Structure, Antimicrobial Activity, Hirshfeld Analysis, and Docking Studies of Three Silver(I) Complexes-Based Pyridine Ligands
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Synthesis
2.3. X-Ray Crystallography
2.4. Computational Details
2.5. Testing of Antimicrobial Activity
2.6. Methodology for Molecular Docking
3. Results and Discussion
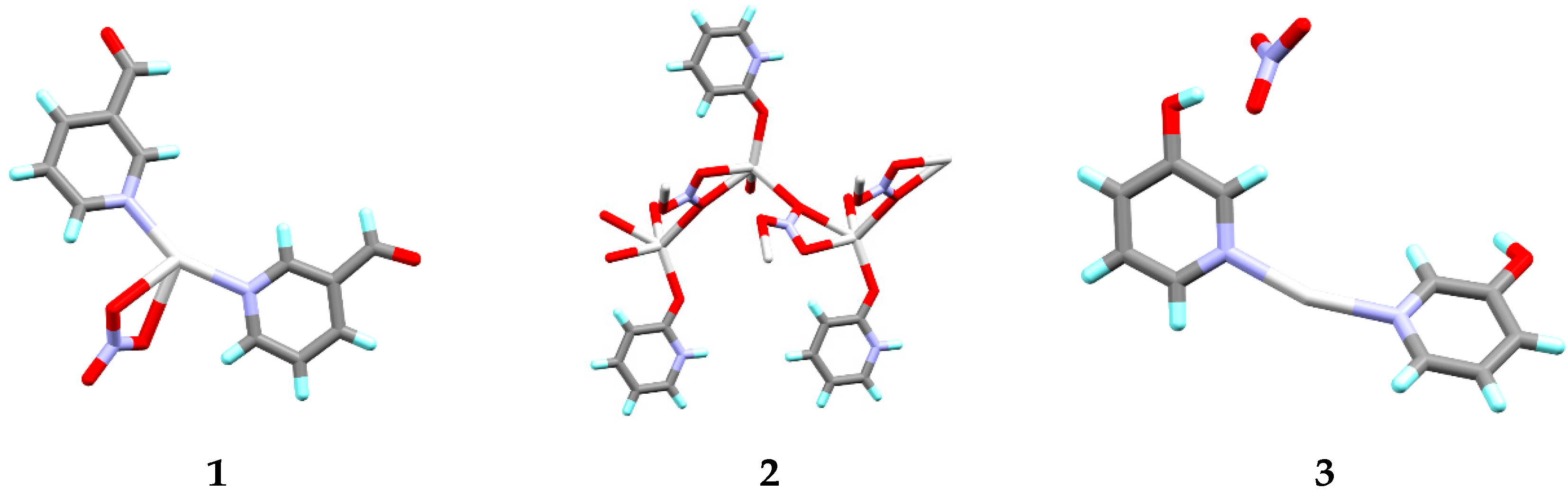
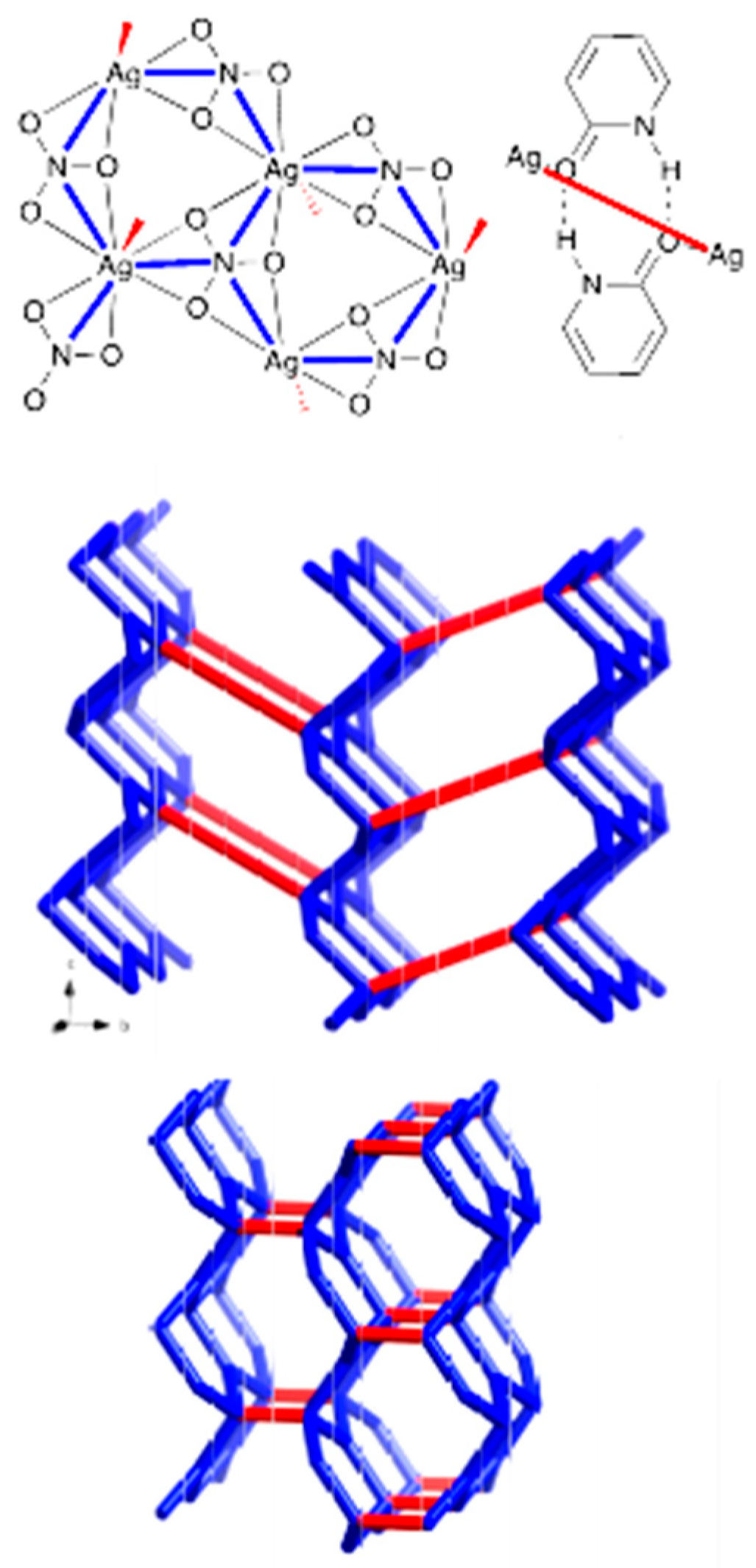
3.1. Crystal Structures
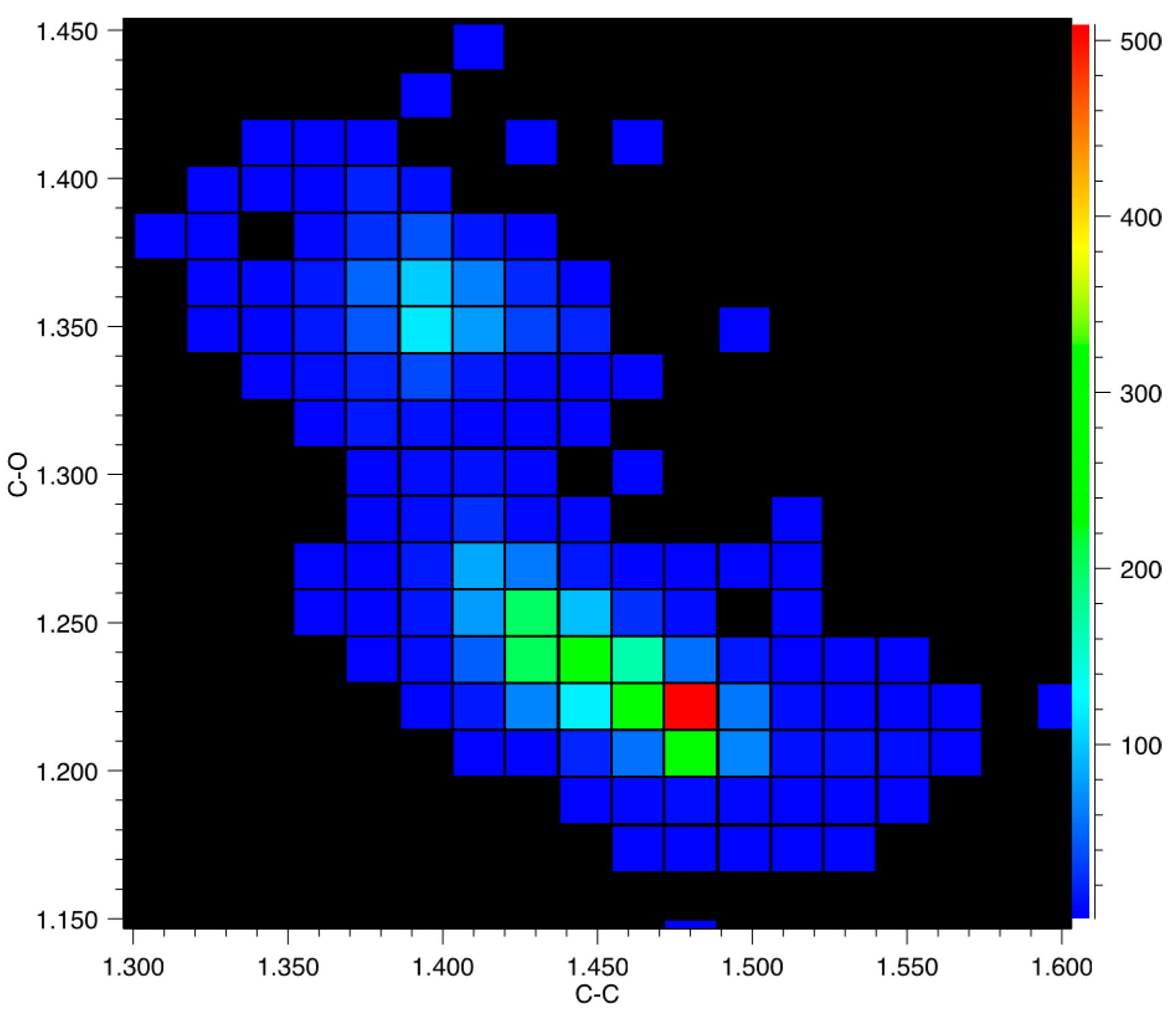
3.2. Tautomerism in 2-Pyridone/2-Hydroxy-Pyridine Systems
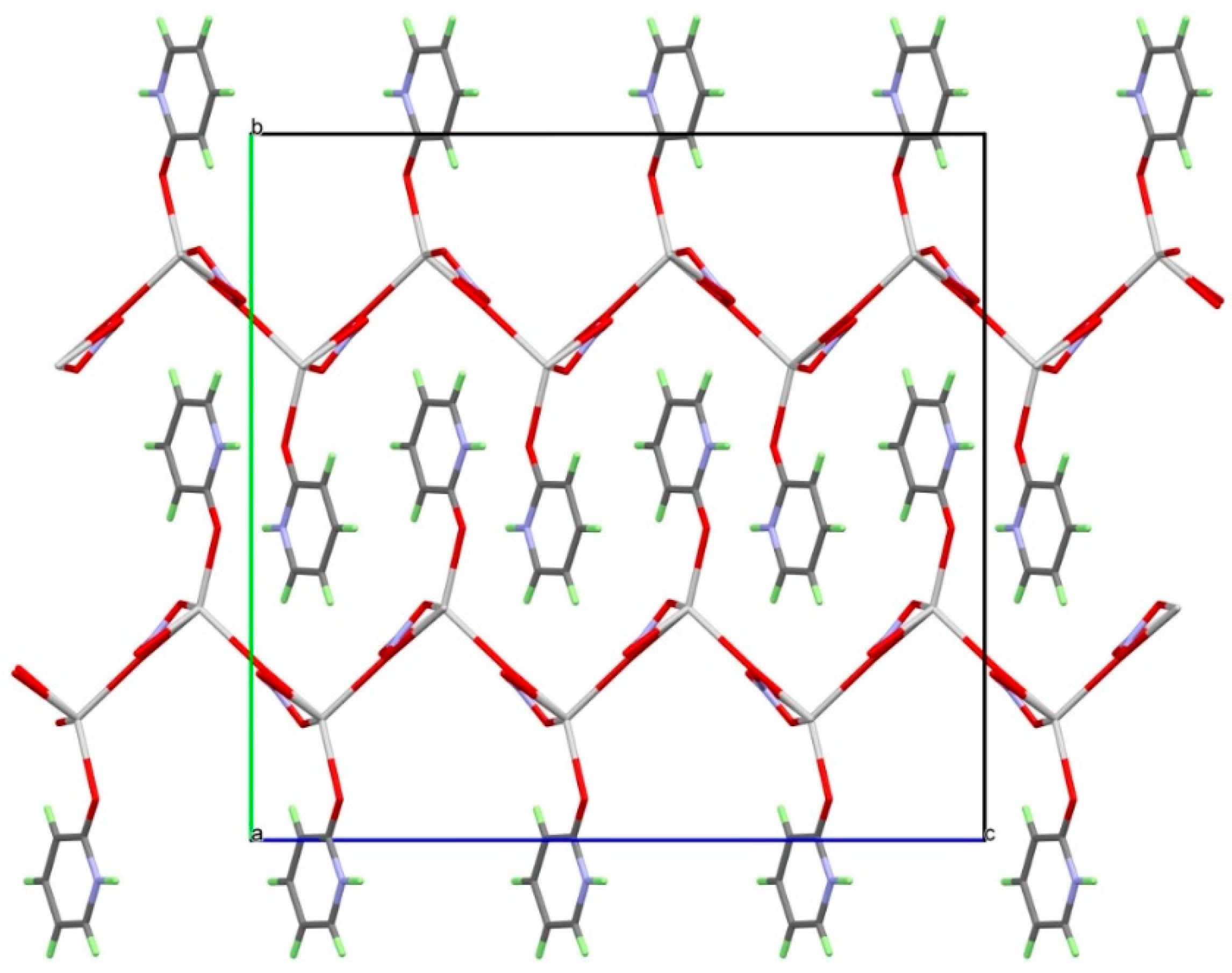
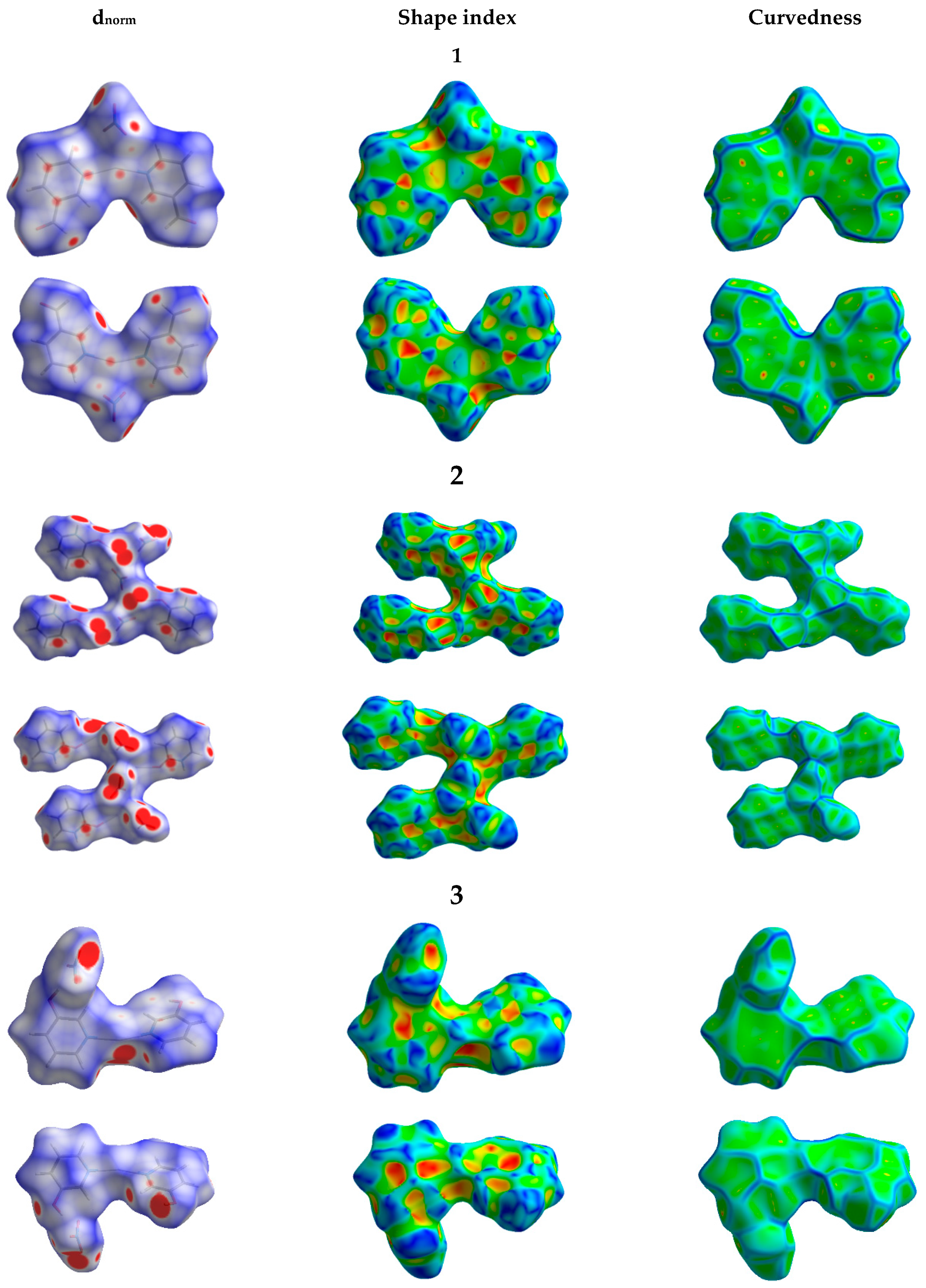
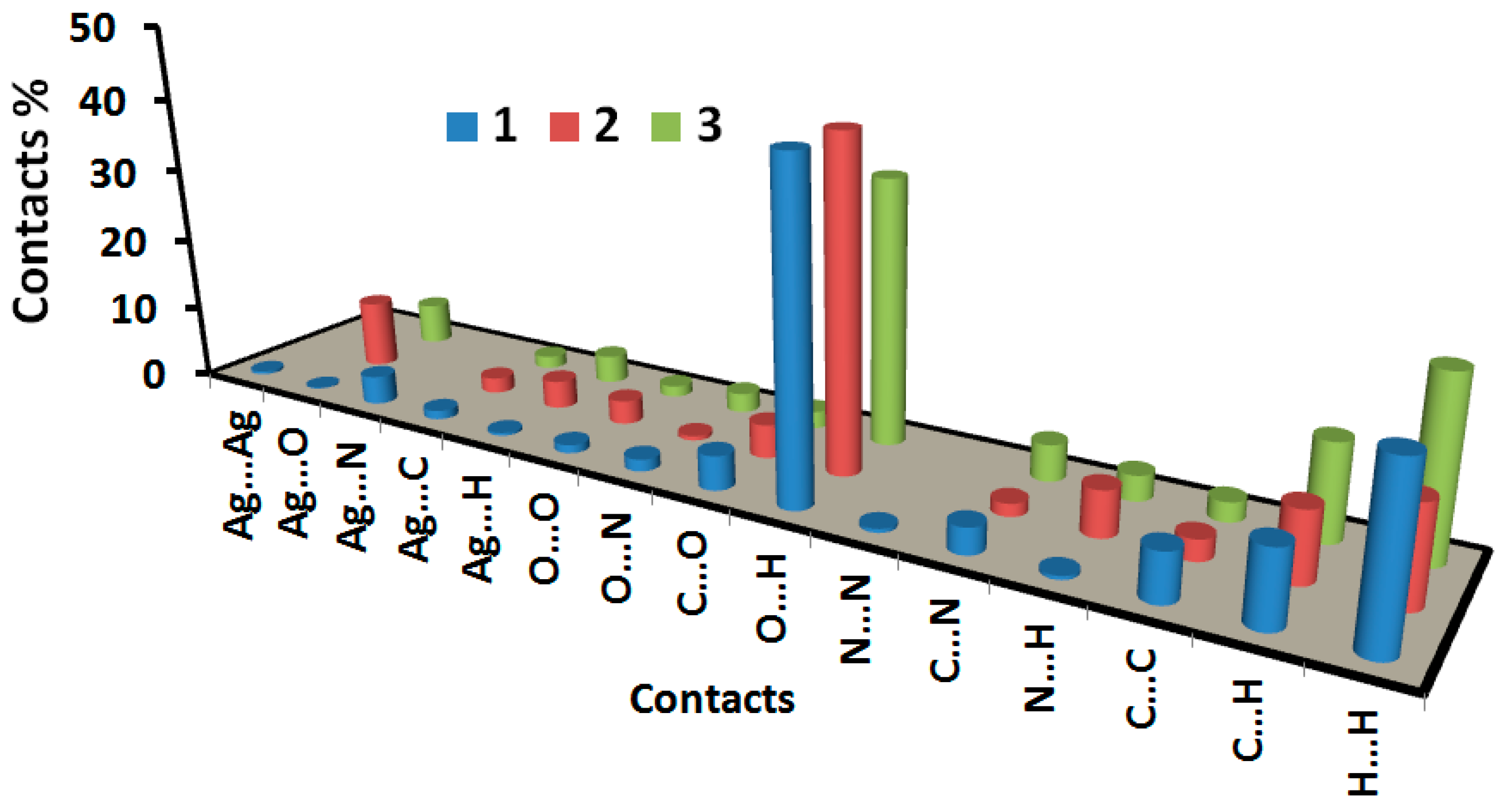
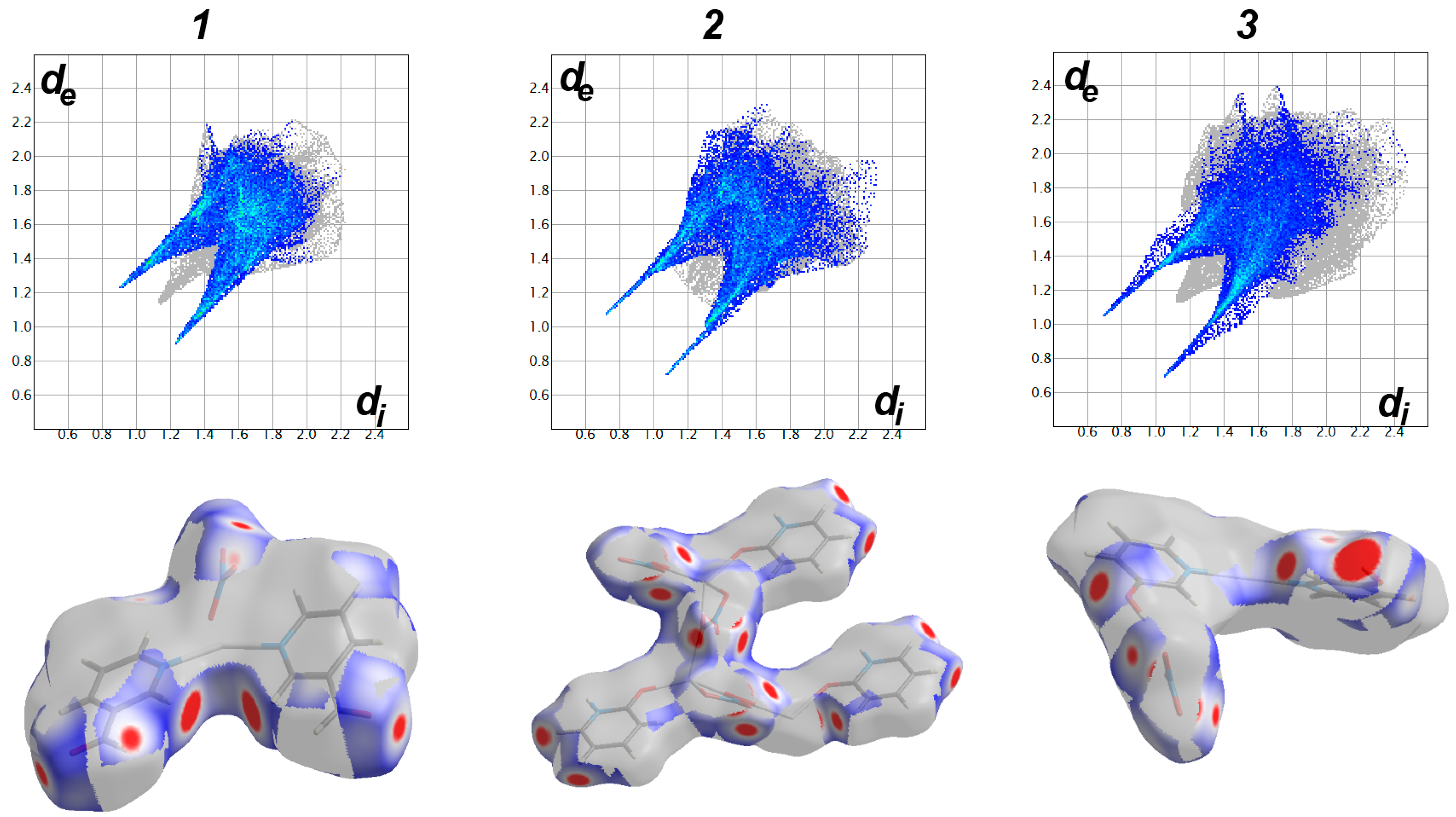
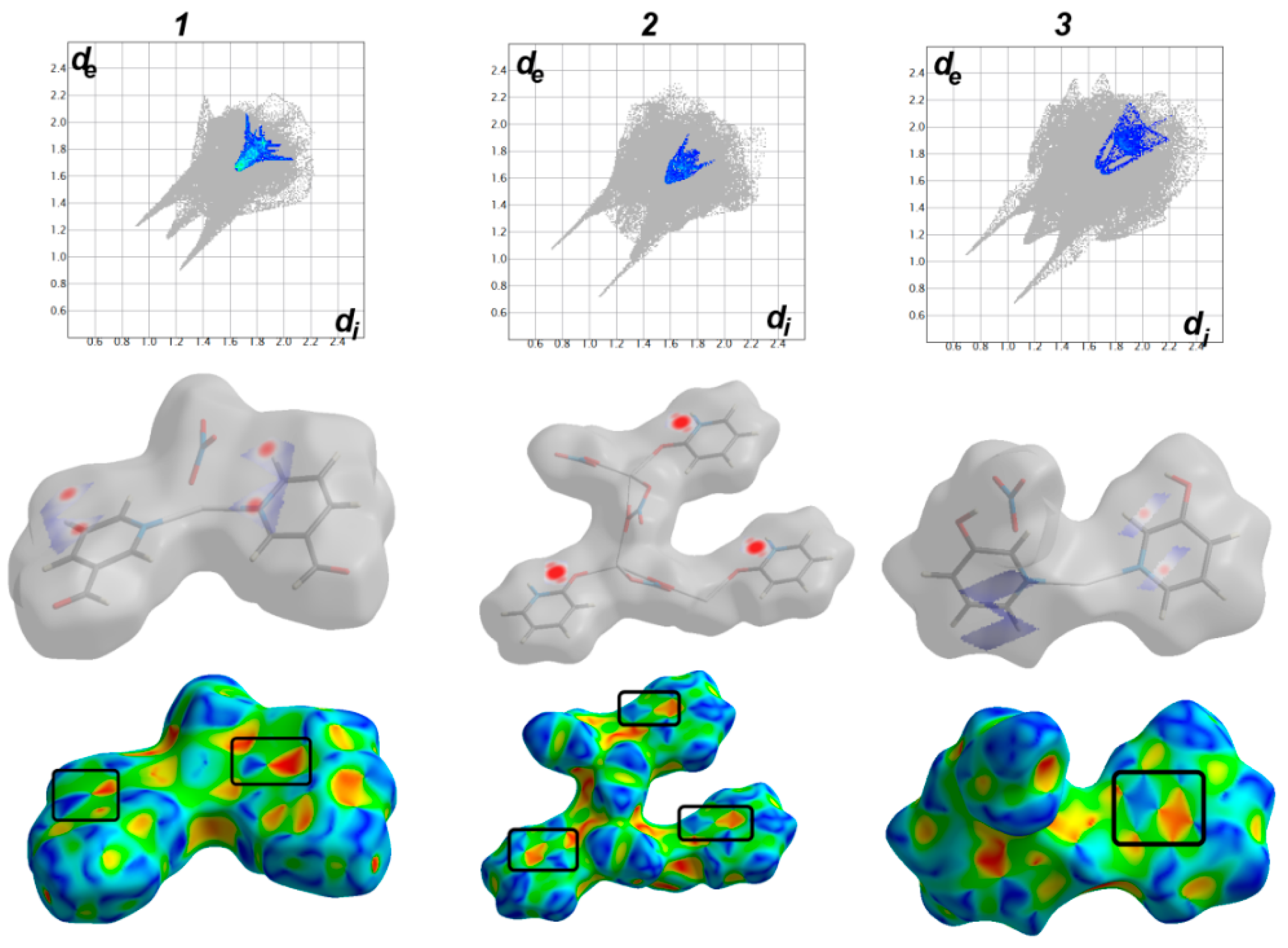
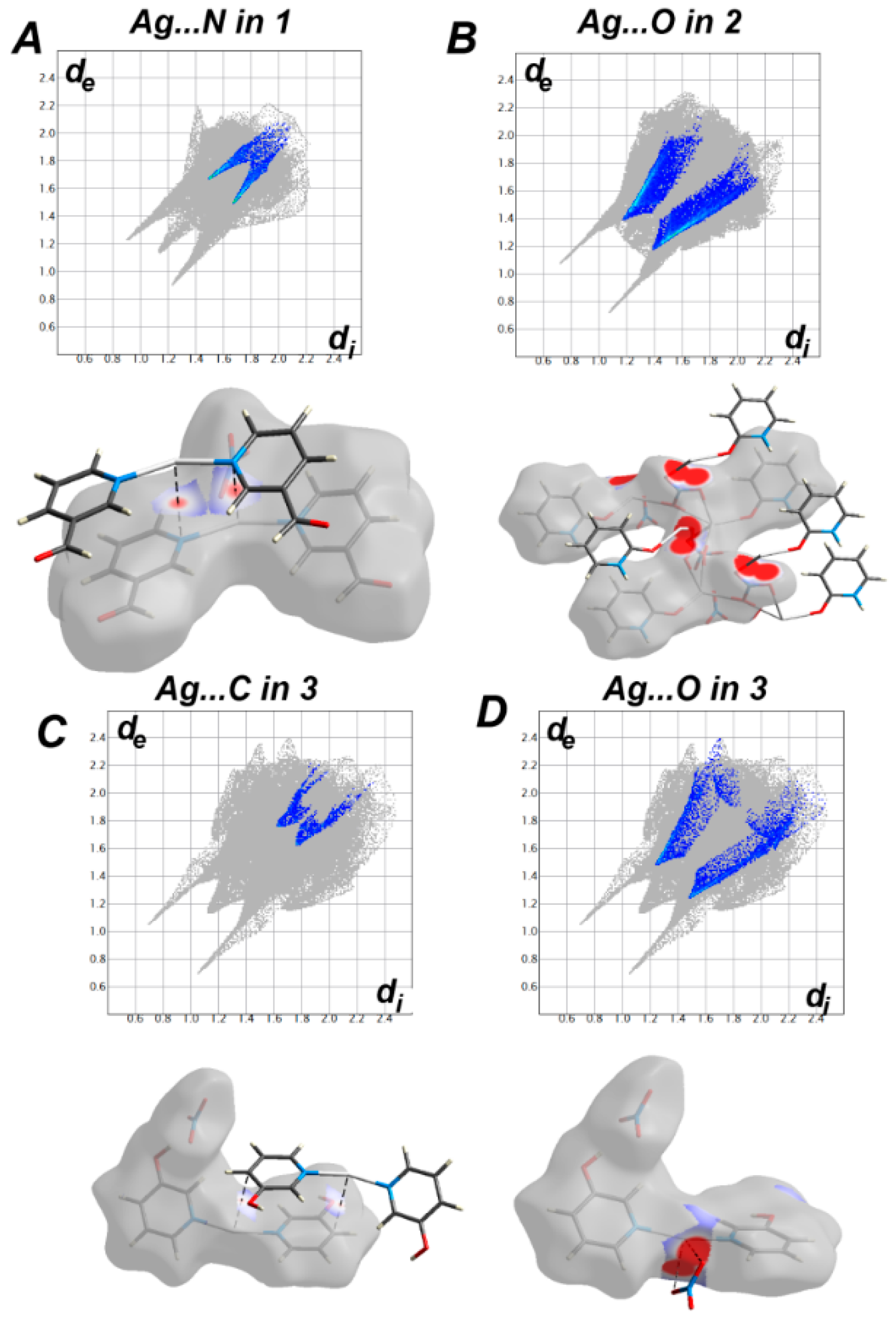
3.3. Analysis of Molecular Packing
3.4. Natural Charge Analysis
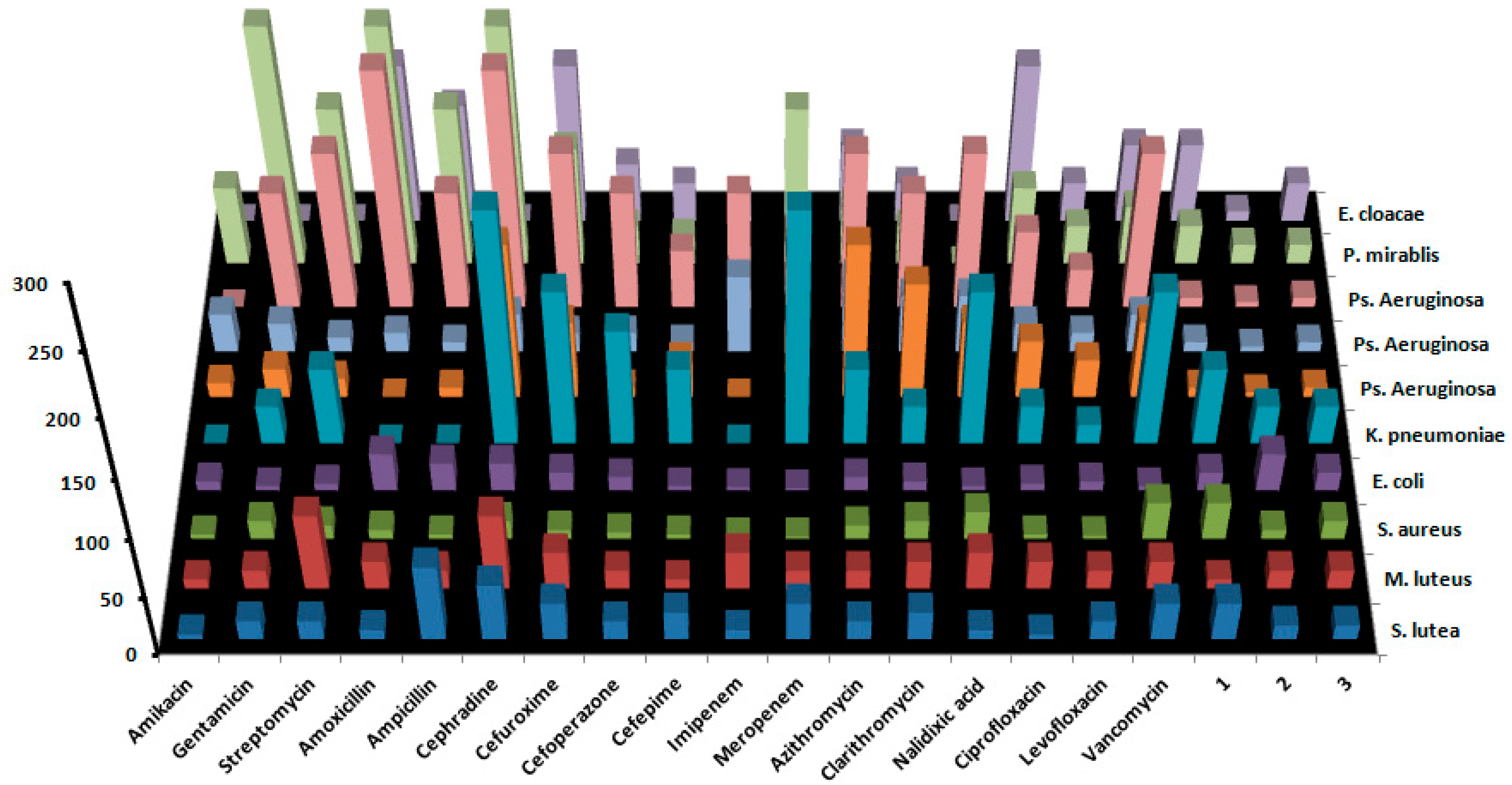
3.5. Antimicrobial Activity
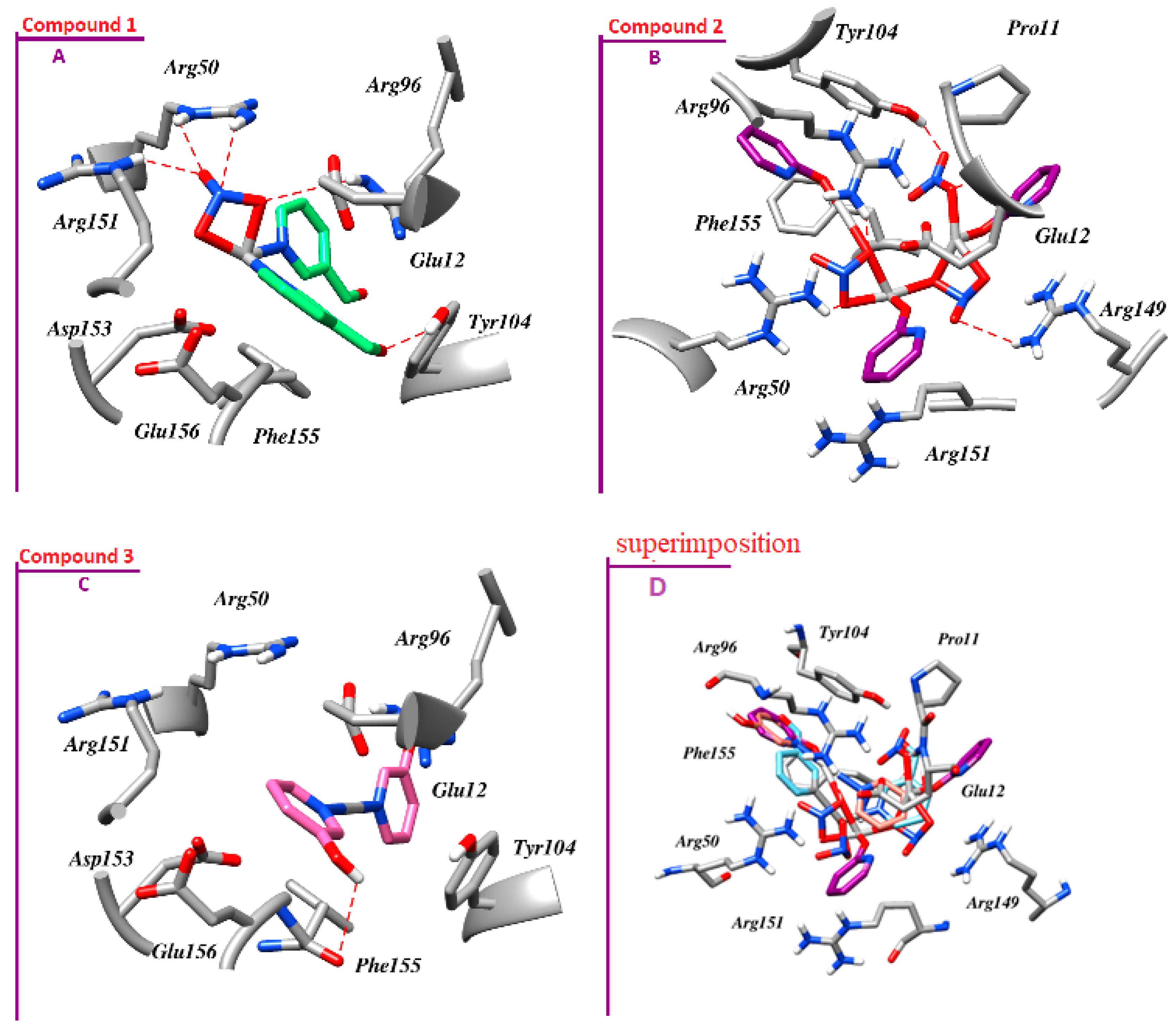
3.6. Molecular Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Savic, N.D.; Milivojevic, D.R.; Glisic, B.D.; Ilic-Tomic, T.; Veselinovic, J.; Pavic, A.; Vasiljevic, B.; Nikodinovic-Runic, J.; Djuran, M.I. A comparative antimicrobial and toxicological study of gold(iii) and silver(i) complexes with aromatic nitrogen-containing heterocycles: Synergistic activity and improved selectivity index of Au(iii)/Ag(i) complexes mixture. Rsc Adv. 2016, 6, 13193–13206. [Google Scholar] [CrossRef][Green Version]
- Marchetti, F.; Palmucci, J.; Pettinari, C.; Pettinari, R.; Scuri, S.; Grappasonni, I.; Cocchioni, M.; Amati, M.; Lelj, F.; Crispini, A. Linkage Isomerism in Silver Acylpyrazolonato Complexes and Correlation with Their Antibacterial Activity. Inorg. Chem. 2016, 55, 5453–5466. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhu, C.F.; Zheng, Z.; He, J.B.; Wang, Y. Synthesis, characterization and antibacterial activity of a biocompatible silver complex based on 2,2′-bipyridine and 5-sulfoisophthalate. Inorg. Chim. Acta 2016, 451, 143–147. [Google Scholar] [CrossRef]
- Glisic, B.D.; Senerovic, L.; Comba, P.; Wadepohl, H.; Veselinovic, A.; Milivojevic, D.R.; Djuran, M.I.; Nikodinovic-Runic, J. Silver(I) complexes with phthalazine and quinazoline as effective agents against pathogenic Pseudomonas aeruginosa strains. J. Inorg. Biochem. 2016, 155, 115–128. [Google Scholar] [CrossRef]
- Fiori, A.T.M.; Nakahata, D.H.; Cuin, A.; Lustri, W.R.; Corbi, P.P. Synthesis, crystallographic studies, high resolution mass spectrometric analyses and antibacterial assays of silver(I) complexes with sulfisoxazole and sulfadimethoxine. Polyhedron 2017, 121, 172–179. [Google Scholar] [CrossRef]
- Cardoso, J.M.S.; Galvao, A.M.; Guerreiro, S.I.; Leitao, J.H.; Suarez, A.C.; Carvalho, M. Antibacterial activity of silver camphorimine coordination polymers. Dalton Trans. 2016, 45, 7114–7123. [Google Scholar] [CrossRef]
- Alisir, S.H.; Dege, N. A new antibacterial silver(I) complex incorporating 2,5-di-methyl-pyrazine and the anti-inflammatory diclofenac. Acta Crystallograp. C 2016, 72, 947–951. [Google Scholar] [CrossRef]
- Adamski, A.; Fik, M.A.; Kubicki, M.; Hnatejko, Z.; Gurda, D.; Fedoruk-Wyszomirska, A.; Wyszko, E.; Kruszka, D.; Dutkiewicz, Z.; Patroniak, V. Full characterization and cytotoxic activity of new silver(i) and copper(i) helicates with quaterpyridine. New J. Chem. 2016, 40, 7943–7957. [Google Scholar] [CrossRef]
