The Effects of Plasma on Plant Growth, Development, and Sustainability
Abstract
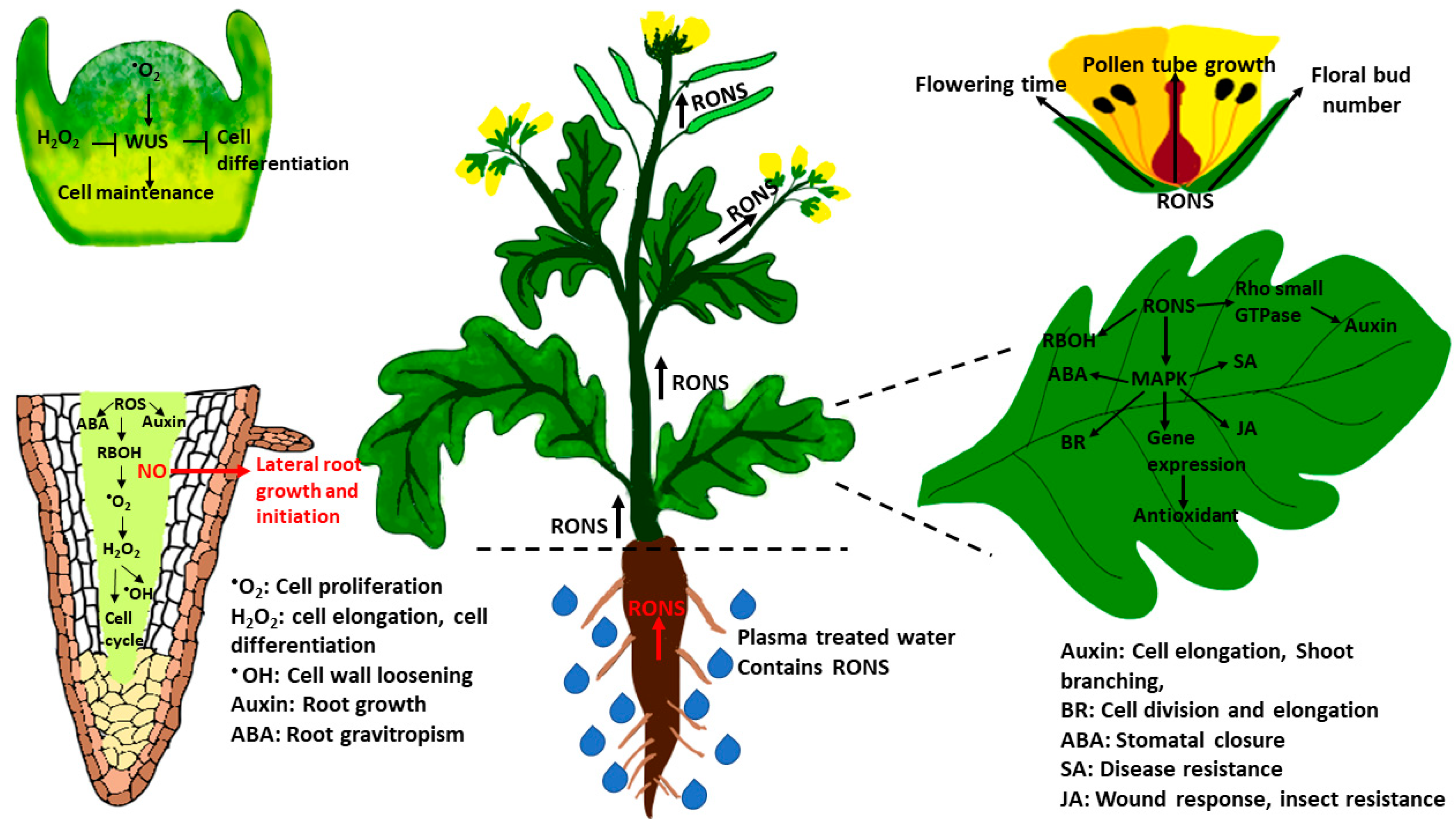
:1. Introduction
2. Effect of Plasma on Seed Germination
3. Effects of Plasma on Plant Vegetative Growth and Reproduction
4. Plasma Technology for Crop Sustainability and Food Processing
5. Future Prospects and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arora, N.K. Impact of climate change on agriculture production and its sustainable solutions. Environ. Sustain. 2019, 2, 95–96. [Google Scholar] [CrossRef] [Green Version]
- Bebber, D.P.; Ramotowski, M.A.T.; Gurr, S.J. Crop pests and pathogens move polewards in a warming world. Nat. Clim. Chang. 2013, 3, 985–988. [Google Scholar] [CrossRef]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant–pathogen warfare under changing climate conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef]
- Pathak, T.; Maskey, M.L.; Dahlberg, J.A.; Kearns, F.; Bali, K.M.; Zaccaria, D. Climate change trends and impacts on California agriculture: A detailed review. Agronomy 2018, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.; Bayer, P.E.; Edwards, D. Climate change and the need for agricultural adaptation. Curr. Opin. Plant Biol. 2019, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Oh, J.-S.; Ohta, T.; Shiratani, M.; Hori, M. Current status and future prospects of agricultural applications using atmospheric-pressure plasma technologies. Plasma Process. Polym. 2017, 2017, e1700073. [Google Scholar] [CrossRef]
- Nonogaki, H. Seed dormancy and germination—Emerging mechanisms and new hypotheses. Front. Plant Sci. 2014, 5, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubinov, A.; Lazarenko, E.; Selemir, V. Effect of glow discharge air plasma on grain crops seed. IEEE Trans. Plasma Sci. 2000, 28, 180–183. [Google Scholar] [CrossRef]
- Volin, J.C.; Denes, F.S.; Young, R.A.; Park, S.M.T. Modification of seed germination performance through cold plasma chemistry technology. Crop Sci. 2000, 40, 1706–1718. [Google Scholar] [CrossRef]
- Meiqiang, Y.; Mingjing, H.; Buzhou, M.; Tengcai, M. Stimulating effects of seed treatment by magnetized plasma on tomato growth and yield. Plasma Sci. Technol. 2005, 7, 3143–3147. [Google Scholar] [CrossRef]
- Šerá, B.; Stranák, V.; Serý, M.; Tichý, M.; Spatenka, P. Germination of Chenopodium Album in response to microwave plasma treatment. Plasma Sci. Technol. 2008, 10, 506–511. [Google Scholar] [CrossRef]
- Šerá, B.; Šerý, M.; Štrañák, V.; Špatenka, P.; Tichý, M. Does cold plasma affect breaking dormancy and seed germination? A study on seeds of Lamb’s quarters (Chenopodium album agg.). Plasma Sci. Technol. 2009, 11, 750–754. [Google Scholar] [CrossRef]
- Šerá, B.; Špatenka, P.; Šerý, M.; Vrchotova, N.; Hruskova, I. Influence of plasma treatment on wheat and oat germination and early growth. IEEE Trans. Plasma Sci. 2010, 38, 2963–2967. [Google Scholar] [CrossRef]
