| – | 75 | 17.3 | 28.3 | 41.6 | 57.9 | 63.1 | 64.4 |
| –* | 95 | 21.0 | 32.7 | 45.7 | 58.7 | 61.4 | 64.5 |
| 3-1a | 102 | 12.0 | 18.5 | 28.2 | 36.7 | 43.0 | 46.2 |
| 3-2a | 95 | 12.2 | 16.8 | 24.4 | 37.5 | 41.2 | 43.4 |
| 4-2a | 90 | 9.3 | 16.5 | 25.5 | 39.7 | 49.0 | 51.1 |
| 5a | 90 | 10.8 | 15.0 | 23.3 | 36.8 | 41.5 | 45.8 |
| 3-1b | 100 | 10.6 | 15.4 | 24.2 | 36.6 | 43.6 | 47.7 |
| 3-2b | 100 | 10.3 | 15.2 | 23.9 | 35.4 | 40.0 | 39.4 |
| 4-1b* | 101 | 11.4 | 18.4 | 28.5 | 38.5 | 42.0 | 45.0 |
| 4-2b | 102 | 11.2 | 17.8 | 29.8 | 49.2 | 58.4 | 65.0 |
| Slag | Initial Setting Time (min) | Final Setting Time (min) |
|---|---|---|
| – | 120 | 170 |
| –* | 135 | 185 |
| 3-1a | 125 | 185 |
| 3-2a | 90 | 170 |
| 4-2a | 75 | 145 |
| 5a | 75 | 125 |
| 3-1b | 75 | 155 |
| 3-2b | 80 | 140 |
| 4-1b* | 90 | 170 |
| 4-2b | 85 | 145 |
| Slag | Expansion (mm) |
|---|---|
| – | 0.0 |
| –* | 0.0 |
| 3-1a | 0.5 |
| 3-2a | 1.3 |
| 4-2a | 2.0 |
| 5a | 0.6 |
| 3-1b | 1.6 |
| 3-2b | 1.0 |
| 4-1b | 2.0 |
| 4-2b | 1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzevelekou, T.; Lampropoulou, P.; Giannakopoulou, P.P.; Rogkala, A.; Koutsovitis, P.; Koukouzas, N.; Petrounias, P. Valorization of Slags Produced by Smelting of Metallurgical Dusts and Lateritic Ore Fines in Manufacturing of Slag Cements. Appl. Sci. 2020, 10, 4670. https://doi.org/10.3390/app10134670
Tzevelekou T, Lampropoulou P, Giannakopoulou PP, Rogkala A, Koutsovitis P, Koukouzas N, Petrounias P. Valorization of Slags Produced by Smelting of Metallurgical Dusts and Lateritic Ore Fines in Manufacturing of Slag Cements. Applied Sciences. 2020; 10(13):4670. https://doi.org/10.3390/app10134670
Chicago/Turabian StyleTzevelekou, Theofani, Paraskevi Lampropoulou, Panagiota P. Giannakopoulou, Aikaterini Rogkala, Petros Koutsovitis, Nikolaos Koukouzas, and Petros Petrounias. 2020. "Valorization of Slags Produced by Smelting of Metallurgical Dusts and Lateritic Ore Fines in Manufacturing of Slag Cements" Applied Sciences 10, no. 13: 4670. https://doi.org/10.3390/app10134670
APA StyleTzevelekou, T., Lampropoulou, P., Giannakopoulou, P. P., Rogkala, A., Koutsovitis, P., Koukouzas, N., & Petrounias, P. (2020). Valorization of Slags Produced by Smelting of Metallurgical Dusts and Lateritic Ore Fines in Manufacturing of Slag Cements. Applied Sciences, 10(13), 4670. https://doi.org/10.3390/app10134670