- Rigamonti, L.; Cotton, C.; Nava, A.; Lang, H.; Ruffer, T.; Perfetti, M.; Sorace, L.; Barra, A.L.; Lan, Y.H.; Wernsdorfer, W.; et al. Diamondoid Structure in a Metal–Organic Framework of Fe4 Single-Molecule Magnets. Chem. Eur. J. 2016, 22, 13705–13714. [Google Scholar] [CrossRef]
- Jaros, S.W.; da Silva, M.; Krol, J.; Oliveira, M.C.; Smolenski, P.; Pombeiro, A.J.L.; Kirillov, A.M. Bioactive Silver–Organic Networks Assembled from 1,3,5-Triaza-7-phosphaadamantane and Flexible Cyclohexanecarboxylate Blocks. Inorg. Chem. 2016, 55, 1486–1496. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I.; Remelli, M.; Zoroddu, M.A. Silver coordination compounds: A new horizon in medicine. Coord. Chem. Rev. 2016, 327, 349–359. [Google Scholar] [CrossRef]
- Storm-Versloot, M.N.; Vos, C.G.; Ubbink, D.T.; Vermeulen, H. Cochrane Database of Systematic Reviews. 2010. [Google Scholar]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed]
- Farrer, N.J.; Sadler, P.J. Medicinal inorganic chemistry: State of the art, new trends, and a vision of the future. Bioinorg. Med. Chem. 2011, 2011, 1–47. [Google Scholar]
- Dai, T.; Huang, Y.Y.; K Sharma, S.; T Hashmi, J.; B Kurup, D.; R Hamblin, M. Topical antimicrobials for burn wound infections. Recent Pat. Anti Infect. Drug Discov. 2010, 5, 124–151. [Google Scholar] [CrossRef] [PubMed]
- Heyneman, A.; Hoeksema, H.; Vandekerckhove, D.; Pirayesh, A.; Monstrey, S. The role of silver sulphadiazine in the conservative treatment of partial thickness burn wounds: A systematic review. Burns 2016, 42, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Savić, N.D.; Vojnovic, S.; Glišić, B.Đ.; Crochet, A.; Pavic, A.; Janjić, G.V.; Pekmezović, M.; Opsenica, I.M.; Fromm, K.M.; Nikodinovic-Runic, J.; et al. Mononuclear silver (I) complexes with 1, 7-phenanthroline as potent inhibitors of Candida growth. Europ. J. Med. Chem. 2018, 156, 760–773. [Google Scholar] [CrossRef]
