Abstract
Cellular communication has a fundamental role in both human physiological and pathological states and various mechanisms are involved in the crosstalk between organs. Among these, microparticles (MPs) have an important involvement. MPs are a subtype of extracellular vesicles produced by a variety of cells following activation or apoptosis. They are normally present in physiological conditions, but their concentration varies in pathological states such as cardiovascular disease, diabetes mellitus, or cancer. Acute and chronic physical exercise are able to modify MPs amounts as well. Among various actions, exercise-responsive MPs affect angiogenesis, the process through which new blood vessels grow from pre-existing vessels. Usually, the neo vascular growth has functional role; but an aberrant neovascularization accompanies several oncogenic, ischemic, or inflammatory diseases. In addition, angiogenesis is one of the key adaptations to physical exercise and training. In the present review, we report evidence regarding the effect of various typologies of exercise on circulating MPs that are able to affect angiogenesis.
1. Introduction
The capacity to communicate between cells, tissues, and organs is a fundamental requisite to ensure appropriate physiological functions [1]. One of the most important mechanisms governed by cell communication and signaling is represented by the formation of vasculature. It is well known that vascular formation and growth are processes strictly regulated by growth factors and bioactive molecules. Hypoxia represents an important stimulus for angiogenesis: it activates hypoxia-inducible factors (HIFs) that upregulate various angiogenic genes, including vascular endothelial growth factor (VEGF) [2]. On the other hand, other different factors (e.g., thrombospondin-1, statins) are involved in the inhibition of angiogenesis [3].
Neovascular growth can be a physiological (e.g., in injured tissues [4]) or a pathological process, as it occurs in diabetic retinopathy, maculopathy, in tumors, and in many other disorders. Physiological angiogenesis represents the results of an accurate balance between pro-angiogenic and anti-angiogenic signals [5]. In pathological conditions, this balance is compromised and the new vessels do not provide a functional vascular perfusion [6].
Small extracellular vesicles, named microparticles (MPs), can be involved in angiogenic processes [7]. MPs are defined as heterogeneous small membranous vesicular structures derived from different cell types [8]. They are lacking nucleus and ranging in size from 0.1 to 1 μm. The most abundant population of MPs derives from platelets, but also other different cell types release these vesicles into the circulation such as endothelial cells [9], leucocytes [10] erythrocytes [11], epithelial cells [12], muscle cells, and various tumor cell lines [12]. Therefore, MPs express surface protein and antigens deriving by their parental cell as well as cytoplasmic and nuclear content (e.g., proteins, mRNAs, microRNAs, small-interfering RNAs, long non-coding RNAs), that are signature of their cellular origin [13]. MPs are released in response to cellular activation or apoptosis, elicited by a variety of stimuli, such as inflammation, oxidative stress, or mechanical/hemodynamic fluctuations depending on the involved parental cell [14].
Physical exercise can induce modifications in MP concentration [15]. In fact, MP concentration is modified by stimuli such hypoxia and shear stress [16], conditions elicited to a different extent by physical exercise. It has been demonstrated that MPs produced in response to physical exercise have a stimulating effect on new vessels growth [17].
The purpose of this review is to summarize the effects of different patterns of physical exercise on the concentration of circulating MPs with a known angiogenic potential.
2. Vasculogenesis, Arteriogenesis, and Angiogenesis
Vessels can be formed mainly by three processes: vasculogenesis, arteriogenesis, and angiogenesis. Vasculogenesis is the formation of new vessels that occurs in the embryo as well in the fetal annexes involved in the transportation of maternal nutrients: the placental villi and the yolk sac [18]; it derives from the differentiation of mesodermal cells into angioblasts, that proliferate, migrate, and couple to produce a primitive tube-like vessel. Consequently, vessels continue to develop as angioblasts differentiate in endothelial cells, form a vascular lumen, and deposit the basal lamina [19]. Various growth factors are involved in the molecular signaling of vasculogenesis: the fibroblast growth factor (FGF)-2, involved in mesodermal induction as well as in the induction of the angioblasts from the mesoderm [20], VEGFs family, that plays a pivotal role for its stimulating effects, [21], and finally the transforming growth factor-β family (TGF-βs) that contributes to vasculogenesis, with a dose dependent effect (low doses stimulate, while high doses inhibit endothelial cell growth [22]).
Arteriogenesis is the process whereby small pre-existing arterioles are transformed in functional arteries. One of the major stimuli inducing arteriogenesis is represented by shear stress, a mechanical signal able to activate transcription factors such as early growth response protein-1 (erg-1), that in turn lead to gene expression of chemokines like monocyte chemoattractant protein-1 (MCP-1), and adhesion molecules like intercellular adhesion molecule-1 (ICAM-1). These molecules are important for the docking of monocytes on endothelial cells, a fundamental step in arteriogenesis. Moreover, also growth factors such as VEGF, FGF, and TGF-β are involved in this process [23,24].
Lastly, angiogenesis is the process in which new blood vessels rising from pre-existing vessels, and it can occur mainly through mechanisms such as sprouting and intussusception (also known as vessel splitting or non-sprouting angiogenesis) [25]. Angiogenesis is a fundamental process during embryonic, fetal, and adult life and plays a pivotal role in many physiological process (e.g., as in skeletal growth, in wound healing, in pregnancy); however, angiogenesis represents also an important hallmark of pathological processes such as cancer and several noncommunicable diseases. Angiogenesis is governed by a precise balance between angiogenic growth factors and inhibitors. VEGF-A and its tyrosine kinase receptors represent the main and best characterized signaling pathway involved in developmental angiogenesis [26]. However, other molecules play a key role in endothelial cell proliferation and migration. They are represented by FGFs, platelet-derived growth factor (PDGF), and epidermal growth factor (EGF) [27]. Therefore, angiogenesis is a complex process controlled by several molecules and can have useful as well as detrimental effects on human health, and for this reason the various underlying mechanisms are object of great interest.
3. Extracellular Vesicles Classification
Human cells are able to release various types of membrane vesicles in response to different stimuli, generally called extracellular vesicles (EVs). EVs include exosomes, MPs (also called microvesicles), and apoptotic bodies [28] (Table 1).

Table 1.
General characteristics of extracellular vesicles (EVs).
Exosomes are vesicles surrounded by a double layer of phospholipids and their size ranges between 50 and 100 nm in diameter. They are released in both physiological condition and upon activation by exocytosis of multivesicular bodies (MVBs) [29]. Exosomes can communicate with other cells either through a direct contact between surface proteins or by vesicles–cell membrane fusion or by endocytosis. They expose phosphatidylserine (PS) on the external membrane and various markers such as LAMP1, TSG101, CD63, CD81, and CD9 [30]. The main functions of exosomes include antigen presentation and immunostimulatory activity. In addition, exosomes are able to horizontally transfer mRNA and miRNA as well as oncogenic receptors [31].
MPs, formerly described as “platelet dust”, are now well known cell–cell communicators and they act as signaling molecules in physiological homeostatic processes or as a consequence of pathological condition including diabetes, chronic kidney disease, rheumatoid arthritis, multiple sclerosis, and cardiovascular diseases [32]. MPs are found in most body fluids such as plasma, saliva, urine, and cerebrospinal fluid. They represent a heterogeneous population of vesicular-like structures composed by a phospholipid bilayer and their sizes range from 100 nm to 1 µm in diameter; however precise cut-off values remain to be established. MPs found in bloodstream may originate from various vascular cells (e.g., platelets, monocytes, erythrocytes, granulocytes) [33]. However, among all circulating MPs, platelet-derived MPs (Plt-MPs) are the most abundant population, representing 70–90% of the total [34]. They are produced by budding of the plasma membrane, in a process called ectocytosis. After their release, MPs carry proteins that are signature of their cell of origin, and transport various enzymes, RNA, and miRNA [35]. MPs generally expose PS on their outer leaflet (Annexin V+), however some evidence shows that MPs can also be PS negative [36]. In addition, a subpopulation of various phenotypes of MPs expose tissue factor [37]. The release of MPs is induced upon cell activation or apoptosis.
Apoptotic bodies are EVs larger than MPs and exosomes. Their sizes range from 500 nm to 3 µm and they expose PS; however, their outer membrane differs from MPs due to its permeability [38]. Apoptotic bodies are released by membrane blebbing of cells undergoing apoptosis and they may horizontally transfer their content, such as cell organelles, fragmented DNA, and oncogenes.
4. Methods of Detection of Microparticles
Flow cytometry (FC) is one of the most common methods of detection, quantification, and size evaluation of MPs [39]. In addition, FC allows the phenotypic characterization of MPs using fluorescent antibodies against the diverse antigens expressed by the cell of origin [40]. However, FC has some limitations. For example, MPs with a size ranging from 0.1 to 0.4 µm are too undetected by most cytometers [41]. In addition, the type of instrument, settings, and sample preparation procedures can cause a high level of variability of the results [42].
The nanoparticle tracking analysis (NTA), on the other hand, is able to detect vesicles smaller than those recognized by FC. Such type of analysis correlates the speed of movements, tracked using a microscope, with the size of MPs [43]. Nevertheless, NTA is unable to discriminate vesicles derived from cells from other particles of small dimensions such as high-density lipoprotein (HDL) [44]. Fluorescent nanoparticle tracking analysis (F-NTA), diversely from NTA, is based on the fluorescence of the particles [45].
