Effect of Burn Joss Paper Ash on Properties of Ground-Granulated Blast Furnace-Based Slag Geopolymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Result and Discussion
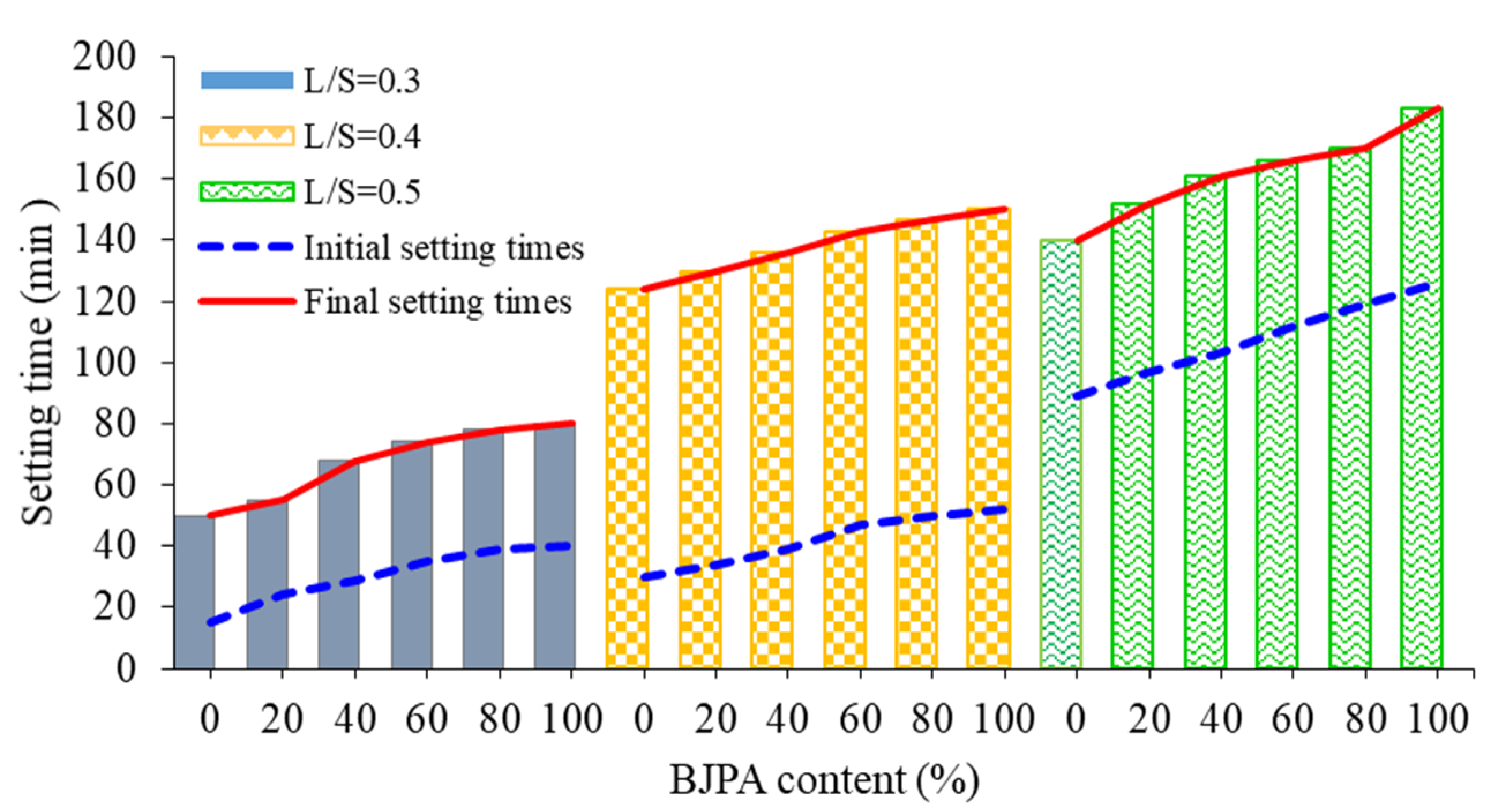
3.1. Setting Time
3.2. Slump
3.3. Compressive Strength
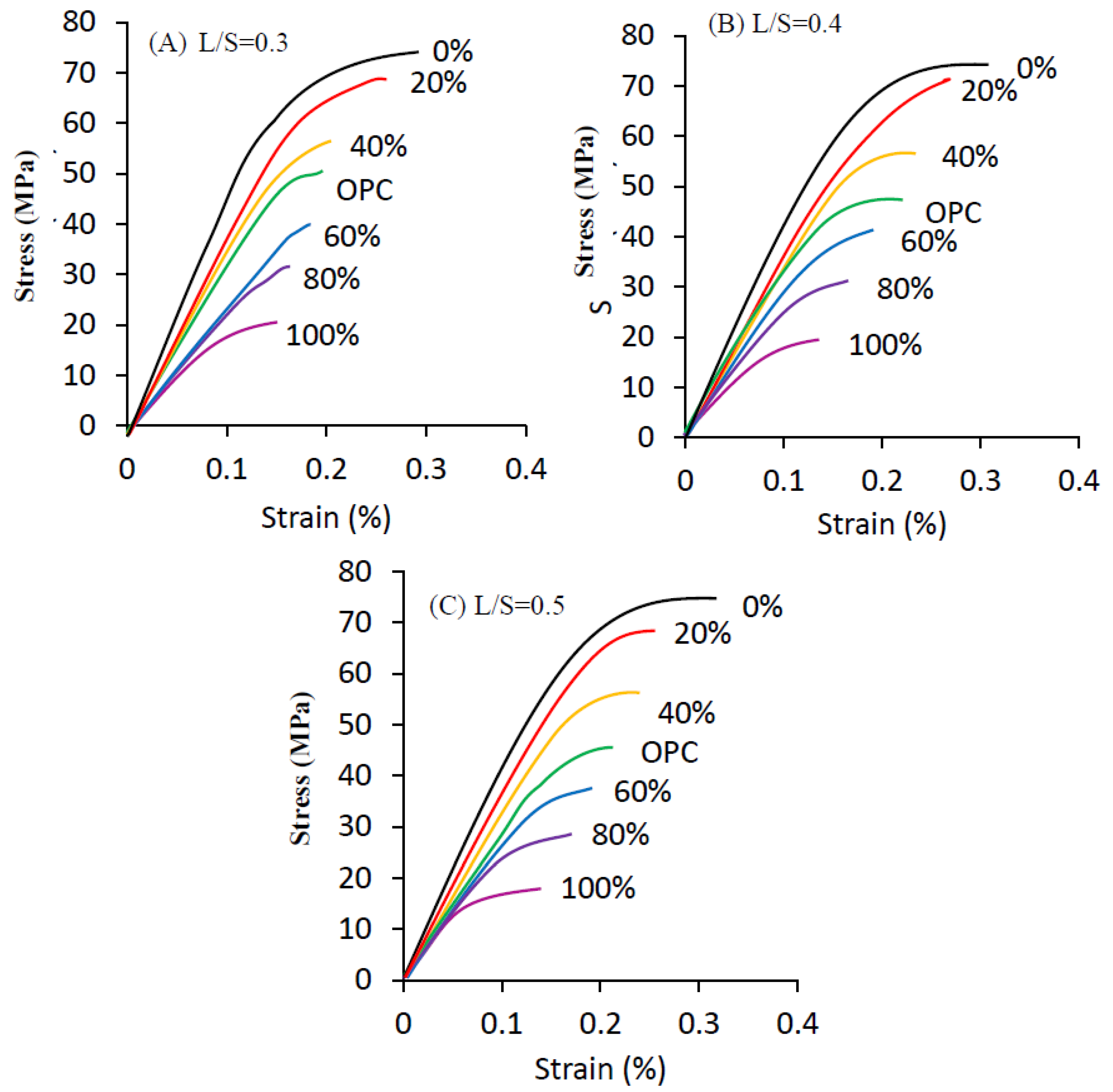
3.4. Stress–Strain Behavior
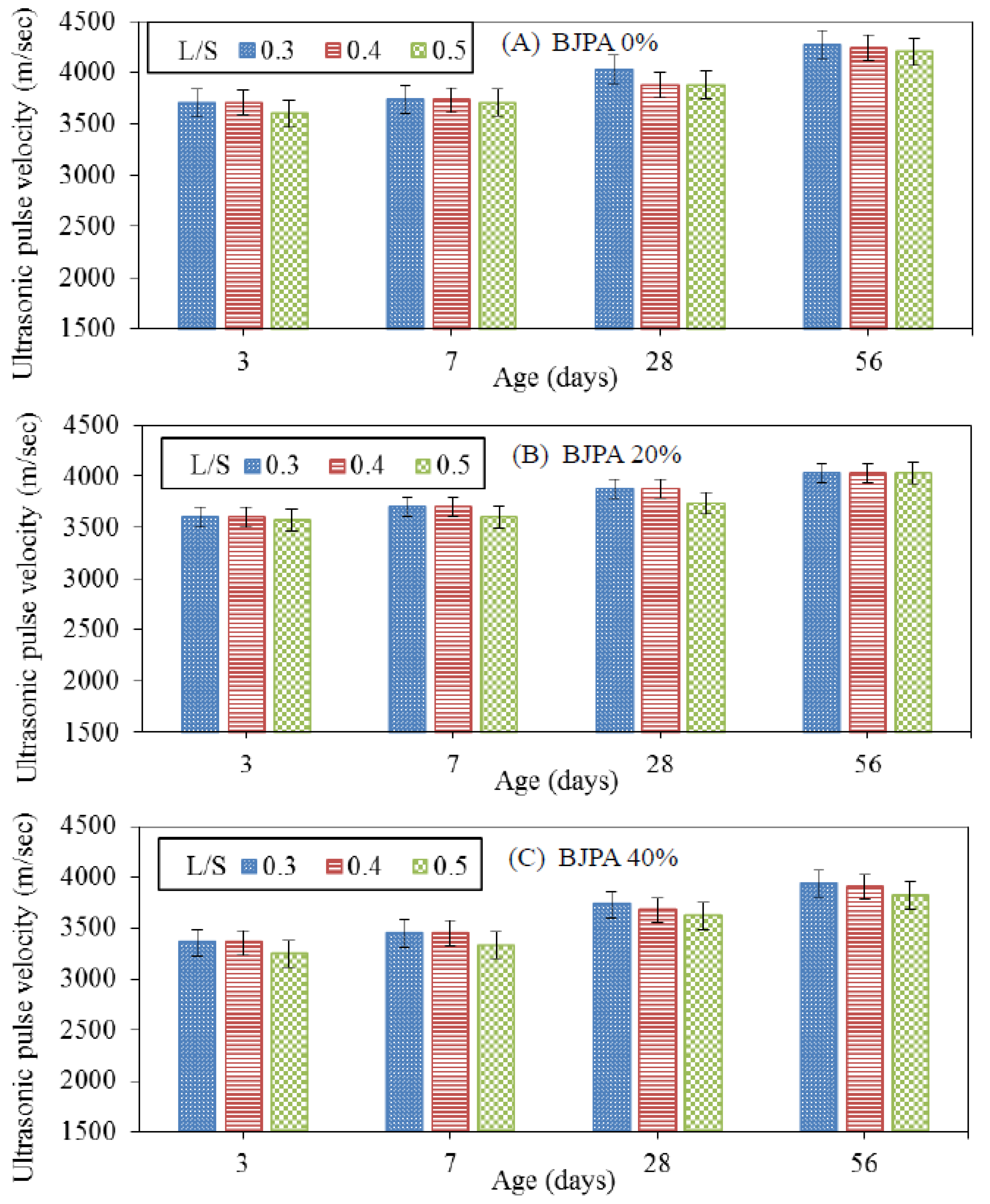
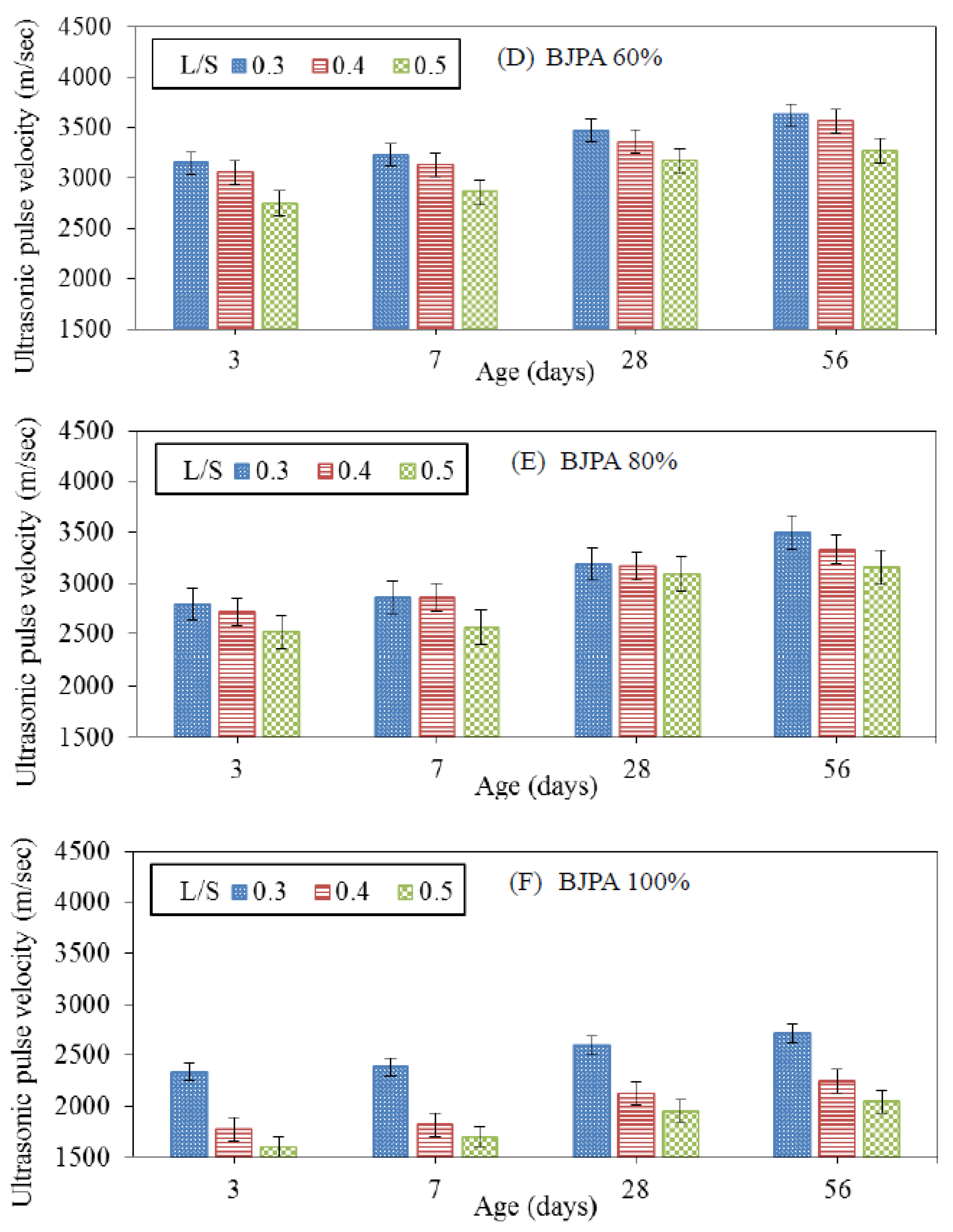
3.5. Ultrasonic Pulse Velocity
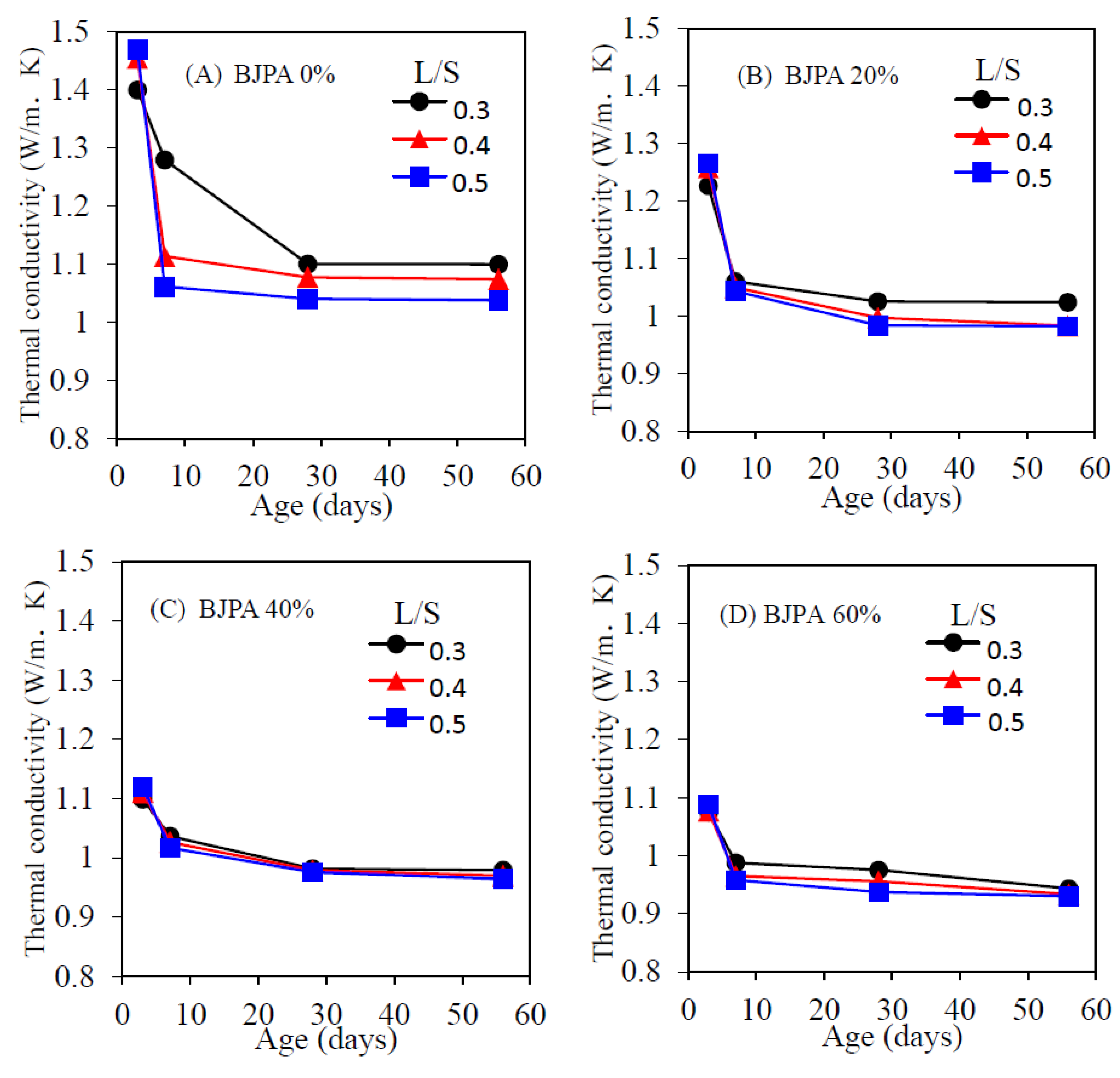
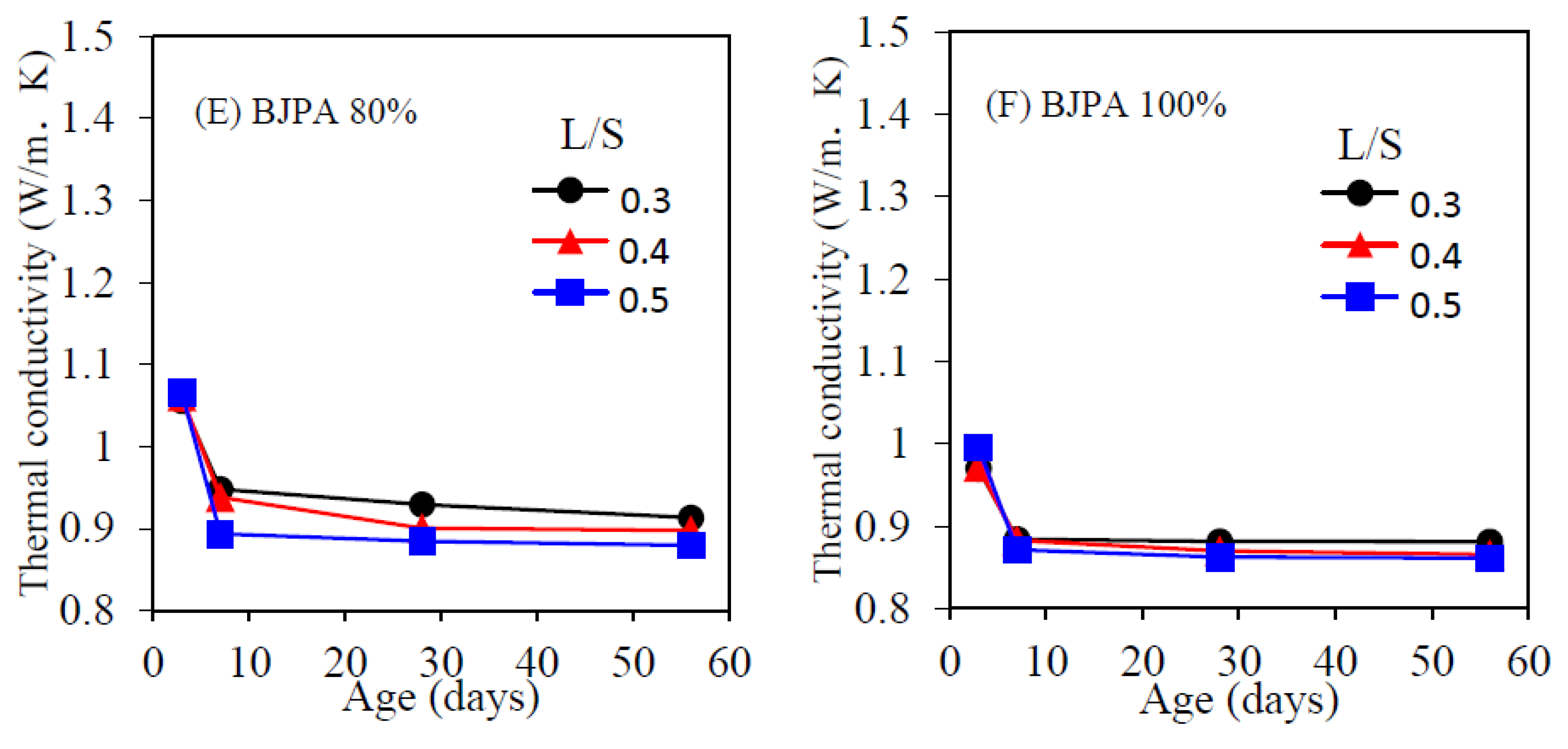
3.6. Thermal Conductivity Coefficient
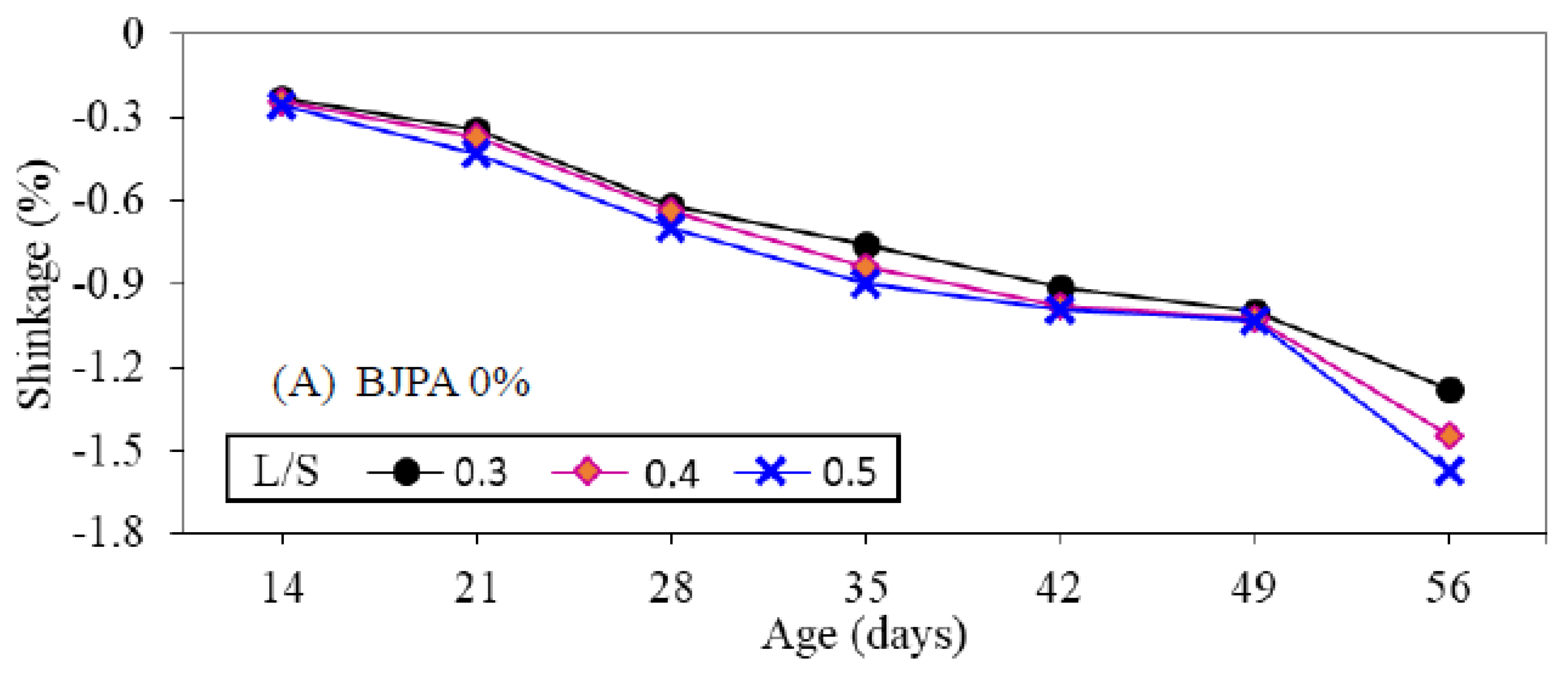
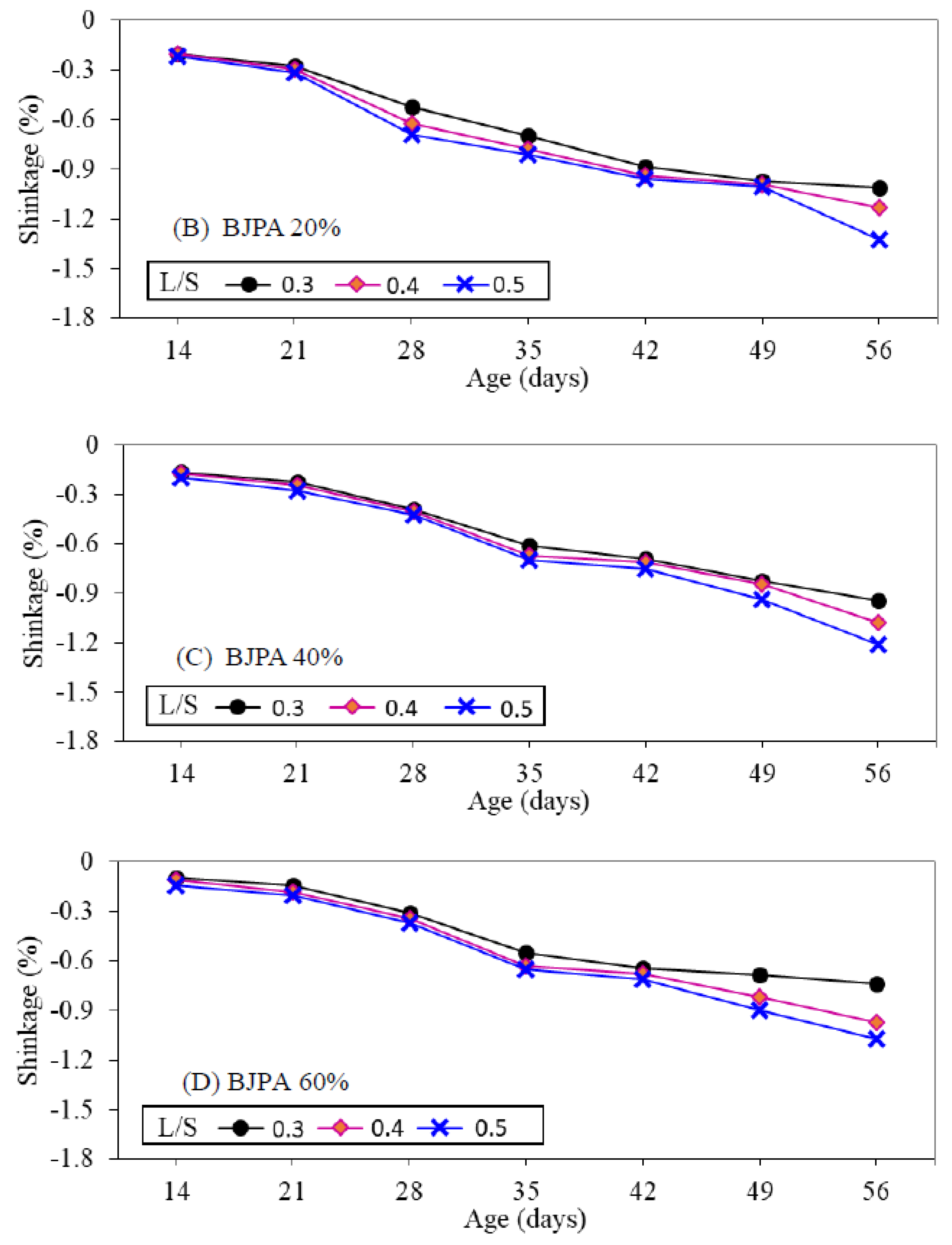
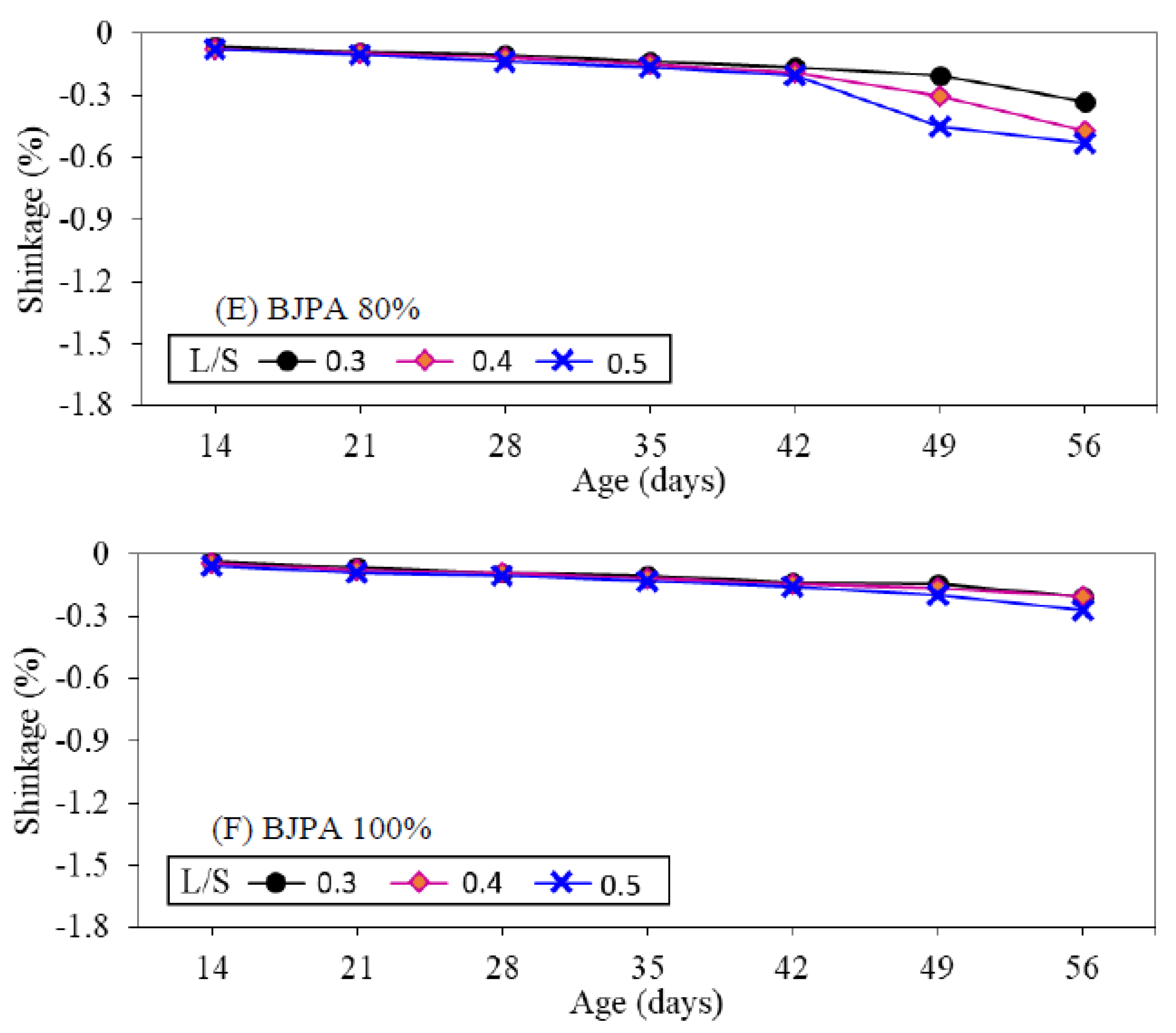
3.7. Shrinkage
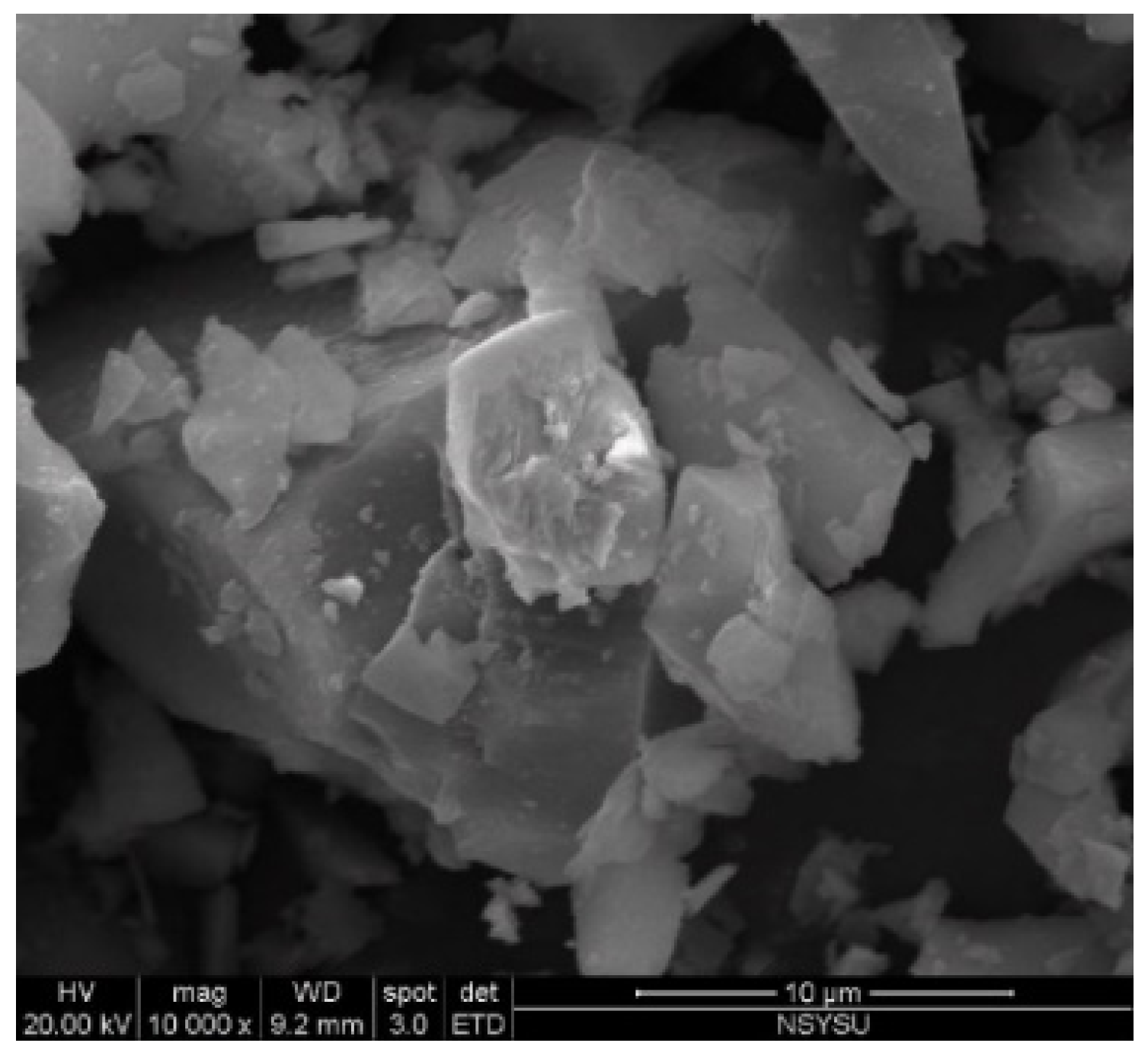
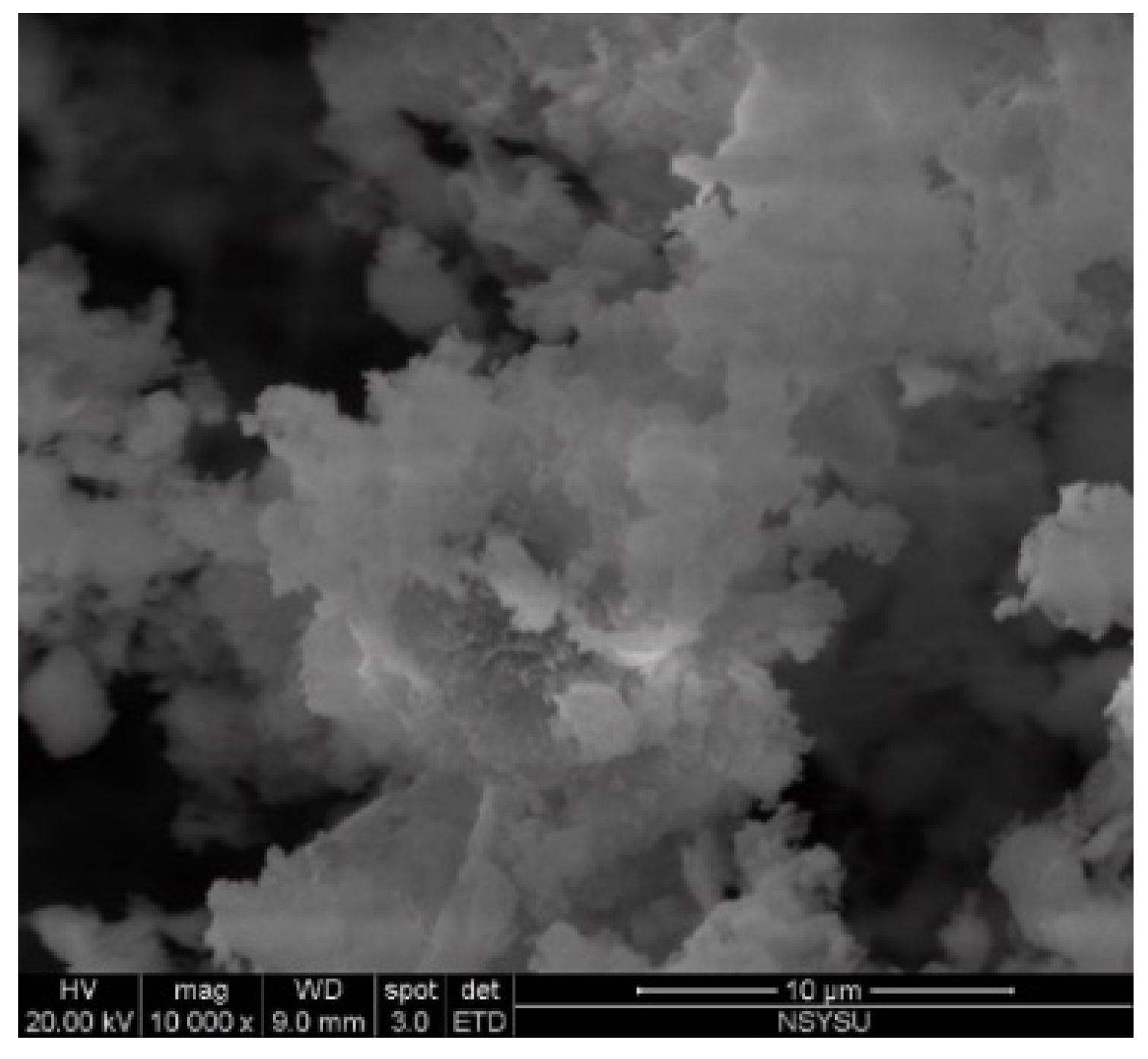
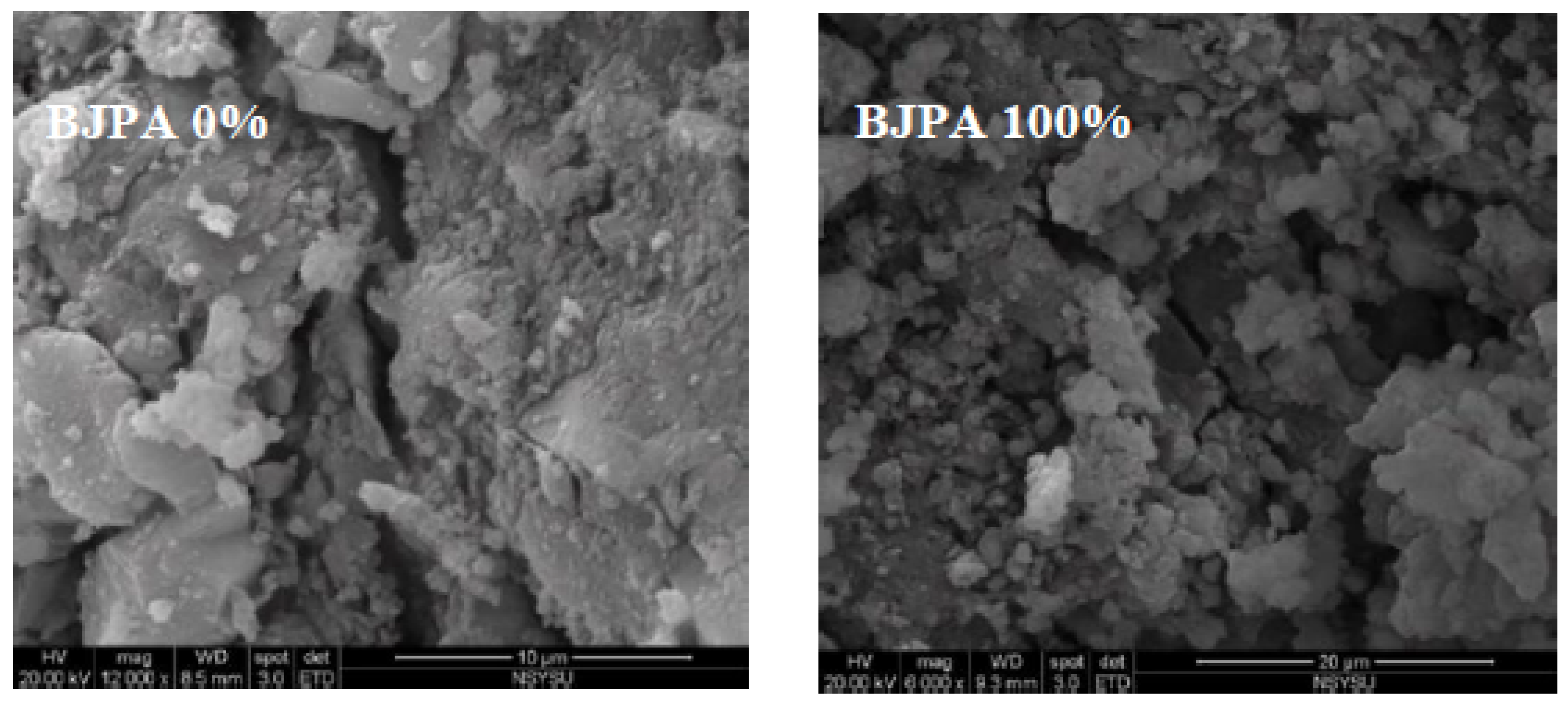
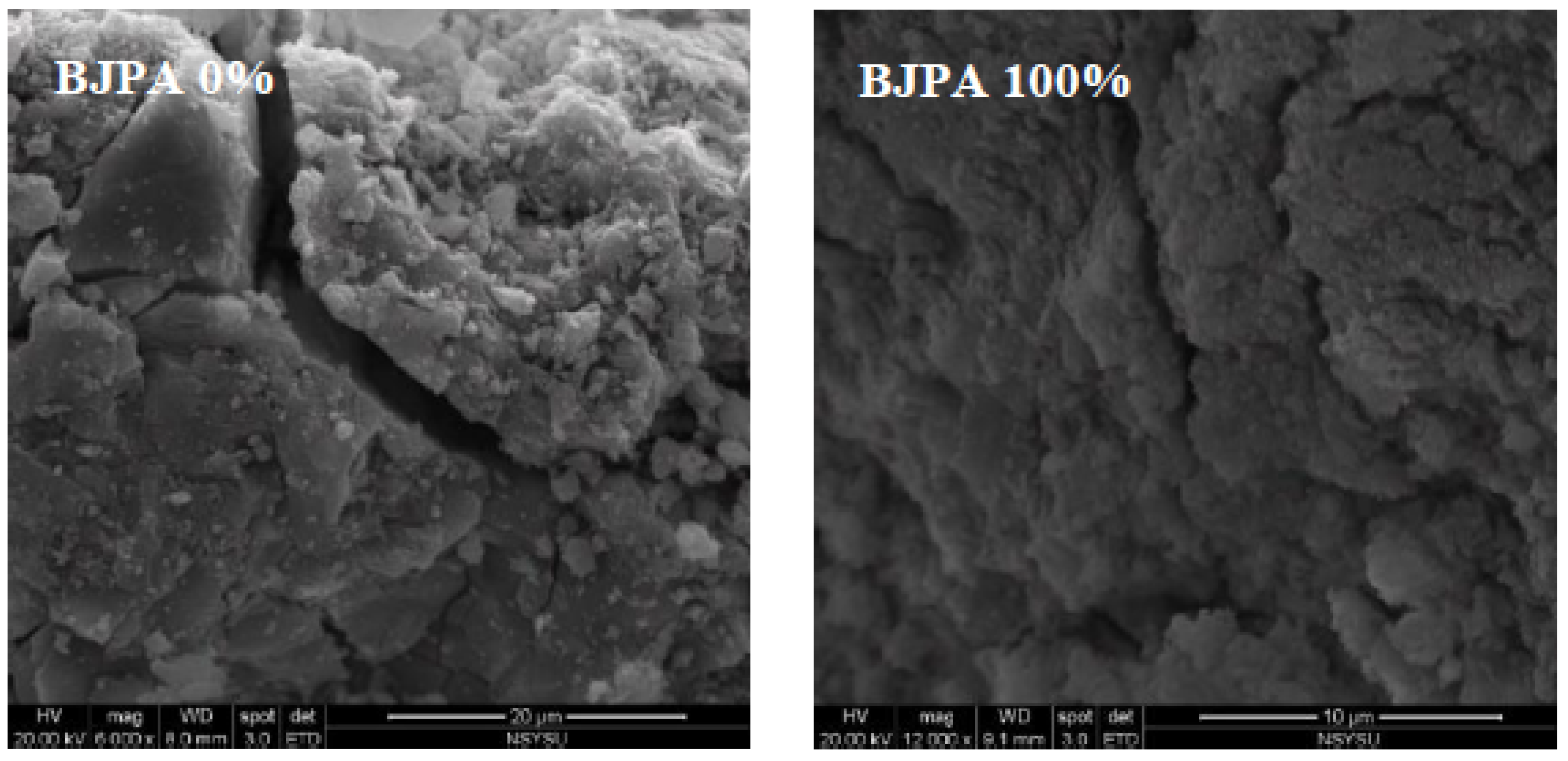
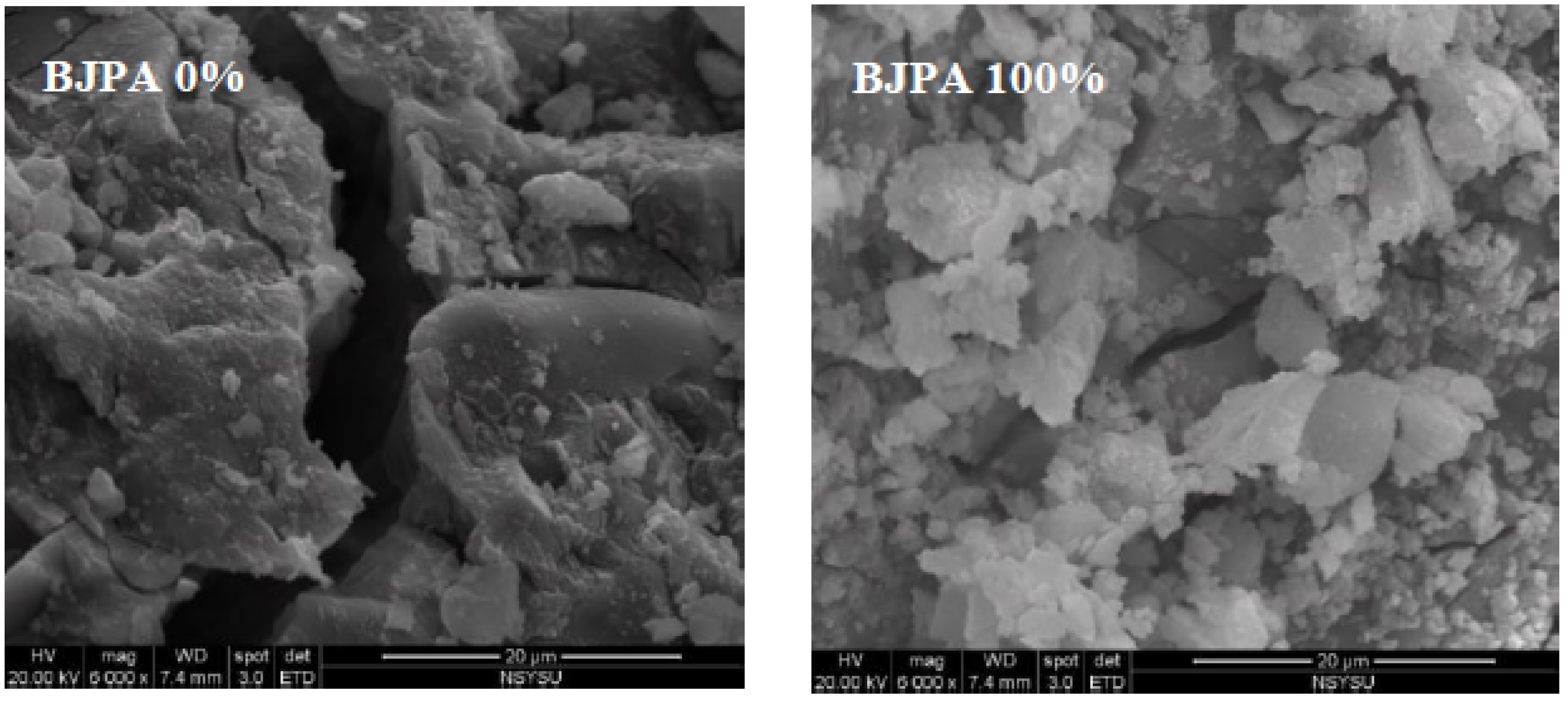
3.8. Scanning Electron Microscope(SEM).
4. Conclusions
- The setting time of the BJPA–GGBFS composite geopolymer increased with a rise in the L/S and BJPA-substitution ratios. An L/S of 0.5 and BJPA-substitution ratio of 100% resulted in the longest initial (126 min) and final (183 min) setting times;
- The slump flow value dramatically decreased at BJPA-substitution ratios of 60%, 80% and 100%, whereas the slump value dropped with a rise in the L/S. The optimal slump value was 11.7 cm and the slump flow value was 21.6 cm;
- At an L/S of 0.3 and a BJPA-substitution ratio of 0%, the maximum compressive strength of a 3-day geopolymer was 33.4 MPa; the maximum compressive strength of a 56-day geopolymer was 81.1 MPa and the strength decreased by 8.5–21.5% with the increasing BJPA-substitution ratio;
