4-Methoxy Sulfonyl Paeonol Inhibits Hepatic Stellate Cell Activation and Liver Fibrosis by Blocking the TGF-β1/Smad, PDGF-BB/MAPK and Akt Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. 4-Methoxy Sulfonyl Paeonol (4-MSP)
2.2. Cell Culture
2.3. TGF-β1, PDGF-BB, and 4-MSP Treatments
2.4. Cytotoxicity Assays
2.5. Wound Healing Assays
2.6. Gene Expression Profiling
2.7. Animals and Experimental Design
2.8. Hydroxyproline Assays
2.9. Western Blotting, IHC Analysis and Blood Biochemical Parameter Measurement
2.10. Quantitative Real-Time PCR (QPCR)
2.11. Statistical Analyses
3. Results
3.1. Compound 4-MSP Inhibits TGF-β1-Induced HSC Activation
3.2. Compound 4-MSP Inhibits HSC Proliferation Through Blocking PDGF-BB-Induced MAPK and Akt Signalings
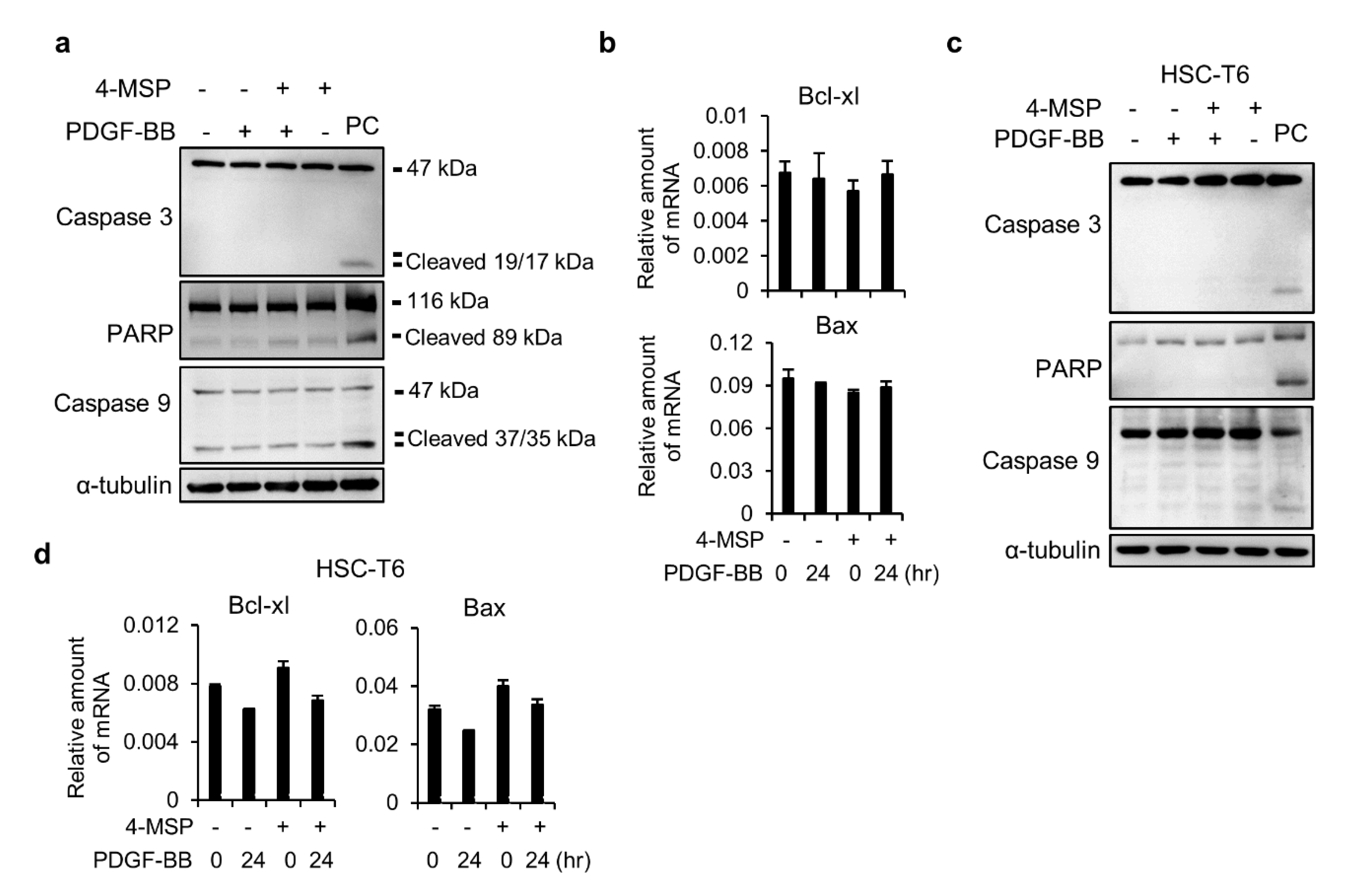
3.3. Compound 4-MSP Treatment Does Not Induce Apoptosis in HSCs
3.4. The Effects of Genes and Biological Functions Caused by 4-MSP-Treated HSCs
3.5. Compound 4-MSP Ameliorates Fibrosis Process in Mice With CCl4-Induced Liver Fibrosis Without Inducing Adverse Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D.; Afdhal, N.H. Liver cirrhosis. Lancet 2008, 371, 838–851. [Google Scholar] [CrossRef]
- Friedman, S.L. Liver fibrosis—From bench to bedside. J. Hepatol. 2003, 38 (Suppl. 1), S38–S53. [Google Scholar] [CrossRef]
- Iredale, J.P.; Thompson, A.; Henderson, N.C. Extracellular matrix degradation in liver fibrosis: Biochemistry and regulation. Biochim. Biophys Acta 2013, 1832, 876–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iredale, J.P. Models of liver fibrosis: Exploring the dynamic nature of inflammation and repair in a solid organ. J. Clin. Investig. 2007, 117, 539–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.Y.; Yuan, W.G.; He, P.; Lei, J.H.; Wang, C.X. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 2016, 22, 10512–10522. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.J.; Parola, M. Liver fibrogenic cells. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 207–217. [Google Scholar] [CrossRef]
- Friedman, S.L. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008, 134, 1655–1669. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Gea, V.; Friedman, S.L. Pathogenesis of liver fibrosis. Annu. Rev. Pathol. 2011, 6, 425–456. [Google Scholar] [CrossRef]
- Friedman, S.L. Hepatic fibrosis—overview. Toxicology 2008, 254, 120–129. [Google Scholar] [CrossRef]
- Lee, U.E.; Friedman, S.L. Mechanisms of hepatic fibrogenesis. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.; Curley, S.A.; Wu, X.; Brown, P.; Hwang, J.P.; Shetty, K.; Yao, Z.X.; He, A.R.; Li, S.; Katz, L.; et al. Hepatic stem cells and transforming growth factor beta in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 530–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inagaki, Y.; Okazaki, I. Emerging insights into transforming growth factor beta smad signal in hepatic fibrogenesis. Gut 2007, 56, 284–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 2009, 17, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Fallowfield, J.A. Therapeutic targets in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G709–G715. [Google Scholar] [CrossRef] [Green Version]