Other techniques to analyze the EVs are based on image analyses obtained by optical and electron microscopy. Optical microscopy permits to assess the size and morphological features of MPs and of apoptotic bodies, while cannot provide information on their phenotype. This kind of information can, anyway, be obtained by fluorescent microscopy labeling MPs with fluorescent probes that recognize specific surface markers [41]. However, this approach does not permit to specify the size of the EVs [41]. An accurate morphological analysis of MPs is obtained by transmission electron microscopy (TEM), that represents the most used method to assess the composition, morphology, size, and membrane structure of MPs [34]. However, TEM requires a considerable amount of time for both preparation and analysis of the sample.
Western blotting (WB) is a commonly used technique that allows to identify the cellular origin of MPs [46]; anyway, this methodological approach presents some important limitations: indeed, it requires a large amount of MPs to be performed and the analysis needs to be completed with complementary methods such as NTA or TEM [47,48].
Finally, another method for MPs detection is the enzyme-linked immunosorbent assay (ELISA). This is also a quantitative analysis, that measures MPs indirectly: indeed, such method relies on the binding of MPs to specific conjugate-antibodies adsorbed to a well plate [49]. ELISA permits the specification of MPs subtypes, but fresh plasma is indispensable, because the freezing-thawing process leads to an increase of annexin-binding MPs [50].
However, is important to note that most of the studies analyzing MPs in exercise settings utilize the flow cytometric approach [17,51,52,53,54,55,56].
5. Production Mechanisms of MPs
It is well known that MPs can be released upon activation or apoptotic stimuli; however, most cells constitutively shed MPs by ectocytosis and significant concentrations of these vesicles can be detected in the plasma [57]. The common mechanism for the production of MPs is represented by a change in the asymmetry of the phospholipids composing the plasma membrane. The lipid bilayer of normal cells presents an asymmetrical composition of phospholipids [58]. PS and phosphatidylethanolamine (PE) are located in the internal layer of the plasma membrane whereas the external layer is composed of phosphatidylinositol (PI), phosphatidylcoline (PC), sphingomyelin (SM), and other glycolipids. In order to allow MPs formation, this precise distribution is disrupted. Such changes in membrane architecture are controlled by three enzymes: flippase, floppase, and scramblase [59]. Flippase is involved in the maintenance of the phospholipid asymmetry between the membrane layers. Floppase controls the ATP-dependent translocation of PS to the external layer in response to cellular activation. Finally, scramblase is responsible for the membrane randomization allowing phospholipids to flow down their concentration gradients. In resting cells, only flippase is active, contributing to the internalization of negatively charged phospholipids (e.g., PS and PE) and to the maintenance of the physiological membrane asymmetry. Nevertheless, various events providing cellular activation are able to increase intracellular calcium concentration, causing the flippase inhibition and the activation of floppase, scramblase, and other calcium-sensitive enzymes such as calpain and gelsolin, resulting in MPs release [60].
An important mediator of apoptotic MPs production is Rho-associated kinase (ROCK I), a protein kinase activated via caspase-mediated cleavage. ROCK I may allow the phosphorylation of myosin light-chains, triggering cytoskeletal rearrangement and formation of apoptotic MPs through the coupling of actin-myosin filaments to the plasma membrane [61].
6. MPs and Angiogenesis
Vesicle-like structures have recently emerged as important mediators for cellular communication in both physiological and pathological processes [30]; in particular, MPs released by various cell types may play an important role in angiogenesiss [62,63], by modulating the production of proangiogenic factors through changes in the secretome of endothelial cells [64,65]. Like exosomes, MPs can communicate with other cells through ligands on their membrane that interact with specific receptors of the target cells; alternatively, MPs can fuse with the plasma membrane of the target cells, delivering its cytoplasmic content (e.g., proteins, miRNAs, RNAs). In this way, MPs may elicit the synthesis of proangiogenic or antiangiogenic factors and affect the main processes involved in formation of new vessels, such as endothelial cells adhesion, migration, and proliferation [66].
Endothelial cells-derived MPs (E-MPs) are clearly involved in the formation of blood vessels. A pioneer in vitro study highlighted that vesicles released by endothelial cells can transport integrin-β1 and active matrix metalloproteinases-2 and 9 (MMP-2 and MMP-9), thus inducing endothelial cells invasion and tube-like structure [67]. In addition, it has been demonstrated that upon stimulation with the proinflammatory interleukin-3, endothelial cells release vesicles containing pro-angiogenic factors such as miR-126-3p and Stat5 [68]. Moreover, another study shows that endothelial MPs are able to transfer miR-126 from endothelial cells to vascular smooth muscle cells, corroborating the angiogenic properties of endothelial MPs [69].
Additionally, Plt-MPs are involved in angiogenesis. These vesicles can promote angiogenic effects by transferring various cytokines, such as PDGF, VEGF, FGF-2, and activating key proteins such as PI3k, ERK, and src kinase [70]. Various studies found that the angiogenic potential of the platelet-derived MPs can have both functional positive and negative effects: when Plt-MPs were infused in a rat model of myocardial ischemia, indeed, they increased the quantity of functional capillaries [70]; on the other hand, MPs shed by platelets can favor angiogenesis involved in tumor progression, upregulating important angiogenic factors, such as VEGF, HGF, and interleukin-8 [71].
7. Angiogenesis Induced by Physical Exercise
Physical activity has beneficial effects on human health [72,73,74,75], and regular physical exercise improves health in both physiological and pathological conditions [76,77,78,79]. Numerous evidence demonstrates the inverse relationship between regular exercise and cardiovascular diseases, hypertension, stroke, type 2 diabetes mellitus, metabolic syndrome, obesity, osteoporosis, and various types of cancer [76,80,81,82,83]. Depending on the type of physical exercise, we can observe different effects on the organism: endurance (or aerobic) exercise is characterized by a high number of muscular contractions, relative low load, and an important involvement of the cardiorespiratory system, whereas resistance exercise is represented by low number of muscular contraction but with relative higher loads and thus it relies mainly on the neuromuscular system [84]. These two types of exercise can be considered two extremes in a line, and between these two, numerous types of exercise exist, based on the modulation of the exercise variables (e.g., intensity, volume, density, rest). Each type of exercise can increase different responses and adaptions, triggering diverse cellular pathways.
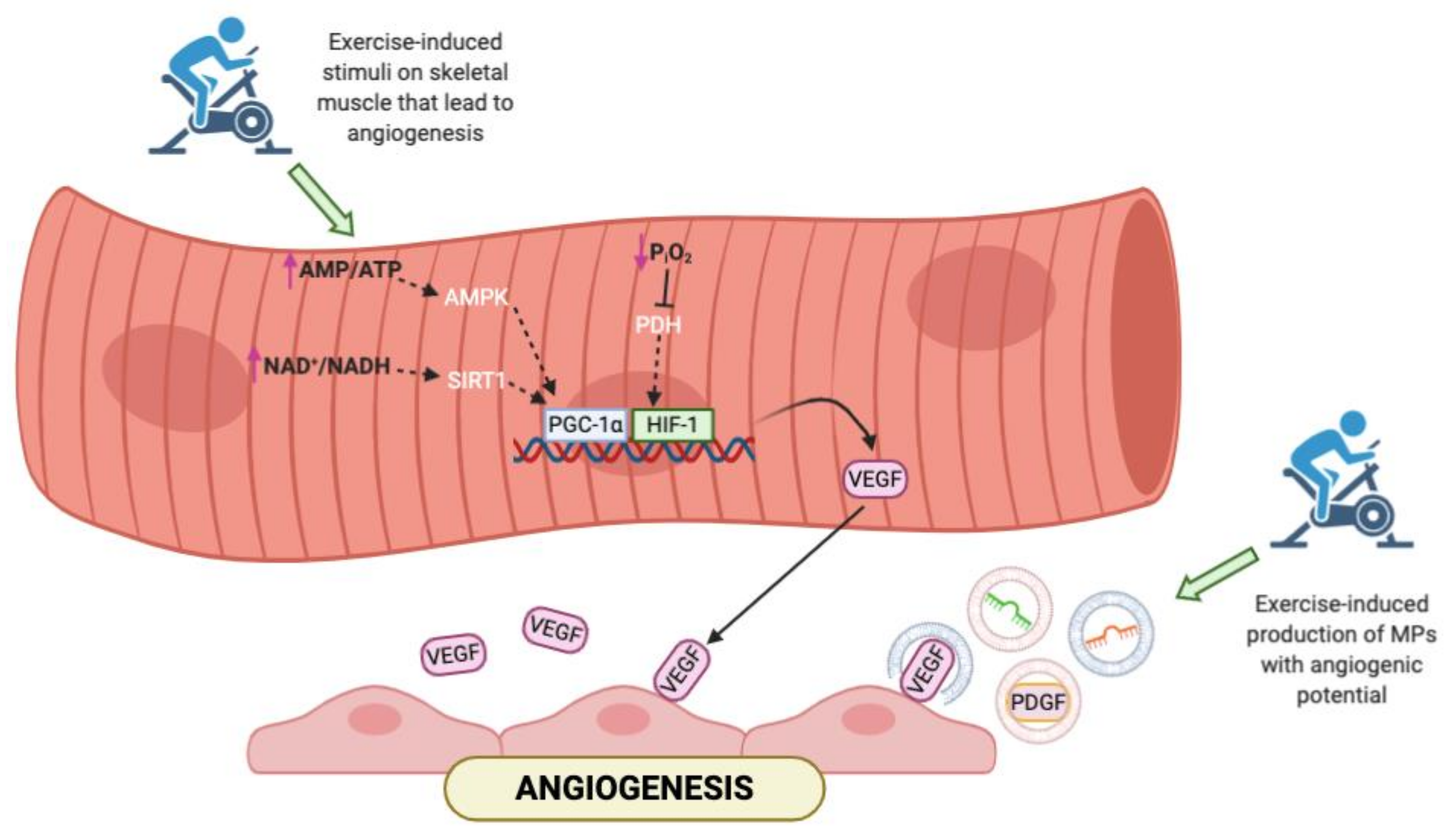
Cardiovascular endurance training is the strongest stimulus for exercise-induced angiogenesis; this results in an improved capillary to fiber ratio in the skeletal muscle to provide an enhanced blood flow and oxygen delivery to the exercising muscles [85,86,87,88,89]. Endurance exercise stimulates the angiogenesis activating several pathways (Figure 1). It induces an important reduction of the partial pressure of oxygen that leads to a local tissue hypoxia. This modification in oxygen levels decreases the hydroxylation of HIF-1alpha trough the inhibition of prolyl hydroxylase domain enzymes (PHD); in this way, HIF-1alpha become more stable and translocate into the nucleus, where forms an active complex with HIF-1beta [90] and activates genes involved in angiogenesis such as VEGF [84]. Another key regulator of VEGF synthesis is the peroxisome proliferator-activated receptor gamma co-activator 1-alpha (PGC-1alpha), which is modulated by aerobic exercise in several ways: intense endurance exercise produces an energy deficit and an increase of the AMP/ATP ratio that activate the AMP-activated protein kinase (AMPK) responsible for the PGC-1alpha phosphorylation. Moreover, an acute bout of exercise increases the NAD+/NADH ratio that promotes the sirtuin (specifically SIRT1) activity and PGC-1alpha deacetylation [91]. Finally, aerobic exercise affects also the estrogen-related receptor gamma (ERR gamma) that controls the skeletal muscle vascularization by modifying the VEGF expression [92].