- In terms of stress–strain behavior, an L/S of 0.3 and BJPA-substitution ratio of 0% obtained a maximum strain of 0.41% and a maximum modulus of elasticity of 53.1 GPa. BJPA-substitution ratios of 0%, 20% and 40% all resulted in higher moduli of elasticity than that of OPC;
- The growth trend of ultrasonic pulse velocity was consistent with that of compressive strength. The pores within the specimen reduced compressive strength and ultrasonic pulse velocity, with the highest and lowest velocity being 4274 m/s and 1695 m/s, respectively. In terms of the thermal conductivity coefficient, although the pores within the specimen reduced the compressive strength, they were effective in thermal insulation: a BJPA-substitution ratio of 100% achieved a minimum thermal conductivity coefficient of 0.8614 W/mK;
- The best ratio result of this study is that when L/S = 0.3, the BJPA substitution amount is 10%;
- The SEM revealed that crack size decreased with an increase in the BJPA-substitution ratio, with the maximum and minimum sizes being 5.80 μm and 176.8 nm, respectively. Because the BJPA was unable to undergo full polymerization, the unreacted and incompletely reacted BJPA indirectly filled the cracks formed by the GGBFS during polymerization.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Islam, A.; Alengaram, U.J.; Jumaat, M.Z.; Bashar, I.I. The development of compressive strength of ground granulated blast furnace slag-palm oil fuel ash-fly ash based geopolymer mortar. Mater. Des. 2014, 56, 833–841. [Google Scholar] [CrossRef]