- Yata, Y.; Gotwals, P.; Koteliansky, V.; Rockey, D.C. Dose-dependent inhibition of hepatic fibrosis in mice by a tgf-beta soluble receptor: Implications for antifibrotic therapy. Hepatology 2002, 35, 1022–1030. [Google Scholar] [CrossRef]
- Borkham-Kamphorst, E.; Weiskirchen, R. The pdgf system and its antagonists in liver fibrosis. Cytokine Growth Factor Rev. 2016, 28, 53–61. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, X.M.; Lui, E.L.; Friedman, S.L.; Cui, W.; Ho, N.P.; Li, L.; Ye, T.; Fan, S.T.; Zhang, H. Therapeutic targeting of the pdgf and tgf-beta-signaling pathways in hepatic stellate cells by ptk787/zk22258. Lab. Investig. 2009, 89, 1152–1160. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Tian, D.A.; Li, P.Y.; He, X.X. Mouse models of liver cancer: Progress and recommendations. Oncotarget 2015, 6, 23306–23322. [Google Scholar] [CrossRef]
- Zhang, L.; Li, D.C.; Liu, L.F. Paeonol: Pharmacological effects and mechanisms of action. Int. Immunopharmacol. 2019, 72, 413–421. [Google Scholar] [CrossRef]
- Wang, Z.; He, C.; Peng, Y.; Chen, F.; Xiao, P. Origins, phytochemistry, pharmacology, analytical methods and safety of cortex moutan (paeonia suffruticosa andrew): A systematic review. Molecules 2017, 22, 946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.; Wu, N.; Zeng, F.; Cheng, C.; Kang, K.; Yang, H. Paeonol induces apoptosis in human ovarian cancer cells. Acta Histochem. 2013, 115, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Li, H.X.; Jin, W.S.; Peng, W.R.; Zhang, C.J.; Bu, L.J.; Du, Y.Y.; Ma, T.; Sun, G.P. The radiosensitizing effect of paeonol on lung adenocarcinoma by augmentation of radiation-induced apoptosis and inhibition of the pi3k/akt pathway. Int. J. Radiat. Biol. 2013, 89, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, Q.; Mei, L.; Lei, H.; Wen, Q.; Miao, J.; Huang, H.; Chen, D.; Du, S.; Zhang, S.; et al. Paeonol attenuates acute lung injury by inhibiting hmgb1 in lipopolysaccharide-induced shock rats. Int. Immunopharmacol. 2018, 61, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Shen, G.; Zhao, W.; Wang, F.; Jiang, X.; Huang, D. Paeonol, the main active principles of paeonia moutan, ameliorates alcoholic steatohepatitis in mice. J. Ethnopharmacol. 2010, 128, 100–106. [Google Scholar] [CrossRef]
- Chen, B.; Ning, M.; Yang, G. Effect of paeonol on antioxidant and immune regulatory activity in hepatocellular carcinoma rats. Molecules 2012, 17, 4672–4683. [Google Scholar] [CrossRef] [Green Version]
- Niwata, S.; Fukami, H.; Sumida, M.; Ito, A.; Kakutani, S.; Saitoh, M.; Suzuki, K.; Imoto, M.; Shibata, H.; Imajo, S.; et al. Substituted 3-(phenylsulfonyl)-1-phenylimidazolidine-2,4-dione derivatives as novel nonpeptide inhibitors of human heart chymase. J. Med. Chem. 1997, 40, 2156–2163. [Google Scholar] [CrossRef]