- Mujahid, M.; Trendafilova, N.; Arfa-Kia, A.F.; Rosair, G.; Kavanagh, K.; Devereux, M.; Walsh, M.; McClean, S.; Creaven, B.S.; Georgieva, I. Novel silver (I) complexes of coumarin oxyacetate ligands and their phenanthroline adducts: Biological activity, structural and spectroscopic characterisation. J. Inorg. Biochem. 2016, 163, 53–67. [Google Scholar] [CrossRef]
- Soliman, S.M.; Badr, A.M.A. Synthesis, structure and Hirshfeld analysis of the potent antimicrobial [Ag(4-bromopyrazole)2ClO4] complex. Polyhedron 2019, 171, 323–329. [Google Scholar] [CrossRef]
- Andrejević, T.P.; Nikolić, A.M.; Glišić, B.Đ.; Wadepohl, H.; Vojnovic, S.; Zlatović, M.; Petković, M.; Nikodinovic-Runic, J.; Opsenica, I.M.; Djuran, M.I. Synthesis, structural characterization and antimicrobial activity of silver (I) complexes with 1-benzyl-1H-tetrazoles. Polyhedron 2018, 154, 325–333. [Google Scholar]
- Eckhardt, S.; Brunetto, P.S.; Gagnon, J.; Priebe, M.; Giese, B.; Fromm, K.M. Nanobio silver: Its interactions with peptides and bacteria, and its uses in medicine. Chem. Rev. 2013, 113, 4708–4754. [Google Scholar] [CrossRef]
- Russell, A.D.; Hugo, W.B. 7 antimicrobial activity and action of silver. Prog. Med. Chem. 1994, 31, 351–370. [Google Scholar] [PubMed]
- Liau, S.Y.; Read, D.C.; Pugh, W.J.; Furr, J.R.; Russell, A.D. Interaction of silver nitrate with readily identifiable groups: Relationship to the antibacterialaction of silver ions. Letters Appl. Microbiol. 1997, 25, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.M.; Albering, J.H.; Barakat, A. Unexpected formation of polymeric silver (I) complexes of azine-type ligand via self-assembly of Ag-salts with isatin oxamohydrazide. R. Soc. Open Sci. 2018, 5, 180434. [Google Scholar] [CrossRef]
