The Effect of a 7-Week Training Period on Changes in Skin NADH Fluorescence in Highly Trained Athletes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Training Characteristics
2.3. Study Design
2.4. Incremental Exercise Test
2.5. Lactic Acid Measurements
2.6. Anthropometric Measure
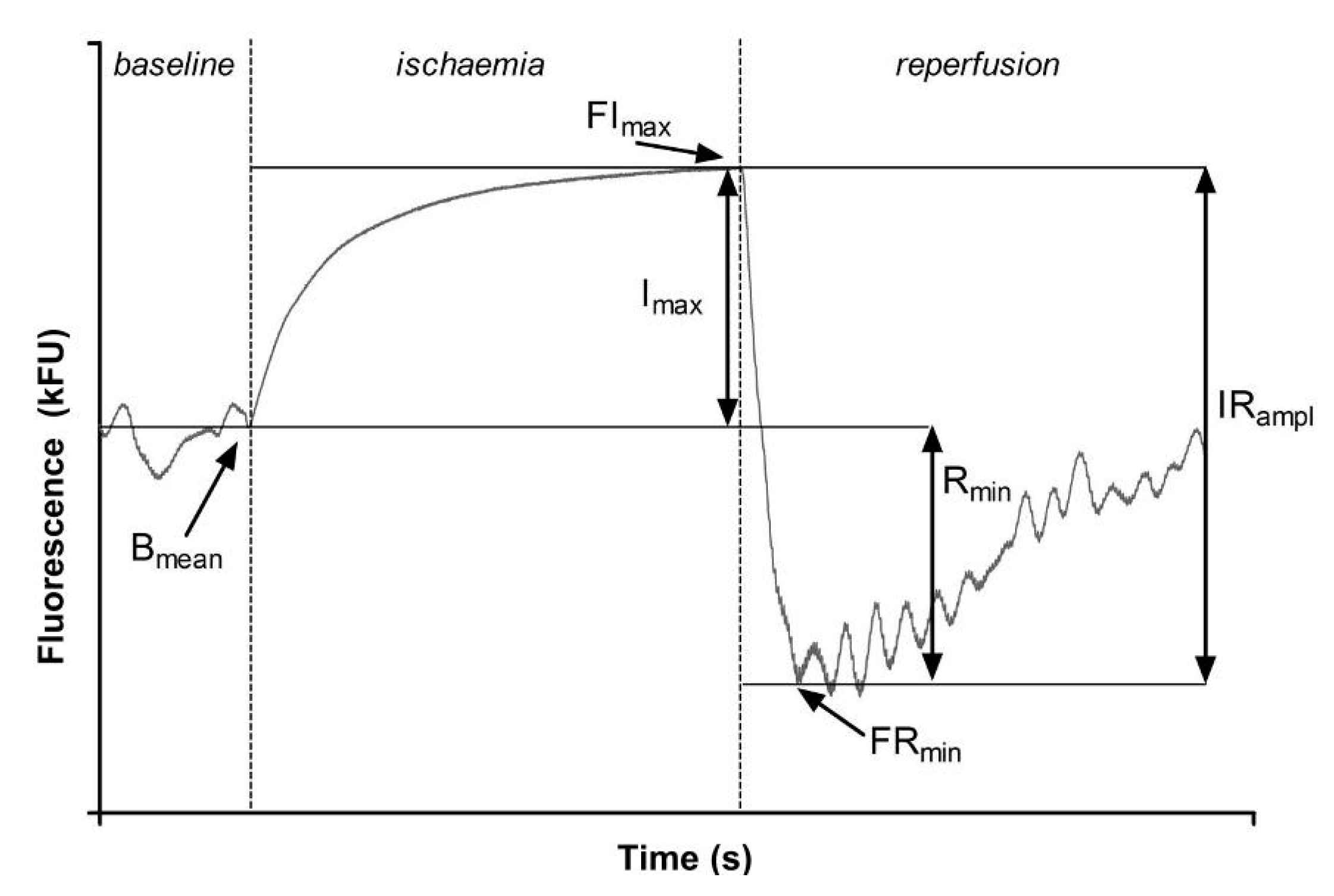
2.7. Nicotinamide Adenine Dinucleotide Fluorescence
- Bmean—Basal fluorescence at the wavelength of 460 nm, recorded at rest at the beginning of the measurement;
- FImax—The maximal increase in fluorescence above the baseline observed during forearm ischemia;
- FRmin—The maximal drop in fluorescence below the baseline observed during reperfusion;
- Imax—The relative increase in fluorescence = the difference between Imax and Bmean;
- Rmin—The relative drop in fluorescence = the difference between Bmean and FRmean;
- IRampl—The maximal range of changes in fluorescence = the sum of Rmin and Imax; and
- CImax—The relative (percentage) contribution of Imax to IRampl [7].