Figure 1.
Representation of the main pathways activated in skeletal muscle in response to exercise. In addition to the canonical molecular pathways, aerobic exercise may affect the release of microparticles (MPs) that carry soluble factors involved in angiogenesis. AMP = adenosine monophosphate; ATP = adenosine triphosphate; NAD = nicotinamide adenine dinucleotide; NADH = reduced nicotinamide adenine dinucleotide; PGC-1α = peroxisome proliferator-activated receptor gamma coactivator 1-alpha; HIF = hypoxia-inducible factor; VEGF = vascular endothelial growth factor; PDGF = platelet-derived growth factor.
In the last years it was evidenced that physical exercise induces modification of the MPs release into the bloodstream [93]. These EVs carry various molecules important for the tissues’ crosstalk during and post exercise [94,95] and can be also a cargo of soluble factors involved in endothelial cell proliferation, migration, and adhesion. This results in the generation of new vessels in response to exercise.
8. MPs and Physical Exercise
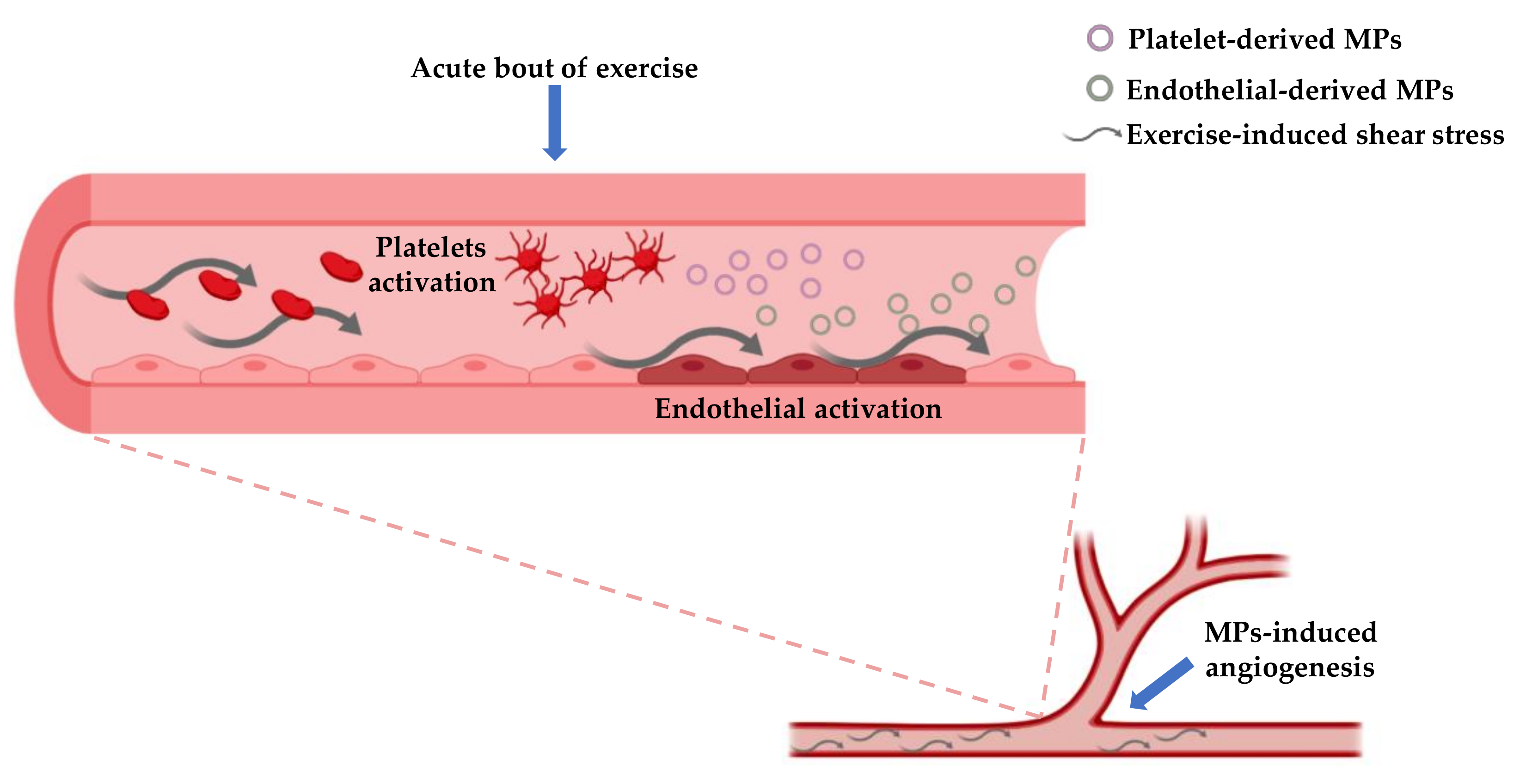
Physical exercise, particularly endurance exercise, is known to stimulate shear stress due to its hemodynamic effects [96]. Intraluminal shear stress has a beneficial effect on vasculature because it is able to ameliorate vascular dilation through mechanisms mediated by nitric oxide [97]. In addition, exercise-induced shear stress can modulate the production of both E-MPs and Plt-MPs. For example, it was seen that high shear stress can reduce the concentration of E-MP while low shear stress is prone to increase such concentration [98]. Moreover, both high and low shear stress can elicit the production of MPs from platelets [99] (Figure 2).
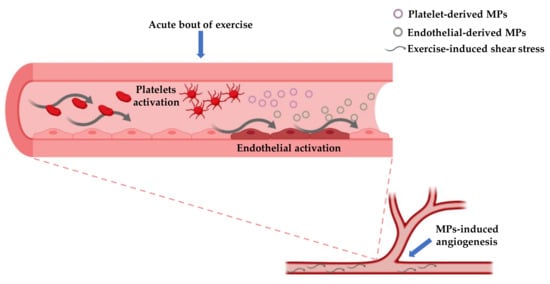
Figure 2.
This figure shows the possible mechanisms involved in the production of platelet-derived MPs and endothelial-derived MPs and their role in stimulating angiogenesis. Both platelets and endothelial cells are activated in response to mechanical and chemical stimuli provided by exercise, that in turn elicit the MPs production by ectocytosis. The branching point refers to the site in which MPs stimulate neo-vascularization.
Acute exercise leads to an augmentation of the sympathetic activity, increasing both epinephrine and norepinephrine concentrations [100]. In this regard, studies report that norepinephrine is able to stimulate Plt-MPs release [101]. Along with hormonal response, acute exercise stimulates the secretion of many cytokines from skeletal muscle, named myokines [102]. Among these myokines, IL-6 is released in the bloodstream and elicits multiorgan effects [103]. It has been reported that IL-6 is also able to stimulate Plt-MPs formation [104], thus exercise can be a potent stimulus to enhance such mechanism.
The following sections provide an overview of various studies (summarized in Table 2) investigating the effects of physical exercise and training on MPs concentration, with an emphasis on endothelial and platelet-derived MPs, due to their specific involvement in angiogenesis [17,105,106,107].

Table 2.
Summary of studies that evaluate MPs concentration in relation with physical exercise/exercise training in various populations.
8.1. The Effect of Physical Exercise on MPs with Angiogenic Potential in Athletes
Endurance athletes have the characteristics to present greater adaptation of the cardiorespiratory and neuromuscular systems resulting in the increase of the delivery and the consumption of oxygen [118]. Two of the sports that highly rely on these adaptations are marathon and half-marathon. Bittencourt et al. compared the amounts of E-MPs and Plt-MPs in a group of half-marathon runners with a control group of gender-matched healthy controls showing that CD51+ E-MPs and CD42+/CD31+ Plt-MPs did not significantly differ between the two groups [52]. This data suggests that athletes exposed to chronic endurance training can benefit from exercise effects but that such type of training does not lead to further decrease in MPs compared with a healthy non-athlete population. On the other hand, E-MPs and Plt-MPs significantly increase following a marathon run and return to baseline values within 2 days; conversely, leukocyte and monocyte-derived MPs decrease after such run [114].