- Abu-Youssef, M.; Soliman, S.; Sharaf, M.; Albering, J.; Öhrström, L. Topology analysis reveals supramolecular organisation of 96 large complex ions into one geometrical object. Cryst. Eng. Comm. 2016, 18, 1883–1886. [Google Scholar] [CrossRef]
- Ivanova, B.; Spiteller, M. AgI and ZnII complexes with possible application as NLO materials—Crystal structures and properties. Polyhedron 2011, 30, 241–245. [Google Scholar] [CrossRef]
- Bowmaker, G.; Effendy, A.; Nitiatmodjo, M.; Skelton, B.W.; White, A.H. Syntheses and structures of some adducts of silver(I) oxyanion salts with some 2-N,O-donor-substituted pyridine bases. Inorg. Chim. Acta 2005, 358, 4327–4341. [Google Scholar] [CrossRef]
- Lu, Z.-Z.; Huo, L.-H.; Gao, S.; Zhao, H.; Zhao, J.-G. Bis(3-hy droxy pyridine-κN)(nitrato-κ2O,O′)silver(I). Acta Cryst. 2005, E61, m1386–m1388. [Google Scholar]
- O’Keeffe, M.; Peskov, M.A.; Ramsden, S.; Yaghi, O.M. The Reticular Chemistry Structure Resource (RCSR) Database of, and Symbols for, Crystal Nets. Acc. Chem. Res. 2008, 41, 1782–1789. [Google Scholar] [CrossRef]
- Öhrström, L. Let’s Talk about MOFs—Topology and Terminology of Metal-Organic Frameworks and Why We Need Them. Crystals 2015, 5, 154. [Google Scholar]
- Ohrstrom, L. Designing, Describing and Disseminating New Materials by using the Network Topology Approach. Chem. Eur. J. 2016, 22, 13758–13763. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. Cryst. Eng. Comm. 2002, 4, 378–392. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J.; Jayatilaka, D. Electrostatic potentials mapped on Hirshfeld surfaces provide direct insight into intermolecular interactions in crystals. Cryst. Eng. Comm. 2008, 10, 377–388. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. Cryst. Eng. Comm. 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acta. 1977, 44, 129–133. [Google Scholar] [CrossRef]