- Bachovchin, D.A.; Zuhl, A.M.; Speers, A.E.; Wolfe, M.R.; Weerapana, E.; Brown, S.J.; Rosen, H.; Cravatt, B.F. Discovery and optimization of sulfonyl acrylonitriles as selective, covalent inhibitors of protein phosphatase methylesterase-1. J. Med. Chem. 2011, 54, 5229–5236. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.J.; Chuang, H.; Liang, Y.C.; Lin, H.H.; Horng, J.C.; Kuo, Y.C.; Chen, C.W.; Tsai, F.Y.; Yen, S.C.; Chou, S.C.; et al. Design, synthesis, and bioevaluation of paeonol derivatives as potential anti-hbv agents. Eur. J. Med. Chem. 2015, 90, 428–435. [Google Scholar] [CrossRef]
- Twu, Y.C.; Lee, T.S.; Lin, Y.L.; Hsu, S.M.; Wang, Y.H.; Liao, C.Y.; Wang, C.K.; Liang, Y.C.; Liao, Y.J. Niemann-pick type c2 protein mediates hepatic stellate cells activation by regulating free cholesterol accumulation. Int. J. Mol. Sci. 2016, 17, 1122. [Google Scholar] [CrossRef]
- Wang, Y.H.; Suk, F.M.; Liu, C.L.; Chen, T.L.; Twu, Y.C.; Hsu, M.H.; Liao, Y.J. Antifibrotic effects of a barbituric acid derivative on liver fibrosis by blocking the nf-kappab signaling pathway in hepatic stellate cells. Front. Pharmacol. 2020, 11, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neff, G.W.; Duncan, C.W.; Schiff, E.R. The current economic burden of cirrhosis. Gastroenterol. Hepatol. 2011, 7, 661–671. [Google Scholar]
- Gressner, O.A.; Weiskirchen, R.; Gressner, A.M. Biomarkers of liver fibrosis: Clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clin. Chim. Acta 2007, 381, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Song, B.; Sun, G.; Ma, T.; Zhong, F.; Wei, W. Endoplasmic reticulum stress-induced resistance to doxorubicin is reversed by paeonol treatment in human hepatocellular carcinoma cells. PLoS ONE 2013, 8, e62627. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Q.; Xu, Y.; Chen, Y.; Deng, Y.; Zhi, F.; Qian, K. Attenuating oxidative stress by paeonol protected against acetaminophen-induced hepatotoxicity in mice. PLoS ONE 2016, 11, e0154375. [Google Scholar] [CrossRef]
- Gong, X.; Yang, Y.; Huang, L.; Zhang, Q.; Wan, R.Z.; Zhang, P.; Zhang, B. Antioxidation, anti-inflammation and anti-apoptosis by paeonol in lps/d-galn-induced acute liver failure in mice. Int. Immunopharmacol. 2017, 46, 124–132. [Google Scholar] [CrossRef]
- Sun, X.; Wang, P.; Yao, L.P.; Wang, W.; Gao, Y.M.; Zhang, J.; Fu, Y.J. Paeonol alleviated acute alcohol-induced liver injury via sirt1/nrf2/nf-kappab signaling pathway. Environ. Toxicol. Pharmacol. 2018, 60, 110–117. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, F.; Wei, D.; Zhu, X.; Zhang, X.; Chen, L.; Lu, Y.; Zheng, S. Paeonol inhibits hepatic fibrogenesis via disrupting nuclear factor-kappab pathway in activated stellate cells: In vivo and in vitro studies. J. Gastroenterol. Hepatol. 2013, 28, 1223–1233. [Google Scholar] [CrossRef]