Like long distance running, road cycling and triathlon are two sports heavily relying on cardiorespiratory endurance [119]. In triathletes/cyclists, high-volume (2 h at 55% of peak power output) and high intensity (four sets of minutes at 90–95% of peak power output interspersed by 3 min of active recovery or four sets of 30 s at maximal effort separated by 7:30 of active recovery) trials on cycle ergometer, significantly decrease CD31+/CD42b− apoptotic E-MPs 60 and 180 min after the termination of the trials [117]. It seems that such decrease was due to an increased uptake of MPs by cells. Indeed, triathletes/cyclists’ serum collected 180 min after high intensities exercises promote the uptake of CD31+/CD42b− apoptotic E-MPs in endothelial cells in vitro [117]. Another recent study involving male athletes showed that an incremental cycling protocol until exhaustion determined a significant increase of CD105+, CD146+ E-MPs, and CD62P+ Plt-MPs at the peak of the exercise session [108].
8.2. The Effect of Physical Exercise on MPs with Angiogenic Potential in Healthy Subjects
The physical exercise performed in a regular manner elicits beneficial effects that include improved cardiorespiratory fitness, body composition, decreased risk of noncommunicable diseases and of all-cause mortality [76,120,121,122,123,124].
Data on the effects of aerobic exercise on E-MPs and Plt-MPs are controversial. After 1 h of treadmill run at 70% of VO2max, a decrease in TF+ Plt-MPs and neutrophil-derived MPs in healthy men [110] has been observed; moreover, a single session of 30 min of moderate intensity aerobic exercise (60–64% of their VO2peak) reduces the number of CD62E+ activated E-MPs and CD31+/CD42b− apoptotic E-MPs [115]. On the other hand, in prehypertensive male and female, aerobic exercise performed at 65% of predicted maximal heart rate for a duration of 6 months, with a frequency of three times per week, decreases the blood concentration of CD62E+ E-MPs and CD31+/CD42a− E-MPs [111]. Some authors also evidenced a possible sex/gender effect on the MPs release: indeed, an acute bout of aerobic exercise at 60–70% of the VO2peak produced a significant increase in CD62E+ E-MPs in men, and an increase in CD34+ MPs in women [51]. However, results of study involving both male and female are inconclusive. Indeed, another study showed CD62E+ E-MPs decrease both during and after moderate intensity continuous exercise (65% of VO2max) in women but not in men [116].
One of the factors that must be taken into account analyzing the effects of physical exercise on the MPs is the timing of the analysis. In response to low to moderate intensity (33% of VO2max) concentric or eccentric cycling, men show higher concentration of CD41+ Plt-MPs 5 min following exercise, but these levels returned to baseline levels after 40 min of recovery, while CD62E+ E-MPs do not show modifications [56]. Another experimental study performed in young males showed that CD41+ Plt-MPs and CD62E+ E-MPs were unaffected by 1 h of cycling at ≈46% of VO2max, while when the intensity were set ≈67% of VO2max an increase of CD41+ Plt-MPs was observed [17]. Interestingly, the MPs released in response to such type of exercise enhanced endothelial proliferation, migration, and tubule-like formation in vitro, compared with MPs present in resting conditions [17].
Another modality of training that is of increasing interest in research is high intensity interval training (HIIT). HIIT is a useful exercise modality to enhance cardiorespiratory endurance, and such type of training can be performed also at strenuous intensities [125,126]. Supramaximal sprint cycling protocol consisting of 10 bouts of 15 s at 120% of peak power output separated by 45 s of active recovery lead to a significant increase of circulating CD105+ and CD106+ E-MPs 90 min after the exercise session, whereas at 180 min post-exercise MPs returned towards resting values [112]. On the other hand, no changes in endothelial MPs concentration was observed after a high intensity interval exercise (95% of VO2max) [116].
Physical inactivity and sedentary behaviors have detrimental effects on vasculature state, contributing to the development of cardiovascular and metabolic diseases [127,128,129]. After 5 days of reduced physical activity (<5000 steps/day), significant increase of CD31+/CD42b− apoptotic E-MPs was registered, whereas CD62E+ activated E-MPs remained unchanged, suggesting that inactivity fosters vascular dysfunction increasing endothelial apoptosis in recreationally active men [53].
8.3. The Effect of Physical Exercise on MPs with Angiogenic Potential in Pathologic Subjects
Physical exercise is a fundamental stimulus for improving metabolic and cardiovascular health. Among the various typology, HIIT has the same, or superior capacity to improve health status in pathological subjects (e.g., presenting diabetes, cardiovascular diseases, or cancer) [130,131]. In overweight/obese men, high-intensity continuous exercise (20 min of cycling at just above the subjective ventilatory threshold) and high-intensity interval exercise (10 bouts of 1 min at ≈90% peak aerobic power) reduce E-MPs concentration 18 h following exercise; different results were observed in overweight/obese women in which high-intensity continuous exercise increased activated CD62E+ E-MPs, leaving unaffected CD31+/CD42b− apoptotic E-MPs, regardless the intensity [15]. However, the chronic exposure of overweight/obese subjects to exercise (2 weeks of HIIT or moderate intensity continuous training) lowered the concentration of activated CD62E+ E-MPs [54]. The effect of exercise on MPs release may also depend on the intensity of the physical activity. Indeed, in a recent study involving both over and normal weight subjects, a bout of moderate intensity (60% of their VO2max) aerobic exercise, reduced the total EVs in the MPs range (130–700 nm); however, the release of MPs in normal weight subjects was higher than in obese and in females than in males. [113].
Polycystic ovary syndrome (PCOS) is an endocrine disorder in which endothelial dysfunction and altered angiogenesis are common. Eight weeks of aerobic training consisting of three sessions per week of 1 h of running on a treadmill at 60% VO2max led to a significant decrease in CD105+ MPs in women with PCOS compared with a control group of healthy women, while did not have an effect on the amount of CD106+ E-MPs [55].
Coronary artery diseases determine a diminished myocardial perfusion that may result in angina, myocardial infarction, and heart failure [132]. In stable coronary heart disease patients, neither moderate-intensity continuous exercise (28.7 min at 70% of peak power output) nor a single bout of high-intensity interval exercise (10 min session with work interval of 15 s at 100% of peak power output interspersed with 15 s of passive recovery) have an effect on the concentration of circulating E-MPs and Plt-MPs [109].
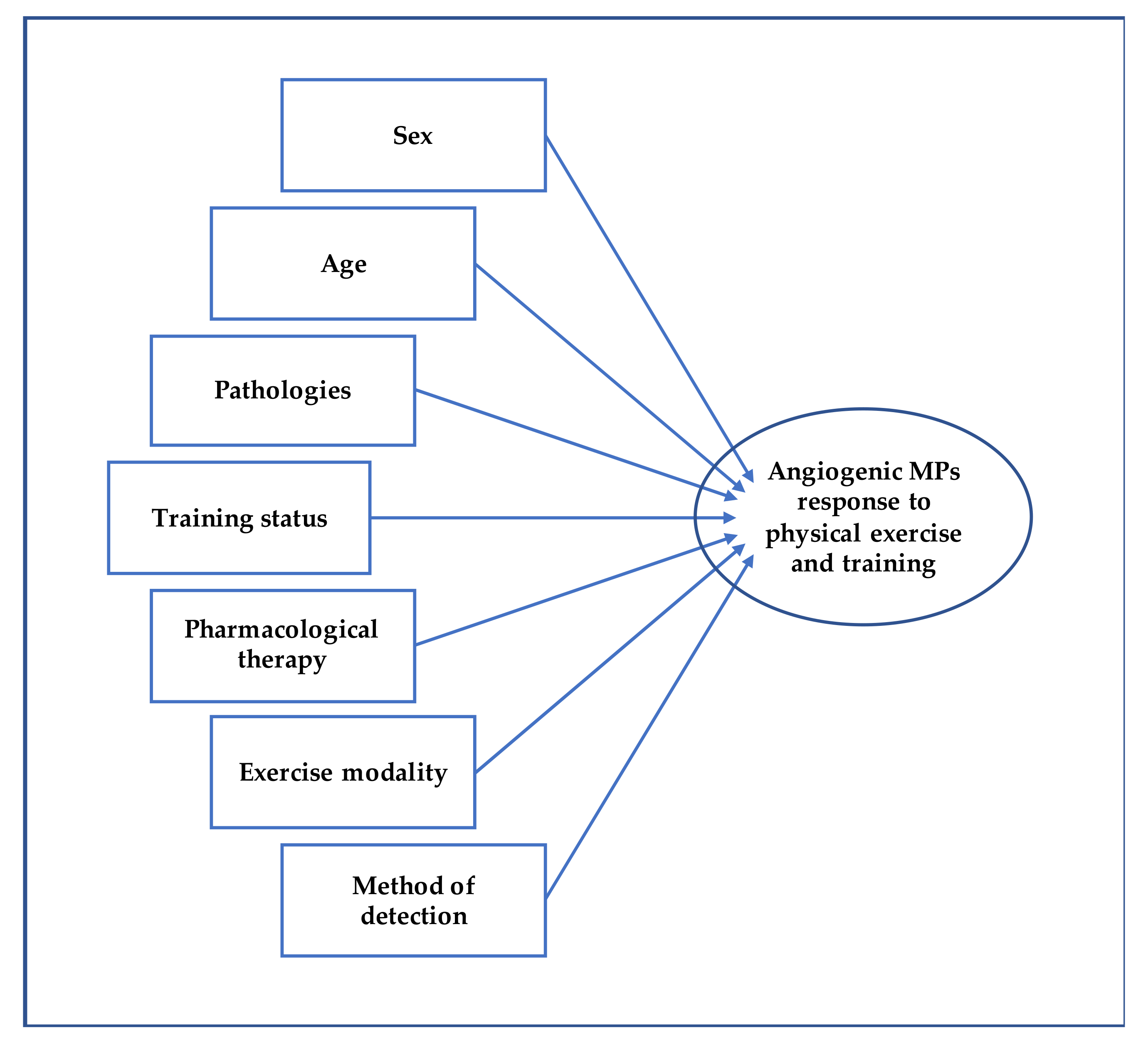
Overall, the numerous studies show contrasting results regarding the qualitative and quantitative changes of MPs in response to acute physical exercise and to exercise training as well. Such divergent conclusions could be due to the heterogeneity of the studies, as they involved different samples in terms of age, sex, training status, possible pharmacological therapies [133], and pathologies [93] (Figure 3). More standardized and harmonized procedures are needed to compare the studies and obtain definitive data on the release of MPs with angiogenic potential in response to physical exercise.