- Martin, A.D.; Hartlieb, K.J.; Sobolev, A.N.; Raston, C.L. Hirshfeld surface analysis of substituted phenols. Cryst. Growth. Des. 2010, 10, 5302–5306. [Google Scholar] [CrossRef]
- SADABS, version 2.10; University of Göttingen: Göttingen, Germany, 2003.
- APEX2; Bruker Nonius BV: Delft, The Netherlands, 2004.
- SHELXTL; Version 6.14; Bruker AXS Inc.: Madison, WI, USA, 2003.
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer17, 2017, University of Western Australia. Available online: http://hirshfeldsurface.net (accessed on 12 June 2017).
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO; Version 3.1; CI, University of Wisconsin: Madison, WI, USA, 1998. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Reversion B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Abu-Youssef, M.A.M.; Soliman, S.M.; Langer, V.; Gohar, Y.M.; Hasanen, A.A.; Makhyoun, M.A.; Zaky, A.H.; Öhrström, L.R. Synthesis, Crystal Structure, Quantum Chemical Calculations, DNA Interactions, and Antimicrobial Activity of [Ag(2-amino-3-methylpyridine)2]NO3 and [Ag(pyridine-2-carboxaldoxime)NO3]. Inorg. Chem. 2010, 49, 9788–9797. [Google Scholar] [CrossRef]
- Sogabe, S.; Masubuchi, M.; Sakata, K.; Fukami, T.A.; Morikami, K.; Shiratori, Y.; Ebiike, H.; Kawasaki, K.; Aoki, Y.; Shimma, N.; et al. Crystal structures of Candida albicans N-myristoyltransferase with two distinct inhibitors. Chem. Biol. 2002, 9, 1119–1128. [Google Scholar] [CrossRef]