- Sata, V.; Sathonsaowaphak, A.; Chindaprasirt, P. Resistance of lignite bottom ash geopolymer mortar to sulfate and sulfuric acid attack. Cem. Concr. Compos. 2012, 34, 700–708. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; El-Enein, S.A.A.; Khater, H.M.; Hasanein, S.A. Resistance of alkali activated water-cooled slag geopolymer to sulphate attack. Hous. Build. Natl. Res. Cent. 2011, 2, 153–160. [Google Scholar]
- Xu, H.; Van Deventer, J.J.S. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Van Deventer, J.S.J.; Lukey, C.G. Effect of alkali metals on the preferential geopolymerization of stilbite/kaolinite mixtures. Ind. Eng. Chem. Res. 2001, 40, 3749–3756. [Google Scholar] [CrossRef]
- Kalaw, M.E.; Culaba, A.; Hinode, H.; Kurniawan, W.; Gallardo, S.; Promentilla, M.A. Optimizing and Characterizing Geopolymers from Ternary Blend of Philippine Coal Fly Ash, Coal Bottom Ash and Rice Hull Ash. Materials 2016, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lorenzen, L. Factors affecting the immobilization of metals in geopolymerized fly ash. Metall. Mater. Trans. B. 1998, 29, 283–291. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.J. (Eds.) Alkali-Activated Materials: State-of-the-Art Report, RILEM TC 224-AAM; Springer/RILEM: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Kumar, S.; García-Triñanes, P.; Teixeira-Pinto, A.; Bao, M. Development of alkali activated cement from mechanically activated silico-manganese (SiMn) slag. Cem. Concr. Compos. 2013, 40, 7–13. [Google Scholar] [CrossRef]