- Wu, S.; Liu, L.; Yang, S.; Kuang, G.; Yin, X.; Wang, Y.; Xu, F.; Xiong, L.; Zhang, M.; Wan, J.; et al. Paeonol alleviates ccl4-induced liver fibrosis through suppression of hepatic stellate cells activation via inhibiting the tgf-beta/smad3 signaling. Immunopharmacol. Immunotoxicol. 2019, 41, 438–445. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Z.; Gong, W.; Zou, Y.; Xu, F.; Chen, L.; Huang, H. Paeonol ameliorates diabetic renal fibrosis through promoting the activation of the nrf2/are pathway via up-regulating sirt1. Front. Pharmacol. 2018, 9, 512. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.H.; Lin, A.H.; Ko, H.K.; Perng, D.W.; Lee, T.S.; Kou, Y.R. Prevention of bleomycin-induced pulmonary inflammation and fibrosis in mice by paeonol. Front. Physiol. 2017, 8, 193. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Qiu, Z.Z.; Yu, Z.H.; Gao, L.; He, J.M.; Zhang, Z.W.; Zheng, J. Paeonol reverses promoting effect of the hotair/mir-124/notch1 axis on renal interstitial fibrosis in a rat model. J. Cell. Physiol. 2019, 234, 14351–14363. [Google Scholar] [CrossRef]
- Friedman, S.L.; Sheppard, D.; Duffield, J.S.; Violette, S. Therapy for fibrotic diseases: Nearing the starting line. Sci. Transl. Med. 2013, 5, 167sr161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leask, A.; Abraham, D.J. Tgf-beta signaling and the fibrotic response. FASEB J. 2004, 18, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, C.; Zhou, D.; Zhang, L. Tgf-beta/smad pathway and its regulation in hepatic fibrosis. J. Histochem. Cytochem. 2016, 64, 157–167. [Google Scholar] [CrossRef]
- Jeong, D.H.; Hwang, M.; Park, J.K.; Goo, M.J.; Hong, I.H.; Ki, M.R.; Ishigami, A.; Kim, A.Y.; Lee, E.M.; Lee, E.J.; et al. Smad3 deficiency ameliorates hepatic fibrogenesis through the expression of senescence marker protein-30, an antioxidant-related protein. Int. J. Mol. Sci. 2013, 14, 23700–23710. [Google Scholar] [CrossRef] [PubMed]
- Latella, G.; Vetuschi, A.; Sferra, R.; Catitti, V.; D’Angelo, A.; Zanninelli, G.; Flanders, K.C.; Gaudio, E. Targeted disruption of smad3 confers resistance to the development of dimethylnitrosamine-induced hepatic fibrosis in mice. Liver Int. 2009, 29, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Kamato, D.; Burch, M.L.; Piva, T.J.; Rezaei, H.B.; Rostam, M.A.; Xu, S.; Zheng, W.; Little, P.J.; Osman, N. Transforming growth factor-beta signalling: Role and consequences of smad linker region phosphorylation. Cell. Signal. 2013, 25, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Hough, C.; Radu, M.; Dore, J.J. Tgf-beta induced erk phosphorylation of smad linker region regulates smad signaling. PLoS ONE 2012, 7, e42513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, K.; Matsuzaki, K.; Mori, S.; Tahashi, Y.; Yamagata, H.; Furukawa, F.; Seki, T.; Nishizawa, M.; Fujisawa, J.; Okazaki, K. Transforming growth factor-beta and platelet-derived growth factor signal via c-jun n-terminal kinase-dependent smad2/3 phosphorylation in rat hepatic stellate cells after acute liver injury. Am. J. Pathol. 2005, 166, 1029–1039. [Google Scholar] [CrossRef]