- G-Dayanandan, N.; Paulsen, J.L.; Viswanathan, K.; Keshipeddy, S.; Lombardo, M.N.; Zhou, W.; Lamb, K.M.; Sochia, A.E.; Alverson, J.B.; Priestley, N.D.; et al. Propargyl-linked antifolates are dual inhibitors of Candida albicans and Candida glabrata. J.Med. Chem. 2014, 57, 2643–2656. [Google Scholar] [CrossRef]
- Oefner, C.; Parisi, S.; Schulz, H.; Lociuro, S.; Dale, G.E. Inhibitory properties and X-ray crystallographic study of the binding of AR-101, AR-102 and iclaprim in ternary complexes with NADPH and dihydrofolate reductase from Staphylococcus aureus. Acta Cryst. D. 2009, 65, 751–757. [Google Scholar] [CrossRef]
- Lu, J.; Patel, S.; Sharma, N.; Soisson, S.M.; Kishii, R.; Takei, M.; Fukuda, Y.; Lumb, K.J.; Singh, S.B. Structures of kibdelomycin bound to Staphylococcus aureus GyrB and ParE showed a novel U-shaped binding mode. Acs Chem. Biol. 2014, 9, 2023–2031. [Google Scholar] [CrossRef]
- Drew, H.R.; Wing, R.M.; Takano, T.; Broka, C.; Tanaka, S.; Itakura, K.; Dickerson, R.E. Structure of a B-DNA dodecamer: Conformation and dynamics. Proc. Natl. Acad. Sci. USA 1981, 78, 2179–2183. [Google Scholar] [CrossRef]
- Choi, J.Y.; Plummer, M.S.; Starr, J.; Desbonnet, C.R.; Soutter, H.; Chang, J.; Miller, J.R.; Dillman, K.; Miller, A.A.; Roush, W.R. Structure guided development of novel thymidine mimetics targeting Pseudomonas aeruginosa thymidylate kinase: From hit to lead generation. J. Med. Chem. 2012, 55, 852–870. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, Y.; Sinko, W.; Hensler, M.E.; Olson, J.; Molohon, K.J.; Lindert, S.; Cao, R.; Li, K.; Wang, K.; et al. Antibacterial drug leads targeting isoprenoid biosynthesis. Proc. Natl. Acad. Sci. USA 2013, 110, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Molecular Operating Environment (MOE); Chemical Computing Group ULC: Montreal, QC, Canada, 2018.