- Shaikh, F.; Haque, S. Behaviour of Carbon and Basalt Fibres Reinforced Fly Ash Geopolymer at Elevated Temperatures. Int. J. Concr. Struct. Mater. 2018, 12, 35. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, V.; Komljenović, M.; Džunuzović, N.; Ivanović, T.; Miladinović, Z. Immobilization of hexavalent chromium by fly ash-based geopolymers. Compos. B Eng. 2017, 112, 213–223. [Google Scholar] [CrossRef]
- Nuaklong, P.; Sata, V.; Chindaprasirt, P. Influence of recycled aggregate on fly ash geopolymer concrete properties. J. Clean. Prod. 2016, 112, 2300–2307. [Google Scholar] [CrossRef]
- Tashima, M.M.; Akasaki, J.L.; Castaldelli, V.N.; Soriano, L.; Monzó, J.; Payá, J.; Borrachero, M.V. New geopolymeric binder based on fluid catalytic cracking catalyst residue (FCC). Mater. Lett. 2012, 80, 50–52. [Google Scholar] [CrossRef]
- Salih, M.A.; Ali, A.A.A.; Farzadnia, N. Characterization of mechanical and microstructural properties of palm oil fuel ash geopolymer cement paste. Constr. Build. Mater. 2014, 65, 592–603. [Google Scholar] [CrossRef]
- Banfill, P.; Frias, M. Rheology and conduction calorimetry of cement modified with calcined paper sludge. Cem. Concr. Res. 2007, 37, 184–190. [Google Scholar] [CrossRef]
- Santa, R.A.A.B.; Bernardin, A.M.; Riella, H.G.; Kuhnen, N.C. Geopolymer synthetized from bottom coal ash and calcined paper sludge. J. Clean. Prod. 2013, 57, 302–307. [Google Scholar] [CrossRef]
- Andini, S.; Cioffi, R.; Colangelo, F.; Grieco, T.; Montagnaro, F.; Santoro, L. Coal fly ash as raw material for the manufacture of geopolymer-based products. Waste Manage. 2008, 28, 416–423. [Google Scholar] [CrossRef]