- Kamato, D.; Ta, H.; Afroz, R.; Xu, S.; Osman, N.; Little, P.J. Mechanisms of par-1 mediated kinase receptor transactivation: Smad linker region phosphorylation. J. Cell Commun. Signal. 2019, 13, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Ramasamy, T.S.; Murphy, N.; Holt, M.K.; Czapiewski, R.; Wei, S.K.; Cui, W. Pi3k/mtorc2 regulates tgf-beta/activin signalling by modulating smad2/3 activity via linker phosphorylation. Nat. Commun. 2015, 6, 7212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marra, F.; Arrighi, M.C.; Fazi, M.; Caligiuri, A.; Pinzani, M.; Romanelli, R.G.; Efsen, E.; Laffi, G.; Gentilini, P. Extracellular signal-regulated kinase activation differentially regulates platelet-derived growth factor’s actions in hepatic stellate cells, and is induced by in vivo liver injury in the rat. Hepatology 1999, 30, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Du, H.; Li, M.; Li, Y.; Liu, S.; Gao, P.; Zhang, X.; Cheng, J. Mapk signal transduction pathway regulation: A novel mechanism of rat hsc-t6 cell apoptosis induced by fuzhenghuayu tablet. Evid. Based Complementary Altern. Med. 2013, 2013, 368103. [Google Scholar] [CrossRef]
- Gabele, E.; Reif, S.; Tsukada, S.; Bataller, R.; Yata, Y.; Morris, T.; Schrum, L.W.; Brenner, D.A.; Rippe, R.A. The role of p70s6k in hepatic stellate cell collagen gene expression and cell proliferation. J. Biol. Chem. 2005, 280, 13374–13382. [Google Scholar] [CrossRef] [Green Version]
- Reif, S.; Lang, A.; Lindquist, J.N.; Yata, Y.; Gabele, E.; Scanga, A.; Brenner, D.A.; Rippe, R.A. The role of focal adhesion kinase-phosphatidylinositol 3-kinase-akt signaling in hepatic stellate cell proliferation and type i collagen expression. J. Biol. Chem. 2003, 278, 8083–8090. [Google Scholar] [CrossRef] [Green Version]
- Son, M.K.; Ryu, Y.L.; Jung, K.H.; Lee, H.; Lee, H.S.; Yan, H.H.; Park, H.J.; Ryu, J.K.; Suh, J.K.; Hong, S.; et al. Hs-173, a novel pi3k inhibitor, attenuates the activation of hepatic stellate cells in liver fibrosis. Sci. Rep. 2013, 3, 3470. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Yang, H.; Wang, N.; Ouyang, Y.; Yi, Y.; Liao, L.; Shen, H.; Hu, G.; Wang, Z.; Tao, L. Fluorofenidone attenuates hepatic fibrosis by suppressing the proliferation and activation of hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G253–G263. [Google Scholar] [CrossRef] [Green Version]
- Bruix, J.; Boix, L.; Sala, M.; Llovet, J.M. Focus on hepatocellular carcinoma. Cancer Cell 2004, 5, 215–219. [Google Scholar] [CrossRef] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.-J.; Wang, Y.-H.; Liu, C.-L.; Fang, C.-C.; Hsu, M.-H.; Suk, F.-M. 4-Methoxy Sulfonyl Paeonol Inhibits Hepatic Stellate Cell Activation and Liver Fibrosis by Blocking the TGF-β1/Smad, PDGF-BB/MAPK and Akt Signaling Pathways. Appl. Sci. 2020, 10, 5941. https://doi.org/10.3390/app10175941
Liao Y-J, Wang Y-H, Liu C-L, Fang C-C, Hsu M-H, Suk F-M. 4-Methoxy Sulfonyl Paeonol Inhibits Hepatic Stellate Cell Activation and Liver Fibrosis by Blocking the TGF-β1/Smad, PDGF-BB/MAPK and Akt Signaling Pathways. Applied Sciences. 2020; 10(17):5941. https://doi.org/10.3390/app10175941
Chicago/Turabian StyleLiao, Yi-Jen, Yuan-Hsi Wang, Chao-Lien Liu, Cheng-Chieh Fang, Ming-Hua Hsu, and Fat-Moon Suk. 2020. "4-Methoxy Sulfonyl Paeonol Inhibits Hepatic Stellate Cell Activation and Liver Fibrosis by Blocking the TGF-β1/Smad, PDGF-BB/MAPK and Akt Signaling Pathways" Applied Sciences 10, no. 17: 5941. https://doi.org/10.3390/app10175941