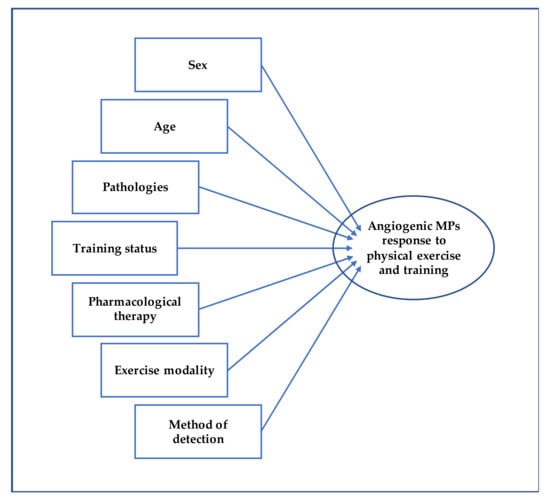
Figure 3.
This figure shows the potential influencing factors affecting MPs following physical exercise and training.
9. Conclusions
Various modalities of cardiovascular endurance-based exercise can modify blood concentration of E-MPs and Plt-MPs, known to affect angiogenic processes. It is well known that physical exercise, among different adaptations, can lead to a functional angiogenesis resulting in a wider vascular bed that increases the blood flow to exercising muscles. Results regarding the effect of exercise on MPs are divergent, but it seems that acute exercise results in an increase of MPs while chronic exposure to exercise results in a decrease in most cases. In addition, MPs sensitivity to exercise can be affected by the training status, sex, age, as well as mode of exercise (e.g., run on a treadmill, road run, cycle ergometer), pharmacological therapies, diseases, and method of detection. While only one study directly showed the pro-angiogenic effects of MPs produced following exercise [17], numerous studies highlight the potential of E-MPs and Plt-MPs in communicating with other cells and stimulating angiogenesis [69,105,106,107]. Thus, future evidence regarding the effect of both acute exercise and training on MPs affecting angiogenesis will be useful to add important insights on the mechanisms by which exercise elicits vascular adaptations and to design specific training programs for various populations.
Author Contributions
A.D.C., P.I., G.G., A.D.B. and B.G. conceived of and designed the outline of the manuscript and examined and ensured the consistency and validity of the contents. A.D.B. and B.G. revised the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Italian Ministry of Education, University and Research (Ministero dell’Istruzione, dell’Università e della Ricerca-MIUR), grant number PRIN 2017ATZ2YK_003.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nair, A.; Chauhan, P.; Saha, B.; Kubatzky, K.F. Conceptual Evolution of Cell Signaling. Int. J. Mol. Sci. 2019, 20, 3292. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Volpert, O.V.; Stellmach, V.; Bouck, N. The modulation of thrombospondin and other naturally occurring inhibitors of angiogenesis during tumor progression. Breast Cancer Res. Treat. 1995, 36, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Folkman, J. Patterns and Emerging Mechanisms of the Angiogenic Switch during Tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch: Angiogenesis. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Gowdak, L.H.W.; Krieger, J.E. Vascular Growth Factors, Progenitor Cells, and Angiogenesis. In Endothelium and Cardiovascular Diseases; Elsevier: Amsterdam, The Netherlands, 2018; pp. 49–62. [Google Scholar]
- Mezentsev, A.; Merks, R.M.H.; O’Riordan, E.; Chen, J.; Mendelev, N.; Goligorsky, M.S.; Brodsky, S.V. Endothelial microparticles affect angiogenesis in vitro: Role of oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1106–H1114. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Deng, F.; Wang, S.; Zhang, L. Endothelial microparticles act as novel diagnostic and therapeutic biomarkers of circulatory hypoxia-related diseases: A literature review. J. Cell. Mol. Med. 2017, 21, 1698–1710. [Google Scholar] [CrossRef]
- He, Z.; Tang, Y.; Qin, C. Increased circulating leukocyte-derived microparticles in ischemic cerebrovascular disease. Thromb. Res. 2017, 154, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Rautou, P.; Bresson, J.; Sainte–Marie, Y.; Vion, A.; Paradis, V.; Renard, J.; Devue, C.; Heymes, C.; Letteron, P.; Elkrief, L.; et al. Abnormal Plasma Microparticles Impair Vasoconstrictor Responses in Patients with Cirrhosis. Gastroenterology 2012, 143, 166–176. [Google Scholar] [CrossRef]
- Batool, S. Microparticles and their Roles in Inflammation: A Review. Open Immunol. J. 2013, 6, 1–14. [Google Scholar] [CrossRef]
- Chen, Y.; Li, G.; Liu, M.-L. Microvesicles as Emerging Biomarkers and Therapeutic Targets in Cardiometabolic Diseases. Genom. Proteom. Bioinform. 2018, 16, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Durrer, C.; Robinson, E.; Wan, Z.; Martinez, N.; Hummel, M.L.; Jenkins, N.T.; Kilpatrick, M.W.; Little, J.P. Differential Impact of Acute High-Intensity Exercise on Circulating Endothelial Microparticles and Insulin Resistance between Overweight/Obese Males and Females. PLoS ONE 2015, 10, e0115860. [Google Scholar] [CrossRef]
- Ayers, L.; Nieuwland, R.; Kohler, M.; Kraenkel, N.; Ferry, B.; Leeson, P. Dynamic microvesicle release and clearance within the cardiovascular system: Triggers and mechanisms. Clin. Sci. 2015, 129, 915–931. [Google Scholar] [CrossRef]
- Wilhelm, E.N.; González-Alonso, J.; Parris, C.; Rakobowchuk, M. Exercise intensity modulates the appearance of circulating microvesicles with proangiogenic potential upon endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1297–H1310. [Google Scholar] [CrossRef]
- Gaggi, G.; Izzicupo, P.; Di Credico, A.; Sancilio, S.; Di Baldassarre, A.; Ghinassi, B. Spare Parts from Discarded Materials: Fetal Annexes in Regenerative Medicine. Int. J. Mol. Sci. 2019, 20, 1573. [Google Scholar] [CrossRef]
- Patan, S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J. Neurooncol. 2000, 50, 1–15. [Google Scholar] [CrossRef]
- Cox, C.M.; Poole, T.J. Angioblast differentiation is influenced by the local environment: FGF-2 induces angioblasts and patterns vessel formation in the quail embryo. Dev. Dyn. 2000, 218, 371–382. [Google Scholar] [CrossRef]
- Lampropoulou, A.; Ruhrberg, C. Neuropilin regulation of angiogenesis. Biochem. Soc. Trans. 2014, 42, 1623–1628. [Google Scholar] [CrossRef]
- Hofer, E.; Schweighofer, B. Signal transduction induced in endothelial cells by growth factor receptors involved in angiogenesis. Thromb. Haemost. 2007, 97, 355–363. [Google Scholar] [PubMed]
- Schaper, W.; Scholz, D. Factors Regulating Arteriogenesis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- D’amico, M.A.; Ghinassi, B.; Izzicupo, P.; Di Ruscio, A.; Di Baldassarre, A. IL-6 Activates PI3K and PKCζ Signaling and Determines Cardiac Differentiation in Rat Embryonic H9c2 Cells: IL-6 and cardiac differentiation of H9c2 cells. J. Cell. Physiol. 2016, 231, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Mentzer, S.J.; Konerding, M.A. Intussusceptive angiogenesis: Expansion and remodeling of microvascular networks. Angiogenesis 2014, 17, 499–509. [Google Scholar] [CrossRef]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef]
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000, 6, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Stoorvogel, W.; Kleijmeer, M.J.; Geuze, H.J.; Raposo, G. The biogenesis and functions of exosomes. Traffic 2002, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, É.; Pap, E.; Kittel, Á.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Hargett, L.A.; Bauer, N.N. On the Origin of Microparticles: From “Platelet Dust” to Mediators of Intercellular Communication. Pulm. Circ. 2013, 3, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Flaumenhaft, R.; Dilks, J.R.; Richardson, J.; Alden, E.; Patel-Hett, S.R.; Battinelli, E.; Klement, G.L.; Sola-Visner, M.; Italiano, J.E. Megakaryocyte-derived microparticles: Direct visualization and distinction from platelet-derived microparticles. Blood 2008, 113, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Goubran, H.A.; Chou, M.-L.; Devos, D.; Radosevic, M. Platelet microparticles: Detection and assessment of their paradoxical functional roles in disease and regenerative medicine. Blood Rev. 2014, 28, 155–166. [Google Scholar] [CrossRef]
- Diehl, P.; Fricke, A.; Sander, L.; Stamm, J.; Bassler, N.; Htun, N.; Ziemann, M.; Helbing, T.; El-Osta, A.; Jowett, J.B.M.; et al. Microparticles: Major transport vehicles for distinct microRNAs in circulation. Cardiovasc. Res. 2012, 93, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Connor, D.E.; Exner, T.; Ma, D.D.F.; Joseph, J.E. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb. Haemost. 2010, 103, 1044–1052. [Google Scholar] [PubMed]