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Allen, F.H.; Motherwell, W.D.S.; Raithby, P.R.; Shields, G.P.; Taylor, R. Systematic analysis of the probabilities of formation of bimolecular hydrogen-bonded ring motifs in organic crystal structures. New J. Chem. 1999, 23, 25–34. [Google Scholar] [CrossRef]
- Carlucci, L.; Ciani, G.; vonGudenberg, D.W.; Proserpio, D.M.; Sironi, A. Self-assembly of a three-dimensional network from two-dimensional layers via metallic spacers: The (3,4)-connected frame of [Ag3(hmt)2][ClO4]3·2H2O (hmt = hexamethylenetetramine). Chem. Commun. 1997, 6, 631–632. [Google Scholar] [CrossRef]
- Yang, H.W.; Craven, B.M. Charge Density Study of 2-Pyridone. Acta Cryst. B 1998, 54, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, S.A.; Osman, O.I.; Alyoubi, A.O.; Aziz, S.G.; Hilal, R.H. The Thermodynamic and Kinetic Properties of 2-Hydroxypyridine/2-Pyridone Tautomerization: A Theoretical and Computational Revisit. Int. J. Mol. Sci. 2016, 17, 1893. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, N.C.; Yamamoto, R.; Hara, A.; Amano, A.; Nomiya, K. Molecular design, crystal structure, antimicrobial activity and reactivity of light-stable and water-soluble Ag–O bonding silver(I) complexes, dinuclear silver(I) N-acetylglycinate. Inorg. Chim. Acta 2006, 359, 4412–4416. [Google Scholar] [CrossRef]
- Barreiro, E.; Casas, J.S.; Couce, M.D.; Sanchez, A.; Seoane, R.; Sordo, J.; Varela, J.M.; Vazquez-Lopez, E.M. Synthesis and antimicrobial activities of silver(I) 3-(substituted phenyl)sulfanylpropenoates. Europ. J. Med. Chem. 2008, 43, 2489–2497. [Google Scholar] [CrossRef]
- Abu-Youssef, M.A.M.; Dey, R.; Gohar, Y.; Massoud, A.A.; Ohrstrom, L.; Langer, V. Synthesis and Structure of Silver Complexes with Nicotinate-Type Ligands Having Antibacterial Activities against Clinically Isolated Antibiotic Resistant Pathogens. Inorg. Chem. 2007, 46, 5893–5903. [Google Scholar] [CrossRef] [PubMed]

| Metal/Ligand | 1 | 2 | 3 |
|---|---|---|---|
| Ag | 0.6222 | 0.7284 | 0.6852 |
| Py-ligand | 0.1071 | 0.0762 | 0.1395 |
| NO3− | −0.8365 | −0.8046 | −0.9642 |
| Antibiotic | Gram-Positive Bacteria | Gram-Negative Bacteria | Fungi | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (µg/mL) | |||||||||||
| S. lutea1 | M. luteus2 | S. aureus3 | E. coli4 | K. pneumoniae2 | Ps. aeruginosa1 | Ps. aeruginosa2 | Ps. aeruginosa3 | P. mirablis2 | E. cloacae2 | C. albicans2 | |
| Amikacin | 4 | 8 | 4 | 8 | >256 | 12 | 32 | >256 | 64 | >256 | - |
| Gentamicin | 16 | 16 | 16 | 4 | 32 | 24 | 24 | 96 | 192 | >256 | - |
| Streptomycin | 16 | 64 | 12 | 6 | 64 | 16 | 12 | 128 | 128 | >256 | - |
| Amoxicillin | 8 | 24 | 8 | 32 | >256 | >256 | 16 | 192 | 192 | 128 | - |
| Ampicillin | 64 | 16 | 4 | 24 | >256 | 8 | 8 | 96 | 128 | 96 | - |
| Cephradinei | 48 | 64 | 16 | 24 | 192 | 128 | 32 | 192 | 192 | >256 | - |
| Cefuroximeii | 32 | 32 | 8 | 16 | 128 | 64 | 16 | 128 | 96 | 128 | - |
| Cefoperazoneiii | 16 | 16 | 6 | 12 | 96 | 8 | 16 | 96 | 32 | 48 | - |
| Cefepimeiv | 24 | 8 | 4 | 4 | 64 | 32 | 8 | 48 | 24 | 32 | - |
| Imipenem | 8 | 32 | 2 | 3 | >256 | >256 | 64 | 96 | >256 | 16 | - |
| Meropenem | 32 | 16 | 2 | 2 | 192 | 128 | 48 | 64 | 128 | 12 | - |
| Azithromycin | 16 | 16 | 12 | 12 | 64 | 128 | 64 | 128 | 48 | 64 | - |
| Clarithromycin | 24 | 24 | 16 | 8 | 32 | 96 | 48 | 96 | 32 | 32 | - |
| Nalidixic acidi | 8 | 32 | 24 | 4 | 128 | 64 | 48 | 128 | >256 | >256 | - |
| Ciprofloxacinii | 4 | 24 | 4 | 6 | 32 | 48 | 24 | 64 | 64 | 128 | - |
| Levofloxaciniii | 16 | 16 | 3 | 8 | 16 | 32 | 16 | 32 | 32 | 32 | - |
| Vancomycin | 32 | 24 | 32 | 4 | 128 | 64 | 32 | 128 | 48 | 64 | - |
| 1 | 32 | 8 | 32 | 16 | 64 | 8 | 8 | 8 | 32 | 64 | 6 |
| 2 | 12 | 16 | 8 | 32 | 32 | 4 | 4 | 4 | 16 | 8 | 8 |
| 3 | 12 | 16 | 16 | 16 | 32 | 8 | 8 | 8 | 16 | 32 | 32 |
| PDB ID Codes | Docking Scores | ||||
|---|---|---|---|---|---|
| RMSD of Control Ligand | Silver (I) Complexes | ||||
| (Å) | Control Ligand | 1 | 2 | 3 | |
| 1IYL | 1.85 | −11.6752 | −7.224 | −10.1026 | −7.7626 |
| 1BNA | - | - | −5.7922 | −7.2206 | −4.3620 |
| 3FYV | 1.23 | −8.4286 | −6.8618 | −9.6077 | −5.3467 |
| 3UWK | 0.9 | −10.9856 | −8.1865 | −11.4224 | −6.2362 |
| 4HOF | 1.5 | −8.6736 | −5.9142 | −7.8969 | −4.9028 |
| 4URM | 1.9 | −9.4327 | −6.7318 | −8.0604 | −4.881 |
| 4H2M | 2.0 | −10.6792 | −5.7233 | −9.2959 | −4.8587 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badr, A.M.A.; Barakat, A.; Albering, J.H.; Sharaf, M.M.; Ul-Haq, Z.; Soliman, S.M. Structure, Antimicrobial Activity, Hirshfeld Analysis, and Docking Studies of Three Silver(I) Complexes-Based Pyridine Ligands. Appl. Sci. 2020, 10, 4853. https://doi.org/10.3390/app10144853
Badr AMA, Barakat A, Albering JH, Sharaf MM, Ul-Haq Z, Soliman SM. Structure, Antimicrobial Activity, Hirshfeld Analysis, and Docking Studies of Three Silver(I) Complexes-Based Pyridine Ligands. Applied Sciences. 2020; 10(14):4853. https://doi.org/10.3390/app10144853
Chicago/Turabian StyleBadr, Ahmed M. A., Assem Barakat, Jörg H. Albering, Mona M. Sharaf, Zaheer Ul-Haq, and Saied M. Soliman. 2020. "Structure, Antimicrobial Activity, Hirshfeld Analysis, and Docking Studies of Three Silver(I) Complexes-Based Pyridine Ligands" Applied Sciences 10, no. 14: 4853. https://doi.org/10.3390/app10144853
APA StyleBadr, A. M. A., Barakat, A., Albering, J. H., Sharaf, M. M., Ul-Haq, Z., & Soliman, S. M. (2020). Structure, Antimicrobial Activity, Hirshfeld Analysis, and Docking Studies of Three Silver(I) Complexes-Based Pyridine Ligands. Applied Sciences, 10(14), 4853. https://doi.org/10.3390/app10144853









