1. Introduction
Studies have revealed that awkward posture, excessive exertion and repetitive work are the primary causes of musculoskeletal injuries [
1]. This includes exposure of the static upper trunk of humans to deeper flexion postures or prolonged stooping in many workplaces [
2,
3,
4]. Such postures are commonly observed in agriculture, manufacturing, mining and construction industries, which are more likely to employ males than females in Taiwan [
5]. Although experts in occupational medicine or ergonomics have called for the use of squatting rather than a stooped posture, a field survey has shown that the stoop is considered to be one of the most effective postures for frontline workers [
6,
7]. When considering the work experience, however, novices are more inclined to use the stooped working postures than the experienced workers, and this may cause higher injury risks [
8,
9].
The mechanism of lower back loading is associated with the flexion–relaxation phenomenon (FRP) of the back muscle, which is generated when the human trunk is bent forward. Floyd and Silver [
10] were the first to propose the existence of FRP. The reason for the occurrence of FRP is that when the trunk is flexed deeply, the role of back muscles (especially lumbar erector spinae, LES) in balancing the torque generated by the trunk on the lumbar region shifts to the passive tissues of the adjacent lumbar spine. Because identical torque needs to be balanced with a shorter arm, it generates more force on the tissues. FRP generally leads to elongated and even damaged passive tissue of the lumbar spine [
11], which may cause lower back injury [
12,
13]. When FRP occurs as a result of trunk flexion, it may be a warning of lower back injury. To determine the occurrence of FRP, an absolute threshold using maximum voluntary contraction (MVC) from electromyography (EMG) is commonly adopted [
2,
12]. A threshold of 5% integrated EMG (IEMG) is a typical threshold for MVC, which is then employed for all testing trials to identify the silences in EMG signals. Jin et al. [
14] developed an algorithm to identify the onset and cessation of FRP in lower back muscle. However, a generally accepted protocol is that when the trunk is flexed forward, the LES EMG value begins to decrease, and thus FRP occurs [
15,
16,
17,
18].
When a person bends the trunk forward from a standing position, the LES and hamstring (HMS) are gradually stretched, which results in FRP. This may imply that individual flexibility in these muscle groups is closely related to FRP. It has been found that when performing identical deep trunk flexion, flexible people exhibited relatively delayed FRP compared with less flexible people [
3,
19,
20]. Hashemirad et al. found that participants with high toe-touch test (TTT) scores had to flex their trunk and rotate their hip more than others, and then back muscle FRP was observed [
21]. This may be because flexibility provides spinal stabilization in back muscle recruitment patterns and the central nervous system strategy. In a recent study, Chen et al. found that participant’s TTT scores significantly affected the FRP of back muscles. When the trunk was bent close to 90°, flexible participants produced a greater ES contraction than less flexible participants. The authors speculated that this was because flexible people have a larger range of motion in the trunk, and thus lumbar lordosis could be maintained, resulting in a slighter degree of FRP [
20].
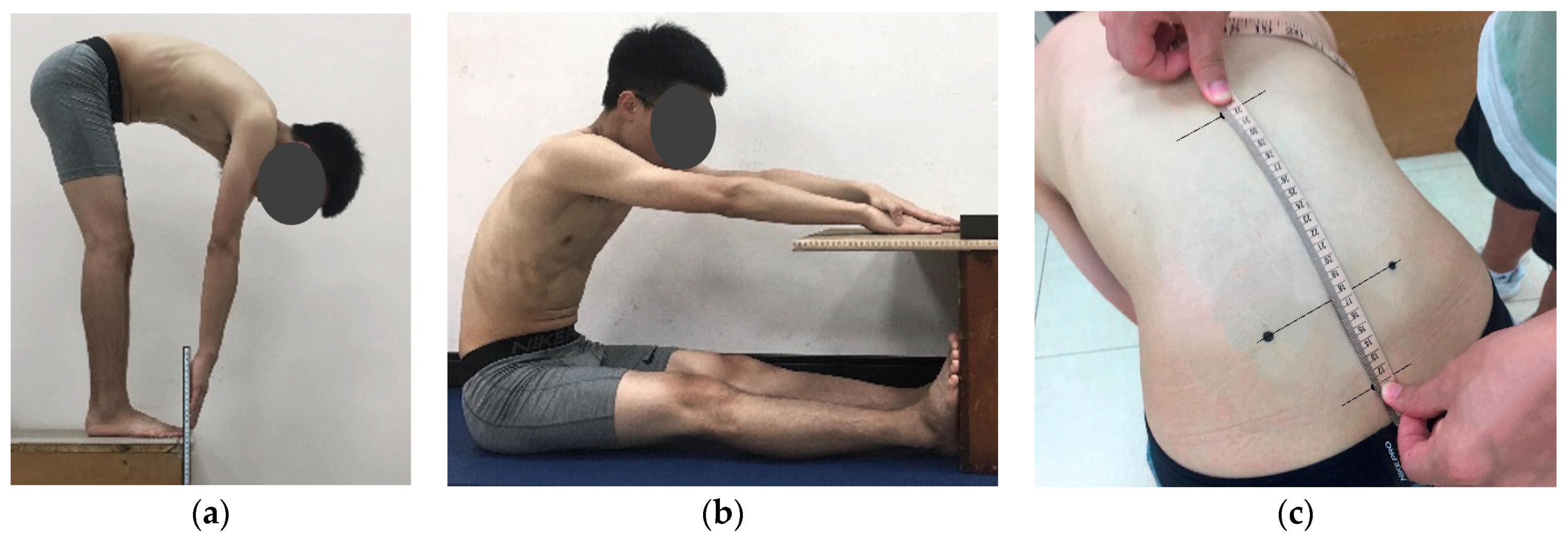
Although the effect of flexibility on FRP has been preliminarily confirmed, flexibility is commonly examined using TTT. Different flexibility measurements, however, can be used to evaluate different stretches of body parts. Generally, TTT and sit-and-reach test (SRT) are primarily used to assess the stretch ability of the HMS muscle, although the two involve different postures. Therefore, although the correlation between them is high, they cannot completely substitute each other [