- Zwicker, J.I.; Trenor, C.C.; Furie, B.C.; Furie, B. Tissue Factor–Bearing Microparticles and Thrombus Formation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Dignat-George, F.; Boulanger, C.M. The Many Faces of Endothelial Microparticles. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 27–33. [Google Scholar] [CrossRef]
- Orozco, A.F.; Lewis, D.E. Flow cytometric analysis of circulating microparticles in plasma. Cytom. Part A 2010, 77A, 502–514. [Google Scholar] [CrossRef]
- Inglis, H.C.; Danesh, A.; Shah, A.; Lacroix, J.; Spinella, P.C.; Norris, P.J. Techniques to improve detection and analysis of extracellular vesicles using flow cytometry: Accuracy and Efficiency of EV Detection Using FCM. Cytom. Part A 2015, 87, 1052–1063. [Google Scholar] [CrossRef]
- Van Der Pol, E.; Hoekstra, A.G.; Sturk, A.; Otto, C.; Van Leeuwen, T.G.; Nieuwland, R. Optical and non-optical methods for detection and characterization of microparticles and exosomes: Detection and characterization of microparticles and exosomes. J. Thromb. Haemost. 2010, 8, 2596–2607. [Google Scholar] [CrossRef]
- Burger, D.; Schock, S.; Thompson, C.S.; Montezano, A.C.; Hakim, A.M.; Touyz, R.M. Microparticles: Biomarkers and beyond. Clin. Sci. 2013, 124, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Gradziuk, M.; Radziwon, P. Methods for detection of microparticles derived from blood and endothelial cells. Acta Haematol. Pol. 2017, 48, 316–329. [Google Scholar] [CrossRef]
- van der Pol, E.; Böing, A.N.; Gool, E.L.; Nieuwland, R. Recent developments in the nomenclature, presence, isolation, detection and clinical impact of extracellular vesicles. J. Thromb. Haemost. 2016, 14, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.P.; Hole, P.; Carr, B.; Redman, C.W.G.; Harris, A.L.; Dobson, P.J.; et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Fritzsching, B.; Schwer, B.; Kartenbeck, J.; Pedal, A.; Horejsi, V.; Ott, M. Release and Intercellular Transfer of Cell Surface CD81 via Microparticles. J. Immunol. 2002, 169, 5531–5537. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E.; Mokhnach, R.E. The approaches to none-invasive detection of cell-derived extracellular vesicles. Biol. Markers Guid. Ther. 2016, 3, 155–175. [Google Scholar] [CrossRef]
- Barteneva, N.S.; Fasler-Kan, E.; Bernimoulin, M.; Stern, J.N.; Ponomarev, E.D.; Duckett, L.; Vorobjev, I.A. Circulating microparticles: Square the circle. BMC Cell Biol. 2013, 14, 23. [Google Scholar] [CrossRef]
- Hnasko, R. ELISA: Methods and Protocols; Humana Press: New York, NY, USA, 2015. [Google Scholar]
- Nomura, S.; Shouzu, A.; Taomoto, K.; Togane, Y.; Goto, S.; Ozaki, Y.; Uchiyama, S.; Ikeda, Y. Assessment of an ELISA Kit for Platelet-Derived Microparticles by Joint Research at Many Institutes in Japan. J. Atheroscler. Thromb. 2010, 16, 878–887. [Google Scholar] [CrossRef]
- Lansford, K.A.; Shill, D.D.; Dicks, A.B.; Marshburn, M.P.; Southern, W.M.; Jenkins, N.T. Effect of acute exercise on circulating angiogenic cell and microparticle populations: Exercise, circulating angiogenic cell and microparticle populations. Exp. Physiol. 2016, 101, 155–167. [Google Scholar] [CrossRef]
- Bittencourt, C.R.d.O.; Izar, M.C.d.O.; França, C.N.; Schwerz, V.L.; Póvoa, R.M.d.S.; Fonseca, F.A.H. Effects of Chronic Exercise on Endothelial Progenitor Cells and Microparticles in Professional Runners. Arq. Bras. Cardiol. 2017, 108, 212–216. [Google Scholar] [CrossRef]
- Boyle, L.J.; Credeur, D.P.; Jenkins, N.T.; Padilla, J.; Leidy, H.J.; Thyfault, J.P.; Fadel, P.J. Impact of reduced daily physical activity on conduit artery flow-mediated dilation and circulating endothelial microparticles. J. Appl. Physiol. 2013, 115, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, H.; Robinson, E.; Barry, J.; Jung, M.E.; Little, J.P. Short-term exercise training reduces glycaemic variability and lowers circulating endothelial microparticles in overweight and obese women at elevated risk of type 2 diabetes. Eur. J. Sport Sci. 2019, 19, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Kirk, R.J.; Madden, L.A.; Peart, D.J.; Aye, M.M.; Atkin, S.L.; Vince, R.V. Circulating Endothelial Microparticles Reduce in Concentration Following an Exercise Programme in Women with Polycystic Ovary Syndrome. Front. Endocrinol. 2019, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Rakobowchuk, M.; Ritter, O.; Wilhelm, E.N.; Isacco, L.; Bouhaddi, M.; Degano, B.; Tordi, N.; Mourot, L. Divergent endothelial function but similar platelet microvesicle responses following eccentric and concentric cycling at a similar aerobic power output. J. Appl. Physiol. 2017, 122, 1031–1039. [Google Scholar] [CrossRef]
- Angelillo-Scherrer, A. Leukocyte-Derived Microparticles in Vascular Homeostasis. Circ. Res. 2012, 110, 356–369. [Google Scholar] [CrossRef]
- Manno, S.; Takakuwa, Y.; Mohandas, N. Identification of a functional role for lipid asymmetry in biological membranes: Phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc. Natl. Acad. Sci. USA 2002, 99, 1943–1948. [Google Scholar] [CrossRef]
- Daleke, D.L. Regulation of transbilayer plasma membrane phospholipid asymmetry. J. Lipid Res. 2003, 44, 233–242. [Google Scholar] [CrossRef]
- Morel, O.; Jesel, L.; Freyssinet, J.-M.; Toti, F. Cellular Mechanisms Underlying the Formation of Circulating Microparticles. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 15–26. [Google Scholar] [CrossRef]
- Coleman, M.L.; Sahai, E.A.; Yeo, M.; Bosch, M.; Dewar, A.; Olson, M.F. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat. Cell Biol. 2001, 3, 339–345. [Google Scholar] [CrossRef]
- Deregibus, M.C.; Cantaluppi, V.; Calogero, R.; Lo Iacono, M.; Tetta, C.; Biancone, L.; Bruno, S.; Bussolati, B.; Camussi, G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 2007, 110, 2440–2448. [Google Scholar] [CrossRef]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120, 1658–1673. [Google Scholar] [CrossRef]
- Soleti, R.; Benameur, T.; Porro, C.; Panaro, M.A.; Andriantsitohaina, R.; Martinez, M.C. Microparticles harboring Sonic Hedgehog promote angiogenesis through the upregulation of adhesion proteins and proangiogenic factors. Carcinogenesis 2009, 30, 580–588. [Google Scholar] [CrossRef]
- Ramakrishnan, D.P.; Hajj-Ali, R.A.; Chen, Y.; Silverstein, R.L. Extracellular Vesicles Activate a CD36-Dependent Signaling Pathway to Inhibit Microvascular Endothelial Cell Migration and Tube Formation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Zernecke, A.; Bidzhekov, K.; Noels, H.; Shagdarsuren, E.; Gan, L.; Denecke, B.; Hristov, M.; Köppel, T.; Jahantigh, M.N.; Lutgens, E.; et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. 2009, 2, ra81. [Google Scholar] [CrossRef] [PubMed]
- Taraboletti, G.; D’Ascenzo, S.; Borsotti, P.; Giavazzi, R.; Pavan, A.; Dolo, V. Shedding of the Matrix Metalloproteinases MMP-2, MMP-9, and MT1-MMP as Membrane Vesicle-Associated Components by Endothelial Cells. Am. J. Pathol. 2002, 160, 673–680. [Google Scholar] [CrossRef]
- Lombardo, G.; Dentelli, P.; Togliatto, G.; Rosso, A.; Gili, M.; Gallo, S.; Deregibus, M.C.; Camussi, G.; Brizzi, M.F. Activated Stat5 trafficking via Endothelial Cell-derived Extracellular Vesicles Controls IL-3 Pro-angiogenic Paracrine Action. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wahl, P.; Wehmeier, U.F.; Jansen, F.J.; Kilian, Y.; Bloch, W.; Werner, N.; Mester, J.; Hilberg, T. Acute Effects of Different Exercise Protocols on the Circulating Vascular microRNAs -16, -21, and -126 in Trained Subjects. Front. Physiol. 2016, 7, 643. [Google Scholar] [CrossRef]
- Brill, A.; Dashevsky, O.; Rivo, J.; Gozal, Y.; Varon, D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc. Res. 2005, 67, 30–38. [Google Scholar] [CrossRef]