3. Results
3.1. Basic Characteristics
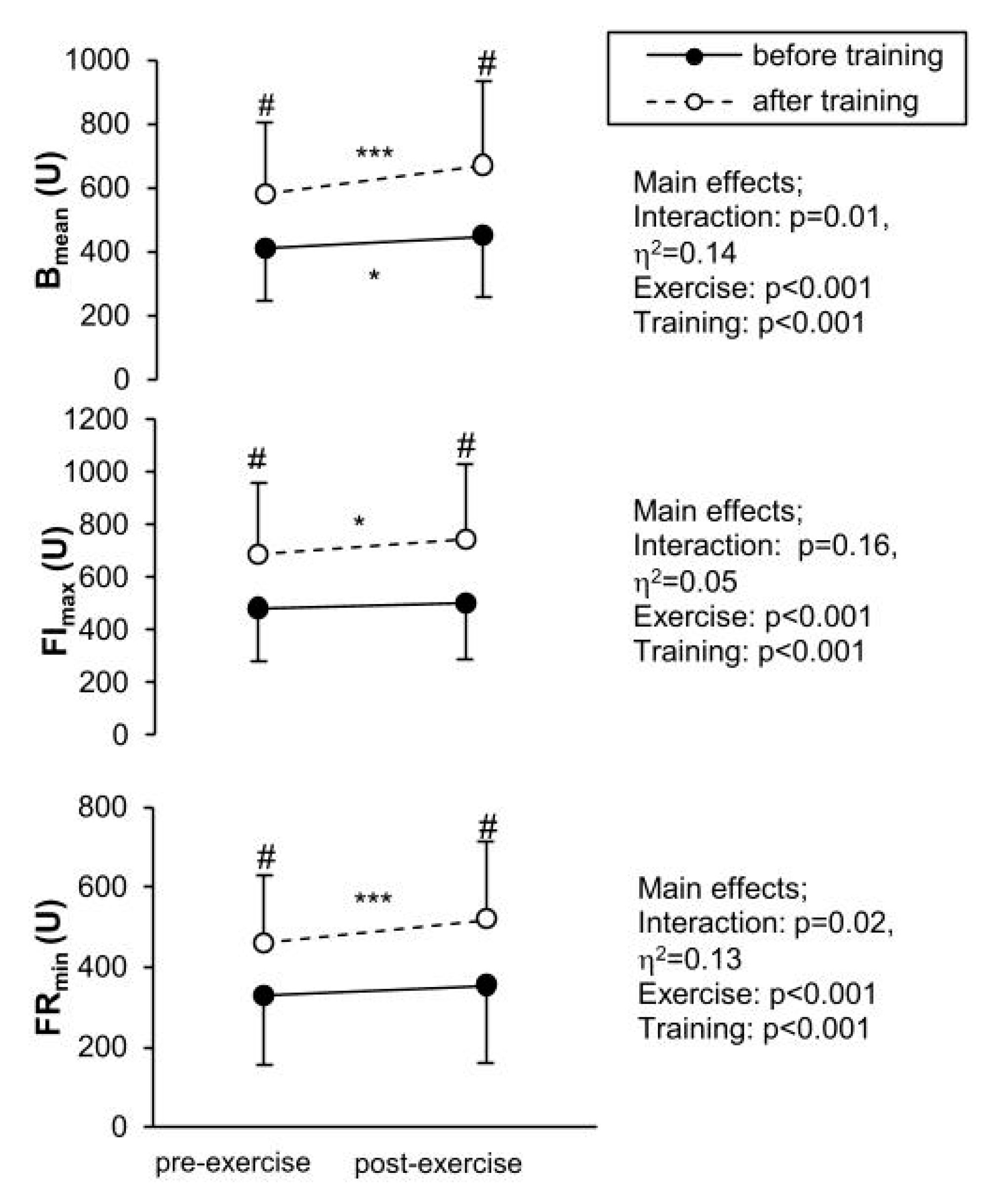
3.2. Measured Parameters
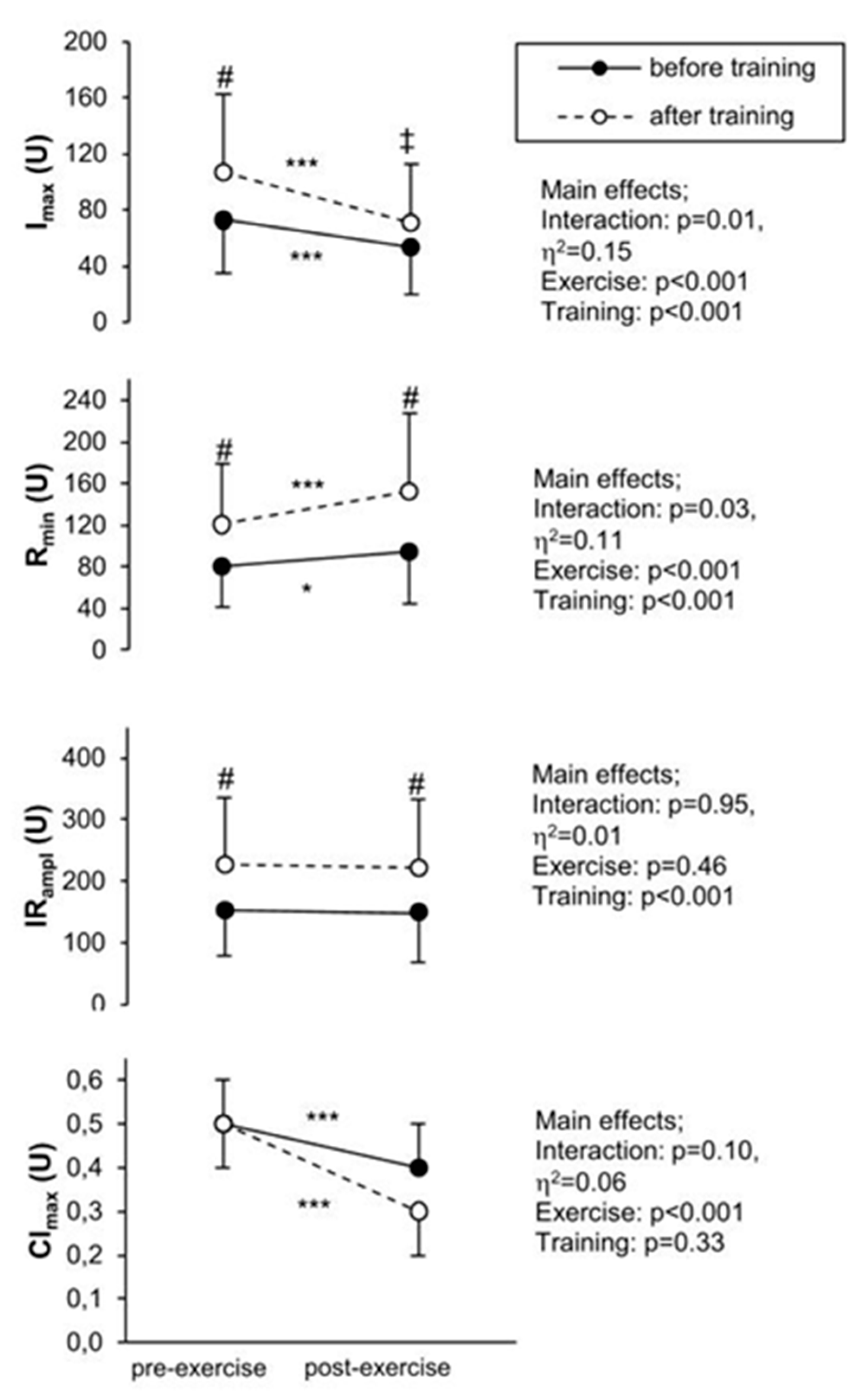
3.3. Calculated Parameters
4. Discussion
4.1. The Effect of Training
4.2. Exercise Response
4.3. Practical Application
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mayevsky, A.; Barbiro-Michaely, E. Use of NADH fluorescence to determine mitochondrial function in vivo. Int. J. Biochem. Cell Biol. 2009, 41, 1977–1988. [Google Scholar] [CrossRef]
- Palero, J.A.; Bader, A.N.; Gerritsen, H.C. In vivo monitoring of protein-bound and free NADH during ischemia by nonlinear spectral imaging microscopy. Biomed. Opt. Express 2011, 2, 1030–1039. [Google Scholar] [CrossRef] [Green Version]
- Pappajohn, D.J.; Penneys, R.; Chance, B. NADH spectrofluorometry of rat skin. J. Appl. Physiol. 1972, 33, 684–687. [Google Scholar] [CrossRef]
- Balu, M.; Mazhar, A.; Hayakawa, C.K.; Mittal, R.; Krasieva, T.B.; König, K.; Venugopalan, V.; Tromberg, B.J. In vivo multiphoton NADH fluorescence reveals depth-dependent keratinocyte metabolism in human skin. Biophys. J. 2013, 104, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Bugaj, O.; Zieliński, J.; Kusy, K.; Kantanista, A.; Wieliński, D.; Guzik, P. The effect of exercise on the skin content of the reduced form of NAD and its response to transient ischemia and reperfusion in highly trained athletes. Front. Physiol. 2019, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- Katarzynska, J.; Lipinski, Z.; Cholewinski, T.; Piotrowski, L.; Dworzynski, W.; Urbaniak, M.; Borkowska, A.; Cypryk, K.; Purgal, R.; Marcinek, A.; et al. Non-invasive evaluation of microcirculation and metabolic regulation using flow mediated skin fluorescence (FMSF): Technical aspects and methodology. Rev. Sci. Instrum. 2019, 90, 104104. [Google Scholar] [CrossRef]
- Sibrecht, G.; Bugaj, O.; Filberek, P.; Niziński, J.; Kusy, K.; Zieliński, J.; Guzik, P. Flow-Mediated Skin Flurescence method for non-invasive measurement of the NADH at 460 nm—A possibility to assess the mitochondrial function. Postepy Biol. Komorki 2017, 44, 333–352. [Google Scholar] [CrossRef]