- Messina, F.; Ferone, C.; Colangelo, F.; Roviello, G.; Cioffi, R. Alkali activated waste fly ash as sustainable composite: Influence of curing and pozzolanic admixtures on the early-age physico-mechanical properties and residual strength after exposure at elevated temperature. Compos. B Eng. 2018, 132, 161–169. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cem. Concr. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- Grzegorz, A.; Piotr, W.; Krzysztof, S. Geopolymer building composites-technology, properties and perspectives. Ochrona Przed Korozją 2018, 61, 57–61. [Google Scholar]
- Qin, L.; Gao, X.; Chen, T. Recycling of raw rice husk to manufacture magnesium oxysulfate cement based lightweight building materials. J. Clean. Prod. 2018, 191, 220–232. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, J.; Wang, J.; Wang, C.; Huang, P.; Fang, C. Sulfate Resistance of Recycled Aggregate Concrete with GGBS and Fly Ash-Based Geopolymer. Materials 2019, 12, 1247. [Google Scholar] [CrossRef] [Green Version]
- Hlaváček, P.; Šmilauer, V.; Škvára, F.; Kopecký, L.; Šulc, R. Inorganic foams made from alkali-activated fly ash: Mechanical, chemical and physical properties. J. Eur. Ceram. Soc. 2015, 35, 703–709. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, R.; Gong, L.; Li, Y.; Cao, W.; Cheng, X. Development of porous fly-ash-based geopolymer with low thermal conductivity. Mater. Des. 2015, 65, 529–533. [Google Scholar] [CrossRef]
- Yang, T.; Yao, X.; Zhang, Z. Geopolymer prepared with high-magnesium nickel slag: Characterization of properties and microstructure. Constr. Build. Mater. 2014, 59, 188–194. [Google Scholar] [CrossRef]
- Hwang, C.L.; Huynh, T.P. Effect of alkali-activator and rice husk ash content on strength development of fly ash and residual rice husk ash-based geopolymers. Constr. Build. Mater. 2015, 101, 1–9. [Google Scholar] [CrossRef]