- Janowska-Wieczorek, A.; Wysoczynski, M.; Kijowski, J.; Marquez-Curtis, L.; Machalinski, B.; Ratajczak, J.; Ratajczak, M.Z. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int. J. Cancer 2005, 113, 752–760. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Bredin, S.S.D. Health benefits of physical activity: A systematic review of current systematic reviews. Curr. Opin. Cardiol. 2017, 32, 541–556. [Google Scholar] [CrossRef]
- Di Blasio, A.; Izzicupo, P.; Di Baldassarre, A.; Gallina, S.; Bucci, I.; Giuliani, C.; Di Santo, S.; Di Iorio, A.; Ripari, P.; Napolitano, G. Walking training and cortisol to DHEA-S ratio in postmenopause: An intervention study. Women Health 2018, 58, 387–402. [Google Scholar] [CrossRef]
- Condello, G.; Capranica, L.; Migliaccio, S.; Forte, R.; Di Baldassarre, A.; Pesce, C. Energy Balance and Active Lifestyle: Potential Mediators of Health and Quality of Life Perception in Aging. Nutrients 2019, 11, 2122. [Google Scholar] [CrossRef] [PubMed]
- Forte, R.; Pesce, C.; Di Baldassarre, A.; Shea, J.; Voelcker-Rehage, C.; Capranica, L.; Condello, G. How Older Adults Cope with Cognitive Complexity and Environmental Constraints during Dual-Task Walking: The Role of Executive Function Involvement. Int. J. Environ. Res. Public Health 2019, 16, 1835. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Condello, G.; Forte, R.; Monteagudo, P.; Ghinassi, B.; Di Baldassarre, A.; Capranica, L.; Pesce, C. Autonomic Stress Response and Perceived Effort Jointly Inform on Dual Tasking in Aging. Brain Sci. 2019, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Pedrinolla, A.; Venturelli, M.; Tamburin, S.; Fonte, C.; Stabile, A.M.; Galazzo, I.B.; Ghinassi, B.; Venneri, M.A.; Pizzini, F.B.; Muti, E.; et al. Non-Aβ-Dependent Factors Associated with Global Cognitive and Physical Function in Alzheimer’s Disease: A Pilot Multivariate Analysis. J. Clin. Med. 2019, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Condello, G.; Forte, R.; Falbo, S.; Shea, J.B.; Di Baldassarre, A.; Capranica, L.; Pesce, C. Steps to Health in Cognitive Aging: Effects of Physical Activity on Spatial Attention and Executive Control in the Elderly. Front. Hum. Neurosci. 2017, 11, 107. [Google Scholar] [CrossRef]
- Di Mauro, M.; Gallina, S.; D’Amico, M.A.; Izzicupo, P.; Lanuti, P.; Bascelli, A.; Di Fonso, A.; Bartoloni, G.; Calafiore, A.M.; Di Baldassarre, A. Functional mitral regurgitation. Int. J. Cardiol. 2013, 163, 242–248. [Google Scholar] [CrossRef]
- Di Francescomarino, S.; Sciartilli, A.; Di Valerio, V.; Di Baldassarre, A.; Gallina, S. The Effect of Physical Exercise on Endothelial Function. Sports Med. 2009, 39, 797–812. [Google Scholar] [CrossRef]
- Morena, F.; Argentati, C.; Trotta, R.; Crispoltoni, L.; Stabile, A.; Pistilli, A.; di Baldassarre, A.; Calafiore, R.; Montanucci, P.; Basta, G.; et al. A Comparison of Lysosomal Enzymes Expression Levels in Peripheral Blood of Mild- and Severe-Alzheimer’s Disease and MCI Patients: Implications for Regenerative Medicine Approaches. Int. J. Mol. Sci. 2017, 18, 1806. [Google Scholar] [CrossRef]
- Filardi, T.; Ghinassi, B.; Di Baldassarre, A.; Tanzilli, G.; Morano, S.; Lenzi, A.; Basili, S.; Crescioli, C. Cardiomyopathy Associated with Diabetes: The Central Role of the Cardiomyocyte. Int. J. Mol. Sci. 2019, 20, 3299. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.; Zierath, J.R. Exercise Metabolism and the Molecular Regulation of Skeletal Muscle Adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Kissane, R.W.; Egginton, S. Exercise-mediated angiogenesis. Curr. Opin. Physiol. 2019, 10, 193–201. [Google Scholar] [CrossRef]
- Izzicupo, P.; D’Amico, M.A.; Di Blasio, A.; Napolitano, G.; Nakamura, F.Y.; Di Baldassarre, A.; Ghinassi, B. Aerobic Training Improves Angiogenic Potential Independently of Vascular Endothelial Growth Factor Modifications in Postmenopausal Women. Front. Endocrinol. 2017, 8, 363. [Google Scholar] [CrossRef]
- Izzicupo, P.; D’Amico, M.A.; Di Blasio, A.; Napolitano, G.; Di Baldassarre, A.; Ghinassi, B. Nordic walking increases circulating VEGF more than traditional walking training in postmenopause. Climacteric 2017, 20, 533–539. [Google Scholar] [CrossRef]
- Izzicupo, P.; Ghinassi, B.; D’Amico, M.A.; Di Blasio, A.; Gesi, M.; Napolitano, G.; Gallina, S.; Di Baldassarre, A. Effects of ACE I/D Polymorphism and Aerobic Training on the Immune–Endocrine Network and Cardiovascular Parameters of Postmenopausal Women. J. Clin. Endocrinol. Metab. 2013, 98, 4187–4194. [Google Scholar] [CrossRef]
- Di Mauro, M.; Izzicupo, P.; Santarelli, F.; Falone, S.; Pennelli, A.; Amicarelli, F.; Calafiore, A.M.; Di Baldassarre, A.; Gallina, S. ACE and AGTR1 Polymorphisms and Left Ventricular Hypertrophy in Endurance Athletes. Med. Sci. Sports Exerc. 2010, 42, 915–921. [Google Scholar] [CrossRef]
- Taylor, C.T. Mitochondria and cellular oxygen sensing in the HIF pathway. Biochem. J. 2008, 409, 19–26. [Google Scholar] [CrossRef]
- Oliveira, A.N.; Hood, D.A. Exercise is mitochondrial medicine for muscle. Sports Med. Health Sci. 2019, 1, 11–18. [Google Scholar] [CrossRef]
- Gorski, T.; De Bock, K. Metabolic regulation of exercise-induced angiogenesis. Vasc. Biol. 2019, 1, H1–H8. [Google Scholar] [CrossRef]
- Highton, P.J.; Martin, N.; Smith, A.C.; Burton, J.O.; Bishop, N.C. Microparticles and Exercise in Clinical Populations. Exerc. Immunol. Rev. 2018, 24, 46–58. [Google Scholar] [PubMed]
- Whitham, M.; Parker, B.L.; Friedrichsen, M.; Hingst, J.R.; Hjorth, M.; Hughes, W.E.; Egan, C.L.; Cron, L.; Watt, K.I.; Kuchel, R.P.; et al. Extracellular Vesicles Provide a Means for Tissue Crosstalk during Exercise. Cell Metab. 2018, 27, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Trovato, E.; Di Felice, V.; Barone, R. Extracellular Vesicles: Delivery Vehicles of Myokines. Front. Physiol. 2019, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, D.H.J.; Dawson, E.A.; Black, M.A.; Hopman, M.T.E.; Cable, N.T.; Green, D.J. Brachial Artery Blood Flow Responses to Different Modalities of Lower Limb Exercise. Med. Sci. Sports Exerc. 2009, 41, 1072–1079. [Google Scholar] [CrossRef]
- Woodman, C.R.; Price, E.M.; Laughlin, M.H. Shear stress induces eNOS mRNA expression and improves endothelium-dependent dilation in senescent soleus muscle feed arteries. J. Appl. Physiol. 2005, 98, 940–946. [Google Scholar] [CrossRef]
- Vion, A.-C.; Ramkhelawon, B.; Loyer, X.; Chironi, G.; Devue, C.; Loirand, G.; Tedgui, A.; Lehoux, S.; Boulanger, C.M. Shear Stress Regulates Endothelial Microparticle Release. Circ. Res. 2013, 112, 1323–1333. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Chen, J.-K.; Wang, J.-S. Strenuous exercise promotes shear-induced thrombin generation by increasing the shedding of procoagulant microparticles from platelets. Thromb. Haemost. 2010, 104, 293–301. [Google Scholar] [CrossRef]
- Fisher, J.P.; Young, C.N.; Fadel, P.J. Autonomic Adjustments to Exercise in Humans. In Comprehensive Physiology; Terjung, R., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 475–512. [Google Scholar]
- Tschuor, C.; Asmis, L.M.; Lenzlinger, P.M.; Tanner, M.; Härter, L.; Keel, M.; Stocker, R.; Stover, J.F. In vitro norepinephrine significantly activates isolated platelets from healthy volunteers and critically ill patients following severe traumatic brain injury. Crit. Care 2008, 12, R80. [Google Scholar] [CrossRef]
- Pedersen, B.K. Muscles and their myokines. J. Exp. Biol. 2011, 214, 337–346. [Google Scholar] [CrossRef]
- Ellingsgaard, H.; Hojman, P.; Pedersen, B.K. Exercise and health—Emerging roles of IL-6. Curr. Opin. Physiol. 2019, 10, 49–54. [Google Scholar] [CrossRef]
- Nomura, S.; Imamura, A.; Okuno, M.; Kamiyama, Y.; Fujimura, Y.; Ikeda, Y.; Fukuhara, S. Platelet-Derived Microparticles in Patients with Arteriosclerosis Obliterans. Thromb. Res. 2000, 98, 257–268. [Google Scholar] [CrossRef]