- Filatova, I.I.; Azharonok, V.V.; Kadyrov, M.A.; Beljavsky, V.; Gvozdov, A.; Shik, A.; Antonuk, A.E.; Belarus, N. The effect of plasma treatment of seeds of some grain and legumes on their sowing quality and productivity. Rom. Rep. Phys. 2011, 56, 139–143. [Google Scholar]
- Zhou, Z.; Huang, Y.; Yang, S.; Chen, W. Introduction of a new atmospheric pressure plasma device and application on tomato seeds. Agric. Sci. 2011, 2, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Drori, E. Cold radiofrequency plasma treatment modifies wettability and germination speed of plant seeds. Sci. Rep. 2012, 2, 741. [Google Scholar] [CrossRef]
- Šerá, B.; Gajdova, I.; Cernak, M.; Gavril, B.; Hnatiuc, E.; Kovacik, D.; Kriha, V.; Slama, J.; Šerý, M.; Špatenka, P. How various plasma sources may affect seed germination and growth. In Proceedings of the 13th International Conference on Optimization of Electrical and Electronic Equipment (OPTIM), Brasov, Romania, 24–26 May 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 1365–1370. [Google Scholar] [CrossRef]
- Ling, L.; Jiafeng, J.; Jiangang, L.; Minchong, S.; Xin, H.; Hanliang, S.; Yuanhua, D. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 2014, 4, 5859. [Google Scholar] [CrossRef] [Green Version]
- Mihai, A.L.; Dobrin, D.; Magureanu, M.; Popa, M.E. Possitive effect of non-thermal plasma treatment on radish seed. Rom. Rep. Phys. 2014, 66, 1110–1117. [Google Scholar]
- Tong, J.; He, R.; Zhang, X.; Zhan, R.; Chen, W.; Yang, S. Effects of atmospheric pressure air plasma pretreatment on the seed germination and early growth of Andrographis paniculata. Plasma Sci. Technol. 2014, 16, 260–266. [Google Scholar] [CrossRef] [Green Version]
- Bußler, S.; Herppich, W.B.; Neugart, S.; Schreiner, M.; Ehlbeck, J.; Rohn, S.; Schlüter, O. Impact of cold atmospheric pressure plasma on physiology and flavonol glycoside profile of peas (Pisum sativum “Salamanca”). Food Res. Int. 2015, 76, 132–141. [Google Scholar] [CrossRef]
- Dobrin, D.; Magureanu, M.; Mandache, N.B.; Ionita, M. The effect of non-thermal plasma treatment on wheat germination and early growth. Innov. Food Sci. Emerg. Technol. 2015, 29, 255–260. [Google Scholar] [CrossRef]
- Hayashi, N.; Ono, R.; Shiratani, M.; Yonesu, A. Antioxidative activity and growth regulation of Brassicaceae induced by oxygen radical irradiation. Jpn. J. Appl. Phys. 2015, 54, 6. [Google Scholar] [CrossRef]
- Ji, S.H.; Kim, T.; Panngom, K.; Hong, Y.J.; Pengkit, A.; Park, D.H.; Kang, M.H.; Lee, S.H.; Im, J.S.; Kim, J.S.; et al. Assessment of the effects of nitrogen plasma and plasma-generated nitric oxide on early development of Coriandum sativum. Plasma Process. Polym. 2015, 12, 1164–1173. [Google Scholar] [CrossRef]
- Ono, R.; Hayashi, N. Variation of antioxidative activity and growth enhancement of Brassicaceae induced by low-pressure oxygen plasma. Jpn. J. Appl. Phys. 2015, 54, 06GD03. [Google Scholar] [CrossRef]
- Peethambaran, B.; Han, J.; Kermalli, K.; Jiaxing, J.; Fridman, G.; Balsamo, R.; Fridman, A.A.; Miller, V. Nonthermal plasma reduces water consumption while accelerating Arabidopsis thaliana growth and fecundity. Plasma Med. 2015, 5, 87–98. [Google Scholar] [CrossRef]
- Stolárik, T.; Henselová, M.; Martinka, M.; Novák, O.; Zahoranová, A.; Černák, M. Effect of low-temperature plasma on the structure of seeds, growth and metabolism of endogenous phytohormones in pea (Pisum sativum L.). Plasma Chem. Plasma Process. 2015, 35, 659–676. [Google Scholar] [CrossRef]
- Ji, S.H.; Choi, K.H.; Pengkit, A.; Im, J.S.; Kim, J.S.; Kim, Y.H.; Park, Y.; Hong, E.J.; Jung, S.K.; Choi, E.H.; et al. Effects of high voltage nanosecond pulsed plasma and micro DBD plasma on seed germination, growth development and physiological activities in spinach. Arch. Biochem. Biophys. 2016, 605, 117–128. [Google Scholar] [CrossRef]
- Junior, C.A.; de Oliveira Vitoriano, J.O.; da Silva, D.L.S.; de Lima Farias, M.; de Lima Dantas, M.B. Water uptake mechanism and germination of Erythrina velutina seeds treated with atmospheric plasma. Sci. Rep. 2016, 6, 33722. [Google Scholar] [CrossRef]
- Park, Y.; Oh, K.S.; Oh, J.; Seok, D.C.; Kim, S.B.; Yoo, S.J.; Lee, M.-J. The biological effects of surface dielectric barrier discharge on seed germination and plant growth with barley. Plasma Process. Polym. 2016, 15, 1600056. [Google Scholar] [CrossRef]
- Sarinont, T.; Amano, T.; Attri, P.; Koga, K.; Hayashi, N.; Shiratani, M. Effects of plasma irradiation using various feeding gases on growth of Raphanus sativus L. Arch. Biochem. Biophys. 2016, 605, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhou, R.; Zhang, X.; Zhuang, J.; Yang, S.; Bazaka, K.; Ostrikov, K. Effects of atmospheric-pressure N2, He, Air, and O2 microplasmas on mung bean seed germination and seedling growth. Sci. Rep. 2016, 6, 32603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubinov, A.E.; Kozhayeva, J.P.; Zuimatch, E.A. Changing germination rate of brown mustard seeds after treatment with plasmas of nanosecond electric discharges. IEEE Trans. Plasma Sci. 2017, 45, 294–300. [Google Scholar] [CrossRef]
- Gómez-Ramírez, A.; López-Santos, C.; Cantos, M.; García, J.L.; Molina, R.; Cotrino, J.; Espinós, J.P.; González-Elipe, A.R. Surface chemistry and germination improvement of Quinoa seeds subjected to plasma activation. Sci. Rep. 2017, 7, 5924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Y.; Qu, G.; Wang, T.; Sun, Q.; Liang, D.; Hu, S. Enhancement of germination and seedling growth of wheat seed using dielectric barrier discharge plasma with various gas sources. Plasma Chem. Plasma Process. 2017, 37, 1105–1119. [Google Scholar] [CrossRef]
- Pawlat, J.; Starek, A.; Sujak, A.; Kwiatkowski, M.; Terebun, P.; Budzeń, M. Effects of atmospheric pressure plasma generated in GlidArc reactor on Lavatera thuringiaca L. seeds’ germination. Plasma Process. Polym. 2017, 15, 1700064. [Google Scholar] [CrossRef]
- Safari, N.; Iranbakhsh, A.; Oraghi Ardebili, Z. Non-thermal plasma modified growth and differentiation process of Capsicum annuum PP805 Godiva in in vitro conditions. Plasma Sci. Technol. 2017, 19, 055501. [Google Scholar] [CrossRef]
- Šerá, B.; Šerý, M.; Gavril, B.; Gajdova, I. Seed germination and early growth responses to seed pre-treatment by non-thermal plasma in hemp cultivars (Cannabis sativa L.). Plasma Chem. Plasma Process. 2017, 37, 207–221. [Google Scholar] [CrossRef]
- Da Silva, A.R.M.; Farias, M.L.; da Silva, D.L.S.; Vitoriano, J.O.; de Sousa, R.C.; Alves-Junior, C. Using atmospheric plasma to increase wettability, imbibition and germination of physically dormant seeds of Mimosa caesalpiniafolia. Colloids Surfaces B 2017, 157, 280–285. [Google Scholar] [CrossRef]
- Zhang, J.J.; Jo, J.O.; Huynh, D.L.; Mongre, R.K.; Ghosh, M.; Singh, A.K.; Lee, S.B.; Mok, Y.S.; Hyuk, P.; Jeong, D.K. Growth-inducing effects of argon plasma on soybean sprouts via the regulation of demethylation levels of energy metabolism-related genes. Sci. Rep. 2017, 7, 41917. [Google Scholar] [CrossRef]
- Matra, K. Atmospheric non-thermal argon–oxygen plasma for sunflower seedling growth improvement. Jpn. J. Appl. Phys. 2018, 57, 01AG03. [Google Scholar] [CrossRef]
- Pawlat, J.; Starek, A.; Sujak, A.; Terebun, P.; Kwiatkowski, M.; Budzeń, M.; Andrejko, D. Effects of atmospheric pressure plasma jet operating with DBD on Lavatera thuringiaca L. seeds’ germination. PLoS ONE 2018, 13, e0194349. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Sajib, S.A.; Rahi, M.S.; Tahura, S.; Roy, N.C.; Parvez, S.; Reza, M.A.; Talukder, M.R.; Kabir, A.H. Mechanisms and signaling associated with LPDBD plasma mediated growth improvement in wheat. Sci. Rep. 2018, 8, 10498. [Google Scholar] [CrossRef] [Green Version]
- Ahn, C.; Gill, J.; Ruzic, D.N. Growth of plasma-treated corn seeds under realistic conditions. Sci. Rep. 2019, 9, 4355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadhlalmawla, S.A.; Mohamed, A.-A.H.; Almarashi, J.Q.; Boutraa, T. The impact of cold atmospheric pressure plasma jet on seed germination and seedlings growth of fenugreek (Trigonella foenum-graecum). Plasma Sci. Technol. 2019, 21, 105503. [Google Scholar] [CrossRef]
- Holc, M.; Primc, G.; Iskra, J.; Titan, P.; Kovač, J.; Mozetič, M.; Junkar, I. Effect of oxygen plasma on sprout and root growth, surface morphology and yield of garlic. Plants 2019, 8, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, T.; Farooq, M.; Afsheen, S.; Abrar, M.; Yousaf, M.; Ijaz, M. Cold plasma treatment and laser irradiation of Triticum spp. seeds for sterilization and germination. J. Laser Appl. 2019, 31, 42013. [Google Scholar] [CrossRef]
- Li, L.; Guo, H.; Zong, J.; Chen, J.; Wang, Y.; Li, J.; Li, D.; Shao, H.; Liu, J. Influence of low-vacuum helium cold plasma pre-treatment on the rooting and root growth of zoysiagrass (Zoysia willd.) stolon cuttings. Plasma Sci. Technol. 2019, 21, 55504. [Google Scholar] [CrossRef]
- Pérez-Pizá, M.C.; Prevosto, L.; Grijalba, P.E.; Zilli, C.G.; Cejas, E.; Mancinelli, B.; Balestrasse, K.B. Improvement of growth and yield of soybean plants through the application of non-thermal plasmas to seeds with different health status. Heliyon 2019, 5, e01495. [Google Scholar] [CrossRef] [Green Version]
- Volkov, A.G.; Hairston, J.S.; Patel, D.; Gott, R.P.; Xu, K.G. Cold plasma poration and corrugation of pumpkin seed coats. Bioelectrochemistry 2019, 128, 175–185. [Google Scholar] [CrossRef]
- Abedi, S.; Iranbakhsh, A.; Oraghi Ardebili, Z.O.; Ebadi, M. Seed priming with cold plasma improved early growth, flowering, and protection of Cichorium intybus against selenium nanoparticle. J. Theor. Appl. Phys. 2020, 14, 113–119. [Google Scholar] [CrossRef]
- Ambrico, P.F.; Šimek, M.; Ambrico, M.; Morano, M.; Prukner, V.; Minafra, A.; Allegretta, I.; Porfido, C.; Senesi, G.S.; Terzano, R. On the air atmospheric pressure plasma treatment effect on the physiology, germination and seedlings of basil seeds. J. Phys. D Appl. Phys. 2020, 53, 104001. [Google Scholar] [CrossRef]
- Ghasempour, M.; Iranbakhsh, A.; Ebadi, M.; Ardebili, Z.O. Seed priming with cold plasma improved seedling performance, secondary metabolism, and expression of deacetylvindoline O-acetyltransferase gene in Catharanthus roseus. Contrib. Plasma Phys. 2020, 60, e201900159. [Google Scholar] [CrossRef]
- Mujahid, Z.; Tounekti, T.; Khemira, H. Cold plasma treatment to release dormancy and improve growth in grape buds: A promising alternative to natural chilling and rest breaking chemicals. Sci. Rep. 2020, 10, 2667. [Google Scholar] [CrossRef] [PubMed]
- Park, D.P.; Davis, K.; Gilani, S.; Alonzo, C.-A.; Dobrynin, D.; Friedman, G.; Fridman, A.; Rabinovich, A.; Fridman, G. Reactive nitrogen species produced in water by non-equilibrium plasma increase plant growth rate and nutritional yield. Curr. Appl. Phys. 2013, 13, S19–S29. [Google Scholar] [CrossRef]
- Lindsay, A.; Byrns, B.; King, W.; Andhvarapou, A.; Fields, J.; Knappe, D.; Fonteno, W.; Shannon, S. Fertilization of radishes, tomatoes, and marigolds using a large-volume atmospheric glow discharge. Plasma Chem. Plasma Process. 2014, 34, 1271–1290. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Khacef, A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: Combined effect of seed and water treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef] [Green Version]
- Bafoil, M.; Jemmat, A.; Martinez, Y.; Merbahi, N.; Eichwald, O.; Dunand, C.; Yousfi, M. Effects of low temperature plasmas and plasma activated waters on Arabidopsis thaliana germination and growth. PLoS ONE 2018, 13, e0195512. [Google Scholar] [CrossRef]
- Judée, F.; Simon, S.; Bailly, C.; Dufour, T. Plasma-activation of tap water using DBD for agronomy applications: Identification and quantification of long lifetime chemical species and production/consumption mechanisms. Water Res. 2018, 133, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.-J.; Khan, M.S.I.; Shim, J.; Kim, Y.-J. Roles of oxides of nitrogen on quality enhancement of soybean sprout during hydroponic production using plasma discharged water recycling technology. Sci. Rep. 2018, 8, 16872. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Park, G.; Choi, E.H. Cold atmospheric plasma-activated water irrigation induces defense hormone and gene expression in tomato seedlings. Sci. Rep. 2019, 9, 16080. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Zhang, A.; Héroux, P.; Sand, W.; Sun, Z.; Zhan, J.; Wang, C.; Hao, S.; Li, Z.; Li, Z.; et al. Effect of dielectric barrier discharge cold plasma on pea seed growth. J. Agric. Food Chem. 2019, 67, 10813–10822. [Google Scholar] [CrossRef] [PubMed]
- Iwata, N.; Gamaleev, V.; Hashizume, H.; Oh, J.; Ohta, T.; Ishikawa, K.; Hori, M.; Ito, M. Simultaneous achievement of antimicrobial property and plant growth promotion using plasma-activated benzoic compound solution. Plasma Process. Polym. 2019, 16, e1900023. [Google Scholar] [CrossRef]
- Kang, M.H.; Jeon, S.S.; Shin, S.M.; Veerana, M.; Ji, S.-H.; Uhm, H.-S.; Choi, E.H.; Shin, J.H.; Park, G. Dynamics of nitric oxide level in liquids treated with microwave plasma-generated gas and their effects on spinach development. Sci. Rep. 2019, 9, 1011. [Google Scholar] [CrossRef]
- Liu, B.; Honnorat, B.; Yang, H.; Arancibia, J.; Rajjou, L.; Rousseau, A. Non-thermal DBD plasma array on seed germination of different plant species. J. Phys. D Appl. Phys. 2019, 52, 025401. [Google Scholar] [CrossRef]
- Zhou, R.; Li, J.; Zhou, R.; Zhang, X.; Yang, S. Atmospheric-pressure plasma treated water for seed germination and seedling growth of mung bean and its sterilization effect on mung bean sprouts. Innov. Food Sci. Emerg. 2019, 53, 36–44. [Google Scholar] [CrossRef]
- Krapivina, S.A.; Filippov, A.K.; Levitskaya, T.N.; Bakhvalov, A. Gas Plasma Treatment of Plant Seeds. U.S. Patent No. US5281315A, 25 January 1994. [Google Scholar]
- Mitra, A.; Li, Y.F.; Klämpfl, T.G.; Shimizu, T.; Jeon, J.; Morfill, G.E.; Zimmermann, J.L. Inactivation of surface-borne microorganisms and increased germination of seed specimen by cold atmospheric plasma. Food Bioprocess Technol. 2014, 7, 645–653. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-Q.; Zhou, R.-W.; de Groot, G.; Bazaka, K.; Murphy, A.B.; Ostrikov, K. Spectral characteristics of cotton seeds treated by a dielectric barrier discharge plasma. Sci. Rep. 2017, 7, 5601. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Lan, Q.; Pritchard, H.W.; Xue, H.; Wang, X. Reactive oxygen species induced by cold stratification promote germination of Hedysarum scoparium seeds. Plant Physiol. Bioch. 2016, 109, 406–415. [Google Scholar] [CrossRef]