- Ngernkham, T.P.; Chindaprasirt, P.; Sata, V.; Hanjitsuwan, S.; Hatanaka, S. The effect of adding nano-SiO2 and nano-Al2O3 on properties of high calcium fly ash geopolymer cured at ambient temperature. Mater. Des. 2014, 55, 58–65. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S. Alkali Activated Materials; Provis, J.L., Ed.; Springer: New York, NY, USA, 2014. [Google Scholar]
- Karakoç, M.B.; Türkmen, İ.; Maraş, M.M.; Kantarci, F.; Demirboğa, R. Sulfate resistance offerrochrome slag based geopolymer concrete. Ceram. Int. 2016, 42, 1254–1260. [Google Scholar] [CrossRef]
- Huang, G.H. Investigations of Burning Joss Papers Ash on Engineering Properties of Cement-Based Materials under Various Curing Temperature. MS. Thesis, National Kaohsiung University of Applied Sciences, Kaohsiung, Taiwan, 2015. [Google Scholar]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Lee, N.K.; Jang, J.G.; Lee, H.K. Shrinkage characteristics of alkali-activated fly ash/slag paste and mortar at early ages. Cem. Concr. Compos. 2014, 53, 239–248. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Effect of pore size distribution on drying shrinkage properties of alkali—Activated slag concrete. Cem. Concr. Res. 2000, 30, 1401–1406. [Google Scholar] [CrossRef]
- Chiu, M. Effect of Ritual Bill Ash Substitute to Part Cement and Fine Aggregate on Properties of Cement Paste and Mortar; National Kaohsiung University of Applied Sciences: Kaohsiung, Taiwan, 2009. [Google Scholar]
- Bouvet, A.; Ghorbel, E.; Ghorbel, E. The mini-conical slump flow test: Analysis and numerical study. Cem. Concr. Res. 2010, 40, 1517–1523. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Wang, Y.; Feng, J. Preparation and Properties of Alkali Activated Metakaolin-Based Geopolymer. Materials 2016, 9, 767. [Google Scholar] [CrossRef] [Green Version]
- ASTM C1254. Standard Test Method for Determination of Uranium in Mineral Acids by X-Ray Fluorescence; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM C191. Standard Test Method for Time of Setting of Hydraulic Cement by Vicat Needle; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- ASTM C109. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- ASTM C597. Standard Test Method for Pulse Velocity Through Concrete; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM C157. Standard Test Method for Length Change of Hardened Hydraulic-Cement Mortar and Concrete; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM C518. Standard Test Method for Steady-State Thermal Transmission Properties by Means of the Heat Flow Meter Apparatus; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Singh, B.; Rahman, M.R.; Paswan, R.; Bhattacharyya, S.K. Effect of activator concentration on the strength, ITZ and drying shrinkage of fly ash/slag geopolymer concrete. Constr. Build Mater. 2016, 118, 171–179. [Google Scholar] [CrossRef]
- Perná, I.; Hanzlícˇek, T. The setting time of a clay-slag geopolymer matrix: The influence of blast-furnace-slag addition and the mixing method. J. Clean Prod. 2016, 112, 1150–1155. [Google Scholar] [CrossRef]
- Ikmal, H.A.; Mohd, M.A.B.A.; Mohd, M.A.A.S.; Emy, A.A.; Jitrin, C.; Andrei, V.S. Strength development of solely ground granulated blast furnace slag geopolymers. Constr. Build. Mater. 2020, 250, 118720. [Google Scholar]


| NaOH | NaCO3 | NaCl | Fe2O3 | SiO2 | Na2O | SiO2/Na2O | |
|---|---|---|---|---|---|---|---|
| Sodium hydroxide | 98.20 | 0.165 | 0.0135 | 0.0004 | – | – | – |
| Sodium silicate | – | – | – | 0.2 | 28 | 9 | 3.11 |
| Material | Specific Surface (cm2/g) | Specific Gravity | Baume Scale | Particle Size (µm) |
|---|---|---|---|---|
| GGBFS | 4000 | 2.80 | – | 45 |
| BJPA | 6753 | 2.75 | – | 0.1 |
| Sodium hydroxide | – | 0.598 | – | – |
| Sodium silicate | – | 1.38 | 37 | – |
| Material | CaO | SiO2 | Al2O3 | MgO | Na2O | K2O | SO3 | Fe2O3 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| GGBFS | 42.11 | 33.65 | 13.67 | 6.53 | 0.57 | 0.57 | 1.87 | 1.18 | 0.58 |
| BJPA | 62.83 | 15.98 | 9.39 | 3.24 | 2.82 | 0.39 | 2.46 | 1.13 | 10.7 |
| BJPA (%) | ||||||
|---|---|---|---|---|---|---|
| L/S | 0 | 20 | 40 | 60 | 80 | 100 |
| 0.3 | 53.1 | 46.5 | 42.1 | 34.8 | 29.6 | 22.5 |
| 0.4 | 51.5 | 47.2 | 42.2 | 35.8 | 31.4 | 27.4 |
| 0.5 | 43.2 | 40.2 | 33.6 | 24.6 | 29 | 29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, W.-T.; Juang, C.-U.; Chen, Z.-R. Effect of Burn Joss Paper Ash on Properties of Ground-Granulated Blast Furnace-Based Slag Geopolymer. Appl. Sci. 2020, 10, 4877. https://doi.org/10.3390/app10144877
Kuo W-T, Juang C-U, Chen Z-R. Effect of Burn Joss Paper Ash on Properties of Ground-Granulated Blast Furnace-Based Slag Geopolymer. Applied Sciences. 2020; 10(14):4877. https://doi.org/10.3390/app10144877
Chicago/Turabian StyleKuo, Wen-Ten, Chuen-Ul Juang, and Zhi-Rong Chen. 2020. "Effect of Burn Joss Paper Ash on Properties of Ground-Granulated Blast Furnace-Based Slag Geopolymer" Applied Sciences 10, no. 14: 4877. https://doi.org/10.3390/app10144877