- Varon, D.; Shai, E. Role of platelet-derived microparticles in angiogenesis and tumor progression. Discov. Med. 2009, 8, 237–241. [Google Scholar]
- Lacroix, R.; Sabatier, F.; Mialhe, A.; Basire, A.; Pannell, R.; Borghi, H.; Robert, S.; Lamy, E.; Plawinski, L.; Camoin-Jau, L.; et al. Activation of plasminogen into plasmin at the surface of endothelial microparticles: A mechanism that modulates angiogenic properties of endothelial progenitor cells in vitro. Blood 2007, 110, 2432–2439. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Song, K.S.; Chung, J.-H.; Lee, K.R.; Lee, S.-N. Platelet microparticles induce angiogenesis in vitro. Br. J. Haematol. 2004, 124, 376–384. [Google Scholar] [CrossRef]
- Brahmer, A.; Neuberger, E.; Esch-Heisser, L.; Haller, N.; Jorgensen, M.M.; Baek, R.; Möbius, W.; Simon, P.; Krämer-Albers, E.-M. Platelets, endothelial cells and leukocytes contribute to the exercise-triggered release of extracellular vesicles into the circulation. J. Extracell. Vesicles 2019, 8, 1615820. [Google Scholar] [CrossRef] [PubMed]
- Guiraud, T.; Gayda, M.; Juneau, M.; Bosquet, L.; Meyer, P.; Théberge-Julien, G.; Galinier, M.; Nozza, A.; Lambert, J.; Rhéaume, E.; et al. A Single Bout of High-Intensity Interval Exercise Does Not Increase Endothelial or Platelet Microparticles in Stable, Physically Fit Men with Coronary Heart Disease. Can. J. Cardiol. 2013, 29, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Highton, P.J.; Goltz, F.R.; Martin, N.; Stensel, D.J.; Thackray, A.E.; Bishop, N.C. Microparticle Responses to Aerobic Exercise and Meal Consumption in Healthy Men. Med. Sci. Sports Exerc. 2019, 51, 1935–1943. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, B.; Lee, H.; Thakkar, S.; Babbitt, D.M.; Eguchi, S.; Brown, M.D.; Park, J.-Y. Shear stress-induced mitochondrial biogenesis decreases the release of microparticles from endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H425–H433. [Google Scholar] [CrossRef]
- Kirk, R.J.; Peart, D.J.; Madden, L.A.; Vince, R.V. Repeated supra-maximal sprint cycling with and without sodium bicarbonate supplementation induces endothelial microparticle release. Eur. J. Sport Sci. 2014, 14, 345–352. [Google Scholar] [CrossRef]
- Rigamonti, A.E.; Bollati, V.; Pergoli, L.; Iodice, S.; De Col, A.; Tamini, S.; Cicolini, S.; Tringali, G.; De Micheli, R.; Cella, S.G.; et al. Effects of an acute bout of exercise on circulating extracellular vesicles: Tissue-, sex-, and BMI-related differences. Int. J. Obes. 2020, 44, 1108–1118. [Google Scholar] [CrossRef]
- Schwarz, V.; Düsing, P.; Liman, T.; Werner, C.; Herm, J.; Bachelier, K.; Krüll, M.; Brechtel, L.; Jungehulsing, G.J.; Haverkamp, W.; et al. Marathon running increases circulating endothelial- and thrombocyte-derived microparticles. Eur. J. Prev. Cardiol. 2018, 25, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Serviente, C.; Burnside, A.; Witkowski, S. Moderate-intensity exercise reduces activated and apoptotic endothelial microparticles in healthy midlife women. J. Appl. Physiol. 2019, 126, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Shill, D.D.; Lansford, K.A.; Hempel, H.K.; Call, J.A.; Murrow, J.R.; Jenkins, N.T. Effect of exercise intensity on circulating microparticles in men and women. Exp. Physiol. 2018, 103, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Wahl, P.; Jansen, F.; Achtzehn, S.; Schmitz, T.; Bloch, W.; Mester, J.; Werner, N. Effects of High Intensity Training and High Volume Training on Endothelial Microparticles and Angiogenic Growth Factors. PLoS ONE 2014, 9, e96024. [Google Scholar] [CrossRef] [PubMed]
- Hackney, A.C. Molecular and Physiological Adaptations to Endurance Training. In Concurrent Aerobic and Strength Training; Schumann, M., Rønnestad, B.R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 19–34. [Google Scholar]
- O’Toole, M.L.; Douglas, P.S. Applied Physiology of Triathlon. Sports Med. 1995, 19, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Izzicupo, P.; D’Amico, M.A.; Bascelli, A.; Di Fonso, A.; D’Angelo, E.; Di Blasio, A.; Bucci, I.; Napolitano, G.; Gallina, S.; Di Baldassarre, A. Walking training affects dehydroepiandrosterone sulfate and inflammation independent of changes in spontaneous physical activity. Menopause J. N. Am. Menopause Soc. 2012, 1. [Google Scholar] [CrossRef]
- Martelli, F.; Ghinassi, B.; Lorenzini, R.; Vannucchi, A.M.; Rana, R.A.; Nishikawa, M.; Partamian, S.; Migliaccio, G.; Migliaccio, A.R. Thrombopoietin Inhibits Murine Mast Cell Differentiation. Stem Cells 2008, 26, 912–919. [Google Scholar] [CrossRef][Green Version]
- Ghinassi, B.; Ferro, L.; Masiello, F.; Tirelli, V.; Sanchez, M.; Migliaccio, G.; Whitsett, C.; Kachala, S.; Riviere, I.; Sadelain, M.; et al. Recovery and Biodistribution of Ex Vivo Expanded Human Erythroblasts Injected into NOD/SCID/IL2R γ null mice. Stem Cells Int. 2011, 2011, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gallina, S.; Di Francescomarino, S.; Di Mauro, M.; Izzicupo, P.; D’Angelo, E.; D’Amico, M.A.; Pennelli, A.; Amicarelli, F.; Di Baldassarre, A. NAD(P)H oxidase p22phox polymorphism and cardiovascular function in amateur runners. Acta Physiol. 2012, 206, 20–28. [Google Scholar] [CrossRef]
- Izzicupo, P.; Di Valerio, V.; D’Amico, M.A.; Di Mauro, M.; Pennelli, A.; Falone, S.; Alberti, G.; Amicarelli, F.; Miscia, S.; Gallina, S.; et al. Nad(P)H Oxidase and Pro-Inflammatory Response during Maximal Exercise: Role of C242T Polymorphism of the P22PHOX Subunit. Int. J. Immunopathol. Pharmacol. 2010, 23, 203–211. [Google Scholar] [CrossRef]
- Buchheit, M.; Laursen, P.B. High-Intensity Interval Training, Solutions to the Programming Puzzle: Part I: Cardiopulmonary Emphasis. Sports Med. 2013, 43, 313–338. [Google Scholar] [CrossRef] [PubMed]
- Di Blasio, A.; Izzicupo, P.; Tacconi, L.; Di Santo, S.; Leogrande, M.; Bucci, I.; Ripari, P.; Di Baldassarre, A.; Napolitano, G. Acute and delayed effects of high intensity interval resistance training organization on cortisol and testosterone production. J. Sports Med. Phys. Fit. 2016, 56, 192–199. [Google Scholar]
- Kohl, H.W.; Craig, C.L.; Lambert, E.V.; Inoue, S.; Alkandari, J.R.; Leetongin, G.; Kahlmeier, S. The pandemic of physical inactivity: Global action for public health. Lancet 2012, 380, 294–305. [Google Scholar] [CrossRef]
- Izzicupo, P.; Di Blasio, A.; Di Credico, A.; Gaggi, G.; Vamvakis, A.; Napolitano, G.; Ricci, F.; Gallina, S.; Ghinassi, B.; Di Baldassarre, A. The Length and Number of Sedentary Bouts Predict Fibrinogen Levels in Postmenopausal Women. Int. J. Environ. Res. Public Health 2020, 17, 3051. [Google Scholar] [CrossRef]
- Biswas, A.; Oh, P.I.; Faulkner, G.E.; Bajaj, R.R.; Silver, M.A.; Mitchell, M.S.; Alter, D.A. Sedentary Time and Its Association with Risk for Disease Incidence, Mortality, and Hospitalization in Adults: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2015, 162, 123. [Google Scholar] [CrossRef]
- Ross, L.M.; Porter, R.R.; Durstine, J.L. High-intensity interval training (HIIT) for patients with chronic diseases. J. Sport Health Sci. 2016, 5, 139–144. [Google Scholar] [CrossRef]
- Lee, K.; Kang, I.; Mack, W.J.; Mortimer, J.; Sattler, F.; Salem, G.; Dieli-Conwright, C.M. Effect of High Intensity Interval Training on Matrix Metalloproteinases in Women with Breast Cancer Receiving Anthracycline-Based Chemotherapy. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Perez-Quilis, C.; Leischik, R.; Lucia, A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann. Transl. Med. 2016, 4, 256. [Google Scholar] [CrossRef]
- Suades, R.; Padró, T.; Alonso, R.; Mata, P.; Badimon, L. Lipid-lowering therapy with statins reduces microparticle shedding from endothelium, platelets and inflammatory cells. Thromb. Haemost. 2013, 110, 366–377. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).