- White, A.T.; Schenk, S. NAD+/NADH and skeletal muscle mitochondrial adaptations to exercise. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E308–E321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayevsky, A.; Chance, B. Oxidation-reduction states of NADH in vivo: From animals to clinical use. Mitochondrion 2007, 7, 330–339. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.M.; Kudej, R.K.; LaNoue, K.F.; Vatner, S.F.; Lewandowski, E.D. Limited transfer of cytosolic NADH into mitochondria at high cardiac workload. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2237–H2242. [Google Scholar] [CrossRef] [Green Version]
- Rajman, L.; Chwalek, K.; Sinclair, D.A. Therapeutic potential of NAD-boosting molecules: The in vivo evidence. Cell Metab. 2018, 27, 529–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayevsky, A.; Rogatsky, G.G. Mitochondrial function in vivo evaluated by NADH fluorescence: From animal models to human studies. Am. J. Physiol Cell Physiol 2007, 292, C615–C640. [Google Scholar] [CrossRef] [PubMed]
- Duboc, D.; Muffat-Joly, M.; Renault, G.; Degeorges, M.; Toussaint, M.; Pocidalo, J.J. In situ NADH laser fluorimetry of rat fast- and slow-twitch muscles during tetanus. J. Appl. Physiol. 1988, 64, 2692–2695. [Google Scholar] [CrossRef] [PubMed]
- Jobsis, F.F.; Stainsby, W.N. Oxidation of NADH during contractions of circulated mammalian skeletal muscle. Respir. Physiol. 1968, 4, 292–300. [Google Scholar] [CrossRef]
- Sahlin, K.; Katz, A.; Henriksson, J. Redox state and lactate accumulation in human skeletal muscle during dynamic exercise. Biochem. J. 1987, 245, 551–556. [Google Scholar] [CrossRef] [Green Version]
- Koltai, E.; Szabo, Z.; Atalay, M.; Boldogh, I.; Naito, H.; Goto, S.; Nyakas, C.; Radak, Z. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech. Ageing Dev. 2010, 131, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Boushel, R.; Gnaiger, E.; Larsen, F.J.; Helge, J.W.; Gonzalez-Alonso, J.; Ara, I.; Munch-Andersen, T.; van Hall, G.; Søndergaard, H.; Saltin, B.; et al. Maintained peak leg and pulmonary VO2 despite substantial reduction in muscle mitochondrial capacity. Scand. J. Med. Sci. Sports 2015, 25, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Guadalupe-Grau, A.; Fernández-Elías, V.E.; Ortega, J.F.; Dela, F.; Helge, J.W.; Mora-Rodriguez, R. Effects of 6-month aerobic interval training on skeletal muscle metabolism in middle-aged metabolic syndrome patients. Scand. J. Med. Sci. Sports 2018, 28, 585–595. [Google Scholar] [CrossRef]
- Granata, C.; Jamnick, N.A.; Bishop, D.J. Training-Induced Changes in Mitochondrial Content and Respiratory Function in Human Skeletal Muscle. Sports Med. 2018, 48, 1809–1828. [Google Scholar] [CrossRef]
- Crane, J.D.; MacNeil, L.G.; Lally, J.S.; Ford, R.J.; Bujak, A.L.; Brar, I.K.; Kemp, B.E.; Raha, S.; Steinberg, G.R.; Tarnopolsky, M.A. Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell 2015, 14, 625–634. [Google Scholar] [CrossRef]
- Busquets-Cortés, C.; Capó, X.; Martorell, M.; Tur, J.A.; Sureda, A.; Pons, A. Training and acute exercise modulates mitochondrial dynamics in football players’ blood mononuclear cells. Eur. J. Appl. Physiol. 2017, 117, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.C.; Wilson, R.J.; Yan, Z. Molecular mechanisms for mitochondrial adaptation to exercise training in skeletal muscle. FASEB J. 2016, 30, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.A.; Lundby, C. Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. J. Appl. Physiol. 2013, 114, 344–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, R.A.; Flück, D.; Bonne, T.C.; Bürgi, S.; Christensen, P.M.; Toigo, M.; Lundby, C. Improvements in exercise performance with high-intensity interval training coincide with an increase in skeletal muscle mitochondrial content and function. J. Appl. Physiol. 2013, 115, 785–793. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Lira, V.A.; Greene, N.P. Exercise training-induced regulation of mitochondrial quality. Exerc. Sport Sci. Rev. 2012, 40, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Di Donato, D.M.; West, D.W.D.; Churchward-Venne, T.A.; Breen, L.; Baker, S.K.; Phillips, S.M. Influence of aerobic exercise intensity on myofibrillar and mitochondrial protein synthesis in young men during early and late postexercise recovery. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1025–E1032. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.M.; Dasari, S.; Konopka, A.R.; Johnson, M.L.; Manjunatha, S.; Esponda, R.R.; Carter, R.E.; Lanza, I.R.; Nair, K.S. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab. 2017, 25, 581–592. [Google Scholar] [CrossRef] [Green Version]
- Edvardsen, E.; Hem, E.; Anderssen, S.A. End criteria for reaching maximal oxygen uptake must be strict and adjusted to sex and age: A cross-sectional study. PLoS ONE 2014, 9, e85276. [Google Scholar] [CrossRef] [Green Version]
- Dunaev, A.V.; Dremin, V.V.; Zherebtsov, E.A.; Rafailov, I.E.; Litvinova, K.S.; Palmer, S.G.; Stewart, N.A.; Sokolovski, S.G.; Rafailov, E.U. Individual variability analysis of fluorescence parameters measured in skin with different levels of nutritive blood flow. Med. Eng. Phys. 2015, 37, 574–583. [Google Scholar] [CrossRef]
- Zarębska, E.A.; Kusy, K.; Słomińska, E.M.; Kruszyna, Ł.; Zieliński, J. Alterations in exercise-induced plasma adenosine triphosphate concentration in highly trained athletes in a one-year training cycle. Metabolites 2019, 9, 230. [Google Scholar] [CrossRef] [Green Version]
- Włodarczyk, M.; Kusy, K.; Słomińska, E.M.; Krasiński, Z.; Zieliński, J. Change in lactate, ammonia, and hypoxanthine concentrations in a 1-year training cycle in highly trained athletes: Applying biomarkers as tools to assess training status. J. Strength Cond. Res. 2020, 34, 355–364. [Google Scholar] [CrossRef]
- Wilkinson, S.B.; Phillips, S.M.; Atherton, P.J.; Patel, R.; Yarasheski, K.E.; Tarnopolsky, M.A.; Rennie, M.J. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J. Physiol. 2008, 586, 3701–3717. [Google Scholar] [CrossRef]
- Finsterer, J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet. Dis. 2012, 13, 218. [Google Scholar] [CrossRef] [Green Version]
- Kane, A.; Sinclair, D. Sirtuins and NADC in the development and treatment of metabolic and cardiovascular diseases. Circ. Res. 2018, 123, 868–885. [Google Scholar] [CrossRef]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R502–R516. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.; Tarnawska, M.; Dudziak, M.; Dorniak, K.; Roustit, M.; Cracowski, J.L. Reproducibility of flow mediated skin fluorescence to assess microvascular function. Microvasc. Res. 2017, 113, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Charkoudian, N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. J. Appl. Physiol. 2010, 109, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Chudecka, M.; Lubkowska, A. The use of thermal imaging to evaluate body temperature changes of athletes during training and a study on the impact of physiological and morphological factors on skin temperature. Hum. Mov. 2012, 13, 33–39. [Google Scholar] [CrossRef]
- Simmons, G.H.; Wong, B.J.; Holowatz, L.A.; Kenney, W.L. Changes in the control of skin blood flow with exercise training: Where do cutaneous vascular adaptations fit in? Exp. Physiol. 2011, 96, 822–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roche, D.M.; Rowland, T.W.; Garrard, M.; Marwood, S.; Unnithan, V.B. Skin microvascular reactivity in trained adolescents. Eur. J. Appl. Physiol. 2010, 108, 1201–1208. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Before Training | After Training |
|---|---|---|
| Age (years) | 22.4 ± 4 | − |
| Training experience (years) | 8 ± 2.3 | − |
| Height (cm) | 178.1 ± 7.3 | 178.1 ± 7.3 |
| Weight (kg) | 69.1 ± 10.3 | 69 ± 10.3 |
| BMI (kg/m2) | 21.6 ± 2.3 | 21.6 ± 2.3 |
| SBPrest (mmHg) | 127.6 ± 14.3 | 119.3 ± 10.8 *** |
| DBPrest (mmHg) | 69.9 ± 7.3 | 72.9 ± 9.3 * |
| SBPexerc (mmHg) | 148 ± 18.3 | 139.2 ± 16.3 ** |
| DBPexerc (mmHg) | 74.5 ± 8.1 | 78.2 ± 8.1 * |
| VO2max (mL/min/kg) | 58.8 ± 8.6 | 59.5 ± 8.6 |
| HRpeak (beats/min) | 191.7 ± 8 | 191.9 ± 8.9 |
| LArest (mmol/L) | 1.2 ± 0.5 | 1.0 ± 0.3 ** |
| LAmax (mmol/L) | 9.9 ± 1.5 | 10.2 ± 1.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugaj, O.; Kusy, K.; Kantanista, A.; Korman, P.; Wieliński, D.; Zieliński, J. The Effect of a 7-Week Training Period on Changes in Skin NADH Fluorescence in Highly Trained Athletes. Appl. Sci. 2020, 10, 5133. https://doi.org/10.3390/app10155133
Bugaj O, Kusy K, Kantanista A, Korman P, Wieliński D, Zieliński J. The Effect of a 7-Week Training Period on Changes in Skin NADH Fluorescence in Highly Trained Athletes. Applied Sciences. 2020; 10(15):5133. https://doi.org/10.3390/app10155133
Chicago/Turabian StyleBugaj, Olga, Krzysztof Kusy, Adam Kantanista, Paweł Korman, Dariusz Wieliński, and Jacek Zieliński. 2020. "The Effect of a 7-Week Training Period on Changes in Skin NADH Fluorescence in Highly Trained Athletes" Applied Sciences 10, no. 15: 5133. https://doi.org/10.3390/app10155133
APA StyleBugaj, O., Kusy, K., Kantanista, A., Korman, P., Wieliński, D., & Zieliński, J. (2020). The Effect of a 7-Week Training Period on Changes in Skin NADH Fluorescence in Highly Trained Athletes. Applied Sciences, 10(15), 5133. https://doi.org/10.3390/app10155133