22]. Nevertheless, the flexibility measurement has been widely employed in the functional evaluation of patients with lower back pain (LBP) or spinal disease [
23,
24,
25]. Additionally, patients with LBP may experience difficulty with trunk forward flexion due to spine rigidity, and the modified Schober’s test (MST) for measuring lumbar spine mobility has thus become a commonly used clinical indicator [
26]. Because FRP mainly occurs in the lumbar muscles, lumbar spine mobility may also affect FRP. However, SRT and MST have not been evaluated in previous FRP studies. To fill the gap, this study was intended to determine the most efficient measurement of flexibility among the three tests for evaluating the occurrence and degree of FRP when participants were requested to statically flex their trunk forward from an upright position (0°) to 90°. The result will serve as a reference for on-site worker recruitment and flexibility training.
4. Discussion
The aim of this study was to determine which flexibility measurement was best able to predict the corresponding FRP. The results indicated that TTT can significantly discriminate differences in LES FRP between high and low flexibility groups, and a positive correlation with the FRP onset was also found. This suggests that the higher the TTT, the slower the occurrence of FRP. The TTT could be considered as an appropriate tool for on-site worker recruitment and flexibility training when work requires a deeper trunk flexion.
The correlations between MST and TTT, and MST and SRT, were relatively low, which may be because MST primarily measures lumbar spine flexibility. Regardless of whether the original Schober’s test or MST used in this study is employed, it has been developed to detect patients with LBP such as ankylosing spondylitis (AS) [
32]. Typical early symptoms include chronic low back pain and spinal stiffness, which cause difficulty in trunk bending [
33]. The Schober’s test is commonly used and is useful for screening the status of AS disease, and also for determining the progression and therapeutic effects of AS [
34,
35]. In a validation study utilizing a Taiwanese sample, Yen et al. [
26] further clarified that the original Schober’s test strongly correlated with MST measurements. Compared with TTT and SRT, which involve different extensibilities of muscle groups throughout the body, especially the HMS, MST only evaluates lumbar spine mobility, which results in a lower correlation with TTT and SRT.
To assess the flexibility of the HMS muscle, field tests such as TTT and SRT are commonly used in sports training and physical fitness evaluation. The procedures of these measurements are simple, easy to administer, and have minimal skill requirements [
36]. However, López-Miñarro and Rodríguez-García [
22] have identified significant differences in thoracic and lumbar spine postures; this may have affected the validity of the measurement and resulted in a correlation coefficient of 0.55–0.61 for TTT and SRT. Ayala et al. [
37] also found that the corresponding correlation coefficient for young Spanish men and women (average 21 years) was 0.829 (
p < 0.001), which was similar to the results of this study. Although the body postures between the two measurements appeared to be alike, it is difficult for them to replace each other. One of the possible reasons for this is that the sitting posture of the SRT anteriorly limits the rotation of the pelvis during measurement, and causes differences in postures between the thoracic and lumbar spine [
28]. López-Miñarro et al. compared lumbar and thoracic spine angles during TTT and SRT measurements, and found that although the lumbar spine angles of the two were not significantly different, the difference in thoracic spine angle was approximately 8°, which can be attributed to the effect of the pelvis being restricted [
28].
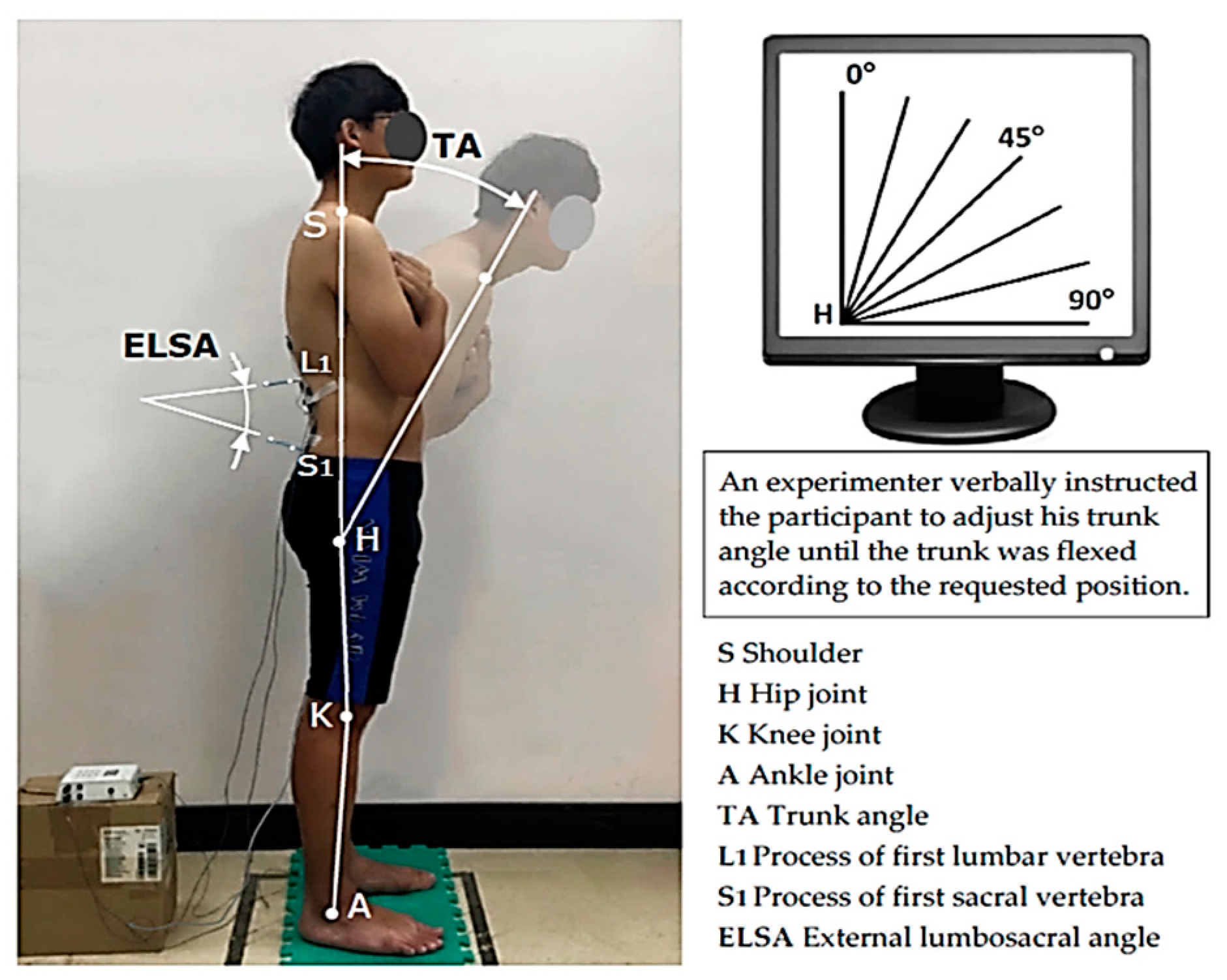
In the current study, when participants flexed their trunk from upright to 45°, all muscle EMG values gradually increased as the trunk angle increased (TES: 5.1–8.3 %MVC, LES: 3.5–10.6 %MVC, HMS: 2.4–8.5 %MVC). Once the trunk was flexed beyond 45°, FRP occurred first at LES (10.6–9.8 %MVC), followed by TES (60°, 9.7–8.4 %MVC) and then HMS (75°, 9.3–8.0 %MVC), as shown in
Figure 4. This indicates a time sequence in the occurrence of FRP among the three muscle groups. This may be due to the roles these muscle groups play. Nordin et al. [
38] observed that in normal trunk flexion, the lumbar vertebral bodies bend forward during the first 50°–60°, and after that, the pelvis rotates anteriorly. At approximately 75–85% trunk flexion, the lumbar spine reaches its maximum range of motion, while the pelvis undergoes terminal flexion to accomplish total trunk flexion [
15]. At this stage, passive soft tissues that are noncontractile, such as intervertebral discs and intervertebral ligaments, begin to provide most of the spinal support and reduce the back muscle activation to maintain the posture [
12,
15]. This leads to the occurrence of FRP. It can be inferred that the FRP of the HMS would occur after the occurrence of back muscle FRP. Sihvonen examined the LES and HMS EMGs during trunk flexion, and found that the TES FRP occurred when the trunk was bent forward at about 80°, whereas the HMS FRP occurred at almost full trunk flexion [
39]. This implies that when the trunk is bent forward, in addition to the elongation of back muscles, the HMS, which is closely related to pelvic movement, is subsequently elongated, and FRP occurs. This has been confirmed by several recent studies [
3,
20].
Gupta found that when TES FRP occurs, all participants display more active HMS contraction. Our result is consistent with that of Gupta, in which the trunk was flexed forward from 45° to 75° [
16]. Gupta postulated that increased HMS contraction occurs because the pelvis turns to stabilize the spine.
Figure 4 shows that the TES FRP occurred after the LES and before the HMS. During trunk flexion, the contribution of lumbar spine bending is greater than that of the thoracic spine [
40], which explains why TES EMG was relatively low at the first 30° of trunk flexion. LSA was maintained at approximately 40° when the trunk was upright. Once flexion began, it gradually decreased until it eventually became negative at 90°. Thus, the lumbar spine changed from lordotic, converting into a kyphotic posture [
20]. Furthermore, when LSA dropped to about 20°, and the trunk was flexed at 45°, LES FRP began to occur.
Different flexibility levels had a significant effect on LES activation for all three measurements (all
p < 0.05,
Table 4). This verified that individual flexibility (high, middle and low) caused different degrees of contraction of LES, within which TTT significantly distinguished groups with high and low flexibility, whereas MST and SRT distinguished between high and non-high flexibility groups (high vs. middle and low), as shown in
Table 6. In previous studies on flexibility and FRP, investigators have tended to employ TTT to classify flexibility levels [
3,
19,
21], and have demonstrated that participants with high and low flexibility exhibit different FRP patterns; however, they rarely evaluated participants whose flexibility was within the full range. The results of this study regarding TTT and FRP conform to those of previous studies, which indicates that compared with the TTT, MST and SRT may be appropriate for discriminating between high and non-high flexibility classifications in FRP assessment.
This study suggests that flexibility grouped by MST did not exert a significant effect on LSA, which was different from the results for TTT and SRT. This is because the measurements of TTT and SRT examine whole-body extensibility, and include the ability to stretch and soften joint tissues, muscles, tendons and ligaments, that is, the joint mobility of various body parts and tissues [
41]. By contrast, MST primarily measures lumbar spine mobility, and the HMS is almost absent. Because LSA is affected by pelvic rotation, and HMS is a crucial factor affecting lumbopelvic movement [
16,
42], this may mean that LSA is not affected by the flexibility groups identified by MST measurement. However, past studies have found that LSA affected back muscle activation [
43], which can cause different lumbar spine extension forces to have different effects on FRP.
This study is the first attempt at developing an onset indicator to quantify the definition of FRP. Determining when FRP occurs is more practical and meaningful. From the perspective of occupational ergonomics, when a flexible worker engages in a deep trunk flexion posture, they bear a relatively low spinal load. This is useful for the prevention of possible lower back injury on site. In this study, when TTT was used to classify the flexibility group, a significant positive correlation was found between LES FRP onset and the flexibility value (r = 0.553,
p < 0.01). The higher the TTT value, the slower the FRP occurrence, which means that FRP can be delayed or even avoided in specific trunk postures. Compared with TTT, the methods of measuring MST and SRT were positively correlated only with the FRP onset of the TES (r = 0.388–0.399, all
p < 0.05). Regarding the HMS, no correlation was observed between the flexibility value and the FRP onset, regardless of the measurement of flexibility. This is reasonable because HMS FRP occurs in the final stage of trunk flexion [
39]. These findings provide a practical reference for screening and health training employees who require deeper trunk flexion during their daily work.
Several study limitations should be highlighted. The sample size (40 young male participants) in this study is relatively small because the sample was divided into three flexibility groups and then analyzed, even though the results may be significant. The results could not be applicable to the female population because of the inherent difference in flexibility between the genders [
44]. Another concern in the study was that static trunk positions were examined, and the results should be further verified through a test of dynamic trunk movement. Because specific trunk positions were performed by the participants, the FRP occurrence was judged by these trunk flexion ranges, instead of a distinct trunk angle. In addition, besides the TTT, the flexibility levels (i.e., high, middle and low) in the study were determined based on the average flexibility values, as suggested by the previous studies, as well as the sample size and the flexibility allocation. This merits further investigation.