- Mildažienė, V.; Aleknavičiūtė, V.; Žūkienė, R.; Paužaitė, G.; Naučienė, Z.; Filatova, I.; Lyushkevich, V.; Haimi, P.; Tamošiūnė, I.; Baniulis, D. Treatment of common sunflower (Helianthus annus L.) seeds with radio-frequency electromagnetic field and cold plasma induces changes in seed phytohormone balance, seedling development and leaf protein expression. Sci. Rep. 2019, 9, 6437. [Google Scholar] [CrossRef]
- Huijser, P.; Schmid, M. The control of developmental phase transitions in plants. Development 2011, 138, 4117–4129. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Thakur, A.K.; Vikram, A.; Vaid, A.; Rane, R. Effect of plasma treatment on growth and yield of okra [Abelmoschus esculentus (L.) under field conditions. Int. J. Bio-Resour. Stress Manag. 2017, 8, 659–667. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Shao, H.; Dong, Y. Effects of low-vacuum helium cold plasma treatment on seed germination, plant growth and yield of oilseed rape. Plasma Sci. Technol. 2018, 20, 095502. [Google Scholar] [CrossRef] [Green Version]
- Seddighinia, F.S.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Nejad Satari, T.; Soleimanpour, S. Seed priming with cold plasma and multi-walled carbon nanotubes modified growth, tissue differentiation, anatomy, and yield in bitter melon (Momordica charantia). J. Plant Growth Regul. 2019, 39, 87–98. [Google Scholar] [CrossRef]
- Mhamdi, A.; Van Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- de Simone, A.; Hubbard, R.; de la Torre, N.V.; Velappan, Y.; Wilson, M.; Considine, M.J.; Soppe, W.J.J.; Foyer, C.H. Redox changes during the cell cycle in the embryonic meristem of Arabidopsis thaliana. Antioxid. Redox Signal. 2017, 27, 1505–1519. [Google Scholar] [CrossRef] [Green Version]
- Tognetti, V.B.; Bielach, A.; Hrtyan, M. Redox regulation at the site of primary growth: Auxin, cytokinin and ROS crosstalk. Plant Cell Environ. 2017, 40, 2586–2605. [Google Scholar] [CrossRef]
- Livanos, P.; Galatis, B.; Quader, H.; Apostolakos, P. Disturbance of reactive oxygen species homeostasis induces atypical tubulin polymer formation and affects mitosis in root-tip cells of Triticum turgidum and Arabidopsis thaliana. Cytoskeleton 2012, 69, 1–21. [Google Scholar] [CrossRef]
- Tsukagoshi, H. Control of root growth and development by reactive oxygen species. Curr. Opin. Plant Biol. 2016, 29, 57–63. [Google Scholar] [CrossRef]
- Hu, L.; Liang, W.; Yin, C.; Cui, X.; Zong, J.; Wang, X.; Hu, J.; Zhang, D. Rice MADS3 regulates ROS homