Synthesis, Characterization and Evaluation of the Antibiofouling Potential of Some Metal and Metal Oxide Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of Nanoparticles (NPs)
2.2.1. Synthesis of Titanium Dioxide Nanoparticles (TiO2-NPs)
2.2.2. Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs)
2.2.3. Synthesis of Copper Oxide Nanoparticle (CuO-NPs)
2.2.4. Synthesis of Silver Nanoparticles (AgNPs)
2.2.5. Synthesis of Ag-Doped Titanium Dioxide Nanoparticles (Ag-TiO2NPs)
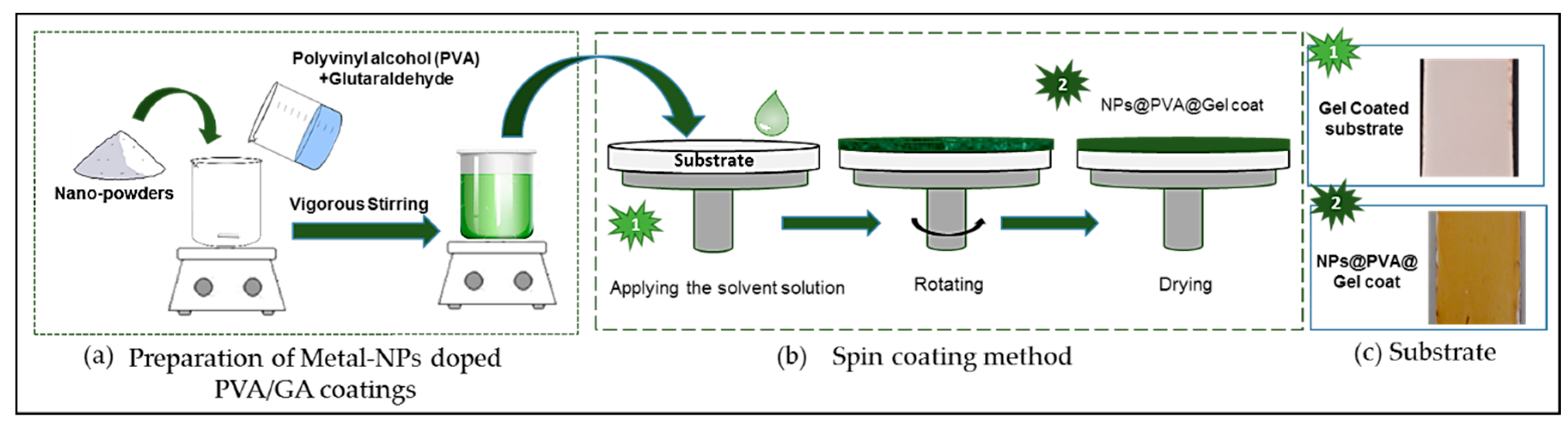
2.3. Coating Procedure
2.4. Characterization Techniques
3. Results and Discussion
3.1. NPs Characterization
3.2. Substrate Characterization
3.3. PVA/GA Conditioning and Characterization
3.4. Substrate Coating
3.4.1. Preparation of Metal-NPs Doped PVA/GA Coatings
3.4.2. Coating Characterization
3.5. Evaluation of the Antifouling Behaviour of NPs Doped Coatings Against Green Algae in Freshwater
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Structural and Morphological Characterization of the Nanoparticles Synthesized

References
- Margaillan, A.; Bressy, C. Fouling release coatings: A nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar]
- Posten, C. Design principles of photo-bioreactors for cultivation of microalgae. Eng. Life Sci. 2009, 9, 165–177. [Google Scholar] [CrossRef]
- Ren, J.; Han, P.; Wei, H.; Jia, L. Fouling-resistant behavior of silver nanoparticle-modi fi ed surfaces against the bioadhesion of microalgae. ACS Appl. Mater. Interfaces 2014, 6, 3829–3838. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Saharudin, K.A.; Sreekantan, S.; Basiron, N.; Khor, Y.L.; Harun, N.H.; Rabiatul, R.B.; Akil, H.M.; Seeni, A.; Vignesh, K. Bacteriostatic activity of LLDPE nanocomposite embedded with sol-gel synthesized TiO2/ZnO coupled oxides at various ratios. Polymers (Basel) 2018, 10, 878. [Google Scholar] [CrossRef] [Green Version]
- Cha, W.; Hyon, S.; Ikada, Y. Transparent poly (viny alcohol) hydrogel with high water content and high strength. Makromol. Chern 1992, 1925, 1913–1925. [Google Scholar] [CrossRef]
- Chem, C.T.; Chang, Y.J.; Chen, C.; Tobolsky, A. V formalized poly (viny alcohol) membranes for reverse osmosis. J. Appl. Polym. Sci. 1973, 17, 789–796. [Google Scholar] [CrossRef]
- Hirai, T.; Asada, Y.; Suzuki, T.; Hayashi, S. Studies on elastic hydrogel membrane. I. Effect of preparation conditions on the membrane performance. J. Appl. Polym. Sci. 1989, 38, 491–502. [Google Scholar] [CrossRef]
- Ibrahim, N.A. Preparation and characterization of carboxylic cation exchange resins from the reaction of poly (viny alcohol) with melamine-formaldehyde and some hydroxy acids. Die Angew. Makromol. Chem. 1993, 210, 7–20. [Google Scholar] [CrossRef]
- Yamaura, K.; Itoh, M.; Tanigami, T. Properties of gels obtained by freezing/thawing of poly (vinyl alcohol)/water/dimethyl sulf oxide solutions. J. Appl. Polym. Sci. 1989, 5, 2709–2718. [Google Scholar] [CrossRef]
- Kumar, R.; Siril, P.F. Preparation and characterization of polyvinyl alcohol stabilized griseofulvin nanoparticles. Mater. Today Proc. 2016, 3, 2261–2267. [Google Scholar] [CrossRef]
- Katz, M.G.; Wydeven, T. Heat-treated membranes. J. Appl. Polym. Sci. 1982, 27, 79–87. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Moreira, A.; Notarfonzo, R. Pervaporation separation of acetic acid-water mixtures using modified membranes. I. Blended polyacrylic acid (PAA). J. Appl. Polym. Sci. 1988, 35, 1191–1200. [Google Scholar] [CrossRef]
- Gebben, B.; van den Berg, H.W.A.; Bargeman, D.; Smolders, C.A. Intramolecular crosslinking of poly(vinyl alcohol). Polymer (Guildf) 1985, 26, 1737–1740. [Google Scholar] [CrossRef] [Green Version]
- Kormeyer, R.W.; Peppas, N.A. Effect of morphology of hydrophilic polimeric matrices on the diffusion and release of water soluble drugs. J. Membr. Sci. 1981, 9, 211–227. [Google Scholar] [CrossRef]
- Gimenez, V.; Mantecon, A.; Cadiz, V. Crosslinking of poly(viny1 alcohol) using dianhydrides as hardeners. J. Appl. Polym. Sci. 1996, 59, 425–431. [Google Scholar] [CrossRef]
- Marin, E.; Rojas, J. Preparation and characterization of crosslinked a films alcohol with waterprof properties. Int. J. Pharm. Pharm. Sci. 2015, 7, 242–248. [Google Scholar]
- Figueiredo, K.C.S.; Alves, T.L.M.; Borges, C.P. Poly (vinyl alcohol) films crosslinked by glutaraldehyde under mild conditions. J. Appl. Polym. Sci. 2008, 111, 3074–3080. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Khan, M.Q.; Kharaghani, D.; Ullah, S.; Waqas, M.; Abbasi, A.M.R.; Saito, Y.; Zhu, C.; Kim, I.S. Self-cleaning properties of electrospun PVA/TiO2 and PVA/ZnO nanofibers composites. Nanomaterials 2018, 8, 644. [Google Scholar] [CrossRef] [Green Version]
- Graziani, L.; Quagliarini, E.; Orazio, M.D. The role of roughness and porosity on the self-cleaning and anti-biofouling efficiency of TiO 2 -Cu and TiO 2 -Ag nanocoatings applied on fired bricks. Constr. Build. Mater. 2016, 129, 116–124. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, C.; Santos-Martinez, M.J.; Radomski, A.; Corrigan, O.I.; Radomski, M.W. Nanoparticles: Pharmacological and toxicological significance. Br. J. Pharmacol. 2007, 150, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wan, Y.; Shi, Y.; Pan, G.; Yan, H.; Xu, J.; Guo, M.; Qin, L.; Liu, Y. Facile modification of thin-film composite nanofiltration membrane with silver nanoparticles for anti-biofouling. J. Polym. Res. 2016, 23, 1–9. [Google Scholar] [CrossRef]
- Loo, C.Y.; Young, P.M.; Lee, W.H.; Cavaliere, R.; Whitchurch, C.B.; Rohanizadeh, R. Non-cytotoxic silver nanoparticle-polyvinyl alcohol hydrogels with anti-biofilm activity: Designed as coatings for endotracheal tube materials. Biofouling 2014, 30, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Rajaeian, B.; Heitz, A.; Tade, M.O.; Liu, S. Improved separation and antifouling performance of PVA thin film nanocomposite membranes incorporated with carboxylated TiO2 nanoparticles. J. Membr. Sci. 2015, 485, 48–59. [Google Scholar] [CrossRef]
- Balta, S.; Sotto, A.; Luis, P.; Benea, L.; Bruggen, B.; Kim, J. A new outlook on membrane enhancement with nanoparticles: The alternative of ZnO. J. Memb. Sci. 2012, 389, 155–161. [Google Scholar] [CrossRef]
- Sawada, I.; Fachrul, R.; Ito, T.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Development of a hydrophilic polymer membrane containing silver nanoparticles with both organic antifouling and antibacterial properties. J. Memb. Sci. 2012, 387–388, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Pourjafar, S.; Rahimpour, A.; Jahanshahi, M. Synthesis and characterization of PVA/PES thin film composite nanofiltration membrane modified with TiO 2 nanoparticles for better performance and surface properties. J. Ind. Eng. Chem. 2012, 18, 1398–1405. [Google Scholar] [CrossRef]
- Hou, X.; Huang, M.; Wu, X.; Liu, A. Preparation and studies of photocatalytic silver-loaded TiO 2 films by hybrid sol—gel method. Chem. Eng. J. 2009, 146, 42–48. [Google Scholar] [CrossRef]
- Sá, J.; Agüera, C.A.; Gross, S.; Anderson, J.A. Applied catalysis B: Environmental photocatalytic nitrate reduction over metal modified TiO 2. Appl. Catal. B Environ. 2009, 85, 192–200. [Google Scholar] [CrossRef]
- Bastús, N.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of highly monodisperse citrate- stabilized silver nanoparticles of up to 200 nm: kinetic control and catalytic properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Pierre, A.C.; Bernard-lyon, U.C. Porous sol-gel ceramics. Ceram. Int. 1997, 23, 229–238. [Google Scholar] [CrossRef]
- Bartley, G.E.; Scolnik, P.A. Plant carotenoids: Pigments for photoprotection, visual attraction, and human health. Plant Cell 1995, 7, 1027–1038. [Google Scholar] [PubMed] [Green Version]
- Abd EI Baky, H.H.; EI-Baroty, G.S. Healthy Benefit of microalgal bioactive substances. J. Aquat. Sci. 2013, 1, 11–23. [Google Scholar]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Guzmán-Velderrain, V.; López, Y.O.; Gutiérrez, J.S.; De Investigación, C.; De Nanotecnología, L.N.; De Materiales, D. TiO 2 films synthesis over polypropylene by Sol-Gel Assisted with hydrothermal treatment for the photocatalytic propane degradation. Green Sustain. Chem. 2014, 4, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Mahshid, S.; Askari, M.; Ghamsari, M.S. Synthesis of TiO2 nanoparticles by hydrolysis and peptization of titanium isopropoxide solution. J. Mater. Process. Technol. 2007, 189, 296–300. [Google Scholar] [CrossRef]
- Hussain, M.; Ceccarelli, R.; Marchisio, D.L.; Fino, D.; Russo, N.; Geobaldo, F. Synthesis, characterization, and photocatalytic application of novel TiO2 nanoparticles. Chem. Eng. J. 2010, 157, 45–51. [Google Scholar] [CrossRef]
- Nyamukamba, P.; Okoh, O.; Mungondori, H.; Taziwa, R.; Zinya, S. Synthetic methods for titanium dioxide nanoparticles: A review. InterchOpen 2018, 8, 151–175. [Google Scholar]
- Ovenstone, J.; Yanagisawa, K. Effect of hydrothermal treatment of amorphous Titania on the Phase change from anatase to rutile during calcination. Chem. Mater. 1999, 32, 2770–2774. [Google Scholar] [CrossRef]
- Vega-Poot, A.G.; Rodrıguez-Gattorno, G.; Soberanis-Domnguez, O.E.; Patiño-Dıaz, R.T.; Espinosa-Pesqueira, M.; Oskam, G. The nucleation kinetics of ZnO nanoparticles from ZnCl 2 in ethanol solutions. Nanoscale 2010, 2, 2710–2717. [Google Scholar] [CrossRef] [PubMed]
- Phiwdang, K.; Suphankij, S.; Mekprasart, W. Synthesis of CuO nanoparticles by precipitation method using different precursors. Energy Procedia 2013, 34, 740–745. [Google Scholar] [CrossRef] [Green Version]
- Ahamed, M.; Khan, M.A.M.; Akhtar, M.J.; Alhadlaq, H.A. Ag-doping regulates the cytotoxicity of TiO2 nanoparticles via oxidative stress in human cancer cells. Sci. Rep. 2017, 7, 1–14. [Google Scholar]
- Durango-Giraldo, G.; Cardona, A.; Zapata, J.F.; Santa, J.F.; Buitrago-Sierra, R. Titanium dioxide modified with silver by two methods for bactericidal applications. Heliyon 2019, 5, e01608. [Google Scholar] [CrossRef] [Green Version]
- Mansur, H.S.; Mansur, A.A.P. Small angle X-Ray scattering, FTIR and SEM characterization of nanostructured PVA/TEOS hybrids by chemical crosslinking. Mater. Res. Soc. Symp. Proc. 2005, 873, 1–6. [Google Scholar] [CrossRef]
- Ranganayaki, T.; Venkatachalam, M.; Vasuki, T.; Shankar, S. Preparation and characterization of nanocrystalline TiO2 thin films prepared by Sol-Gel spin coating method. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 16708–16711. [Google Scholar]
- Clayton, K.N.; Salameh, J.W.; Wereley, S.T.; Kinzer-Ursem, T.L. Physical characterization of nanoparticle size and surface modification using particle scattering diffusometry. Biomicrofluidics 2016, 10, 054107. [Google Scholar] [CrossRef] [Green Version]
- Howell, D.; Behrends, B. A review of surface roughness in antifouling coatings illustrating the importance of cutoff length. Biofouling 2006, 22, 401–410. [Google Scholar] [CrossRef]
- dos Reis, E.F.; Campos, F.S.; Lage, A.P.; Leite, R.C.; Heneine, L.G.; Vasconcelos, W.L.; Lobato, Z.I.P.; Mansur, H.S. Synthesis and characterization of poly (vinyl alcohol) hydrogels and hybrids for rMPB70 protein adsorption. Mater. Res. 2006, 9, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Sugumaran, S.; Bellan, C.S. Transparent nano composite PVA–TiO2 and PMMA–TiO2 thin films: Optical and dielectric properties. Opt. Int. J. Light Electron Opt. 2014, 125, 5128–5133. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO nanoparticles to escherichia Coli: Mechanism and the influence of medium components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Tantillo, G.; Ghibelli, L.; Sabbatini, L.; Bleve-Zacheo, T.; D’Alessio, M.; Zambonin, P.G.; Traversa, E. Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chem. Mater. 2005, 17, 5255–5262. [Google Scholar] [CrossRef]








| Metal-NPs | Size (nm) | Polydispersity Index (PDI) |
|---|---|---|
| TiO2 | 110.0 | 0.127 |
| ZnO | 112.9 | 0.100 |
| CuO | 104.2 | 0.101 |
| AgNPs | 100.9 | 0.152 |
| Ag-TiO2 | 135.2 | 0.144 |
| Gel-Coat (Surface) | Cutoff Length | Roughness (Ra) | Total Roughness (Ra) | Standard Deviation |
|---|---|---|---|---|
| (cm) | Average (µm) | Average (µm) | ||
| Substrate 1 | 1.65 | 0.42 | ||
| 3.30 | 0.41 | 0.42 | 0.015 | |
| 4.95 | 0.44 | |||
| Substrate 2 | 1.65 | 0.46 | ||
| 3.30 | 0.48 | 0.46 | 0.025 | |
| 4.95 | 0.43 | |||
| Substrate 3 | 1.65 | 0.57 | ||
| 3.30 | 0.40 | 0.49 | 0.085 | |
| 4.95 | 0.50 | |||
| Mean value | 0.46 |
| Coating Surface | Cutoff Length | Roughness (Ra) 1 | Total Roughness (Ra) | Standard Deviation |
|---|---|---|---|---|
| (cm) | Average (µm) | Average (µm) | ||
| PVA/GA | 1.65 | 0,89 | ||
| 3.30 | 0,95 | 0.95 | 0.06 | |
| 4.95 | 1,02 | |||
| PVA/GA/AgNPs | 1.65 | 4.90 | ||
| 3.30 | 5.12 | 5.0 | 0.2 | |
| 4.95 | 4.94 | |||
| PVA/GA/TiO2-NPs | 1.65 | 2.00 | ||
| 3.30 | 2.31 | 2.1 | 0.2 | |
| 4.95 | 1.98 | |||
| PVA/GA/ZnO-NPs | 1.65 | 9.74 | ||
| 3.30 | 9.91 | 9.8 | 0.1 | |
| 4.95 | 9.68 | |||
| PVA/GA/Ag-TiO2-NPs | 1.65 | 1.43 | ||
| 3.30 | 1.52 | 1.46 | 0.06 | |
| 4.95 | 1.41 | |||
| PVA/GA/CuO-NPs | 1.65 | 9.22 | ||
| 3.30 | 9.35 | 9.28 | 0.07 | |
| 4.95 | 9.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Covián, L.; Campello-García, J.R.; Blanco-López, M.C.; Miranda-Martínez, M. Synthesis, Characterization and Evaluation of the Antibiofouling Potential of Some Metal and Metal Oxide Nanoparticles. Appl. Sci. 2020, 10, 5864. https://doi.org/10.3390/app10175864
Blanco-Covián L, Campello-García JR, Blanco-López MC, Miranda-Martínez M. Synthesis, Characterization and Evaluation of the Antibiofouling Potential of Some Metal and Metal Oxide Nanoparticles. Applied Sciences. 2020; 10(17):5864. https://doi.org/10.3390/app10175864
Chicago/Turabian StyleBlanco-Covián, Lucía, José Ramón Campello-García, María Carmen Blanco-López, and Manuel Miranda-Martínez. 2020. "Synthesis, Characterization and Evaluation of the Antibiofouling Potential of Some Metal and Metal Oxide Nanoparticles" Applied Sciences 10, no. 17: 5864. https://doi.org/10.3390/app10175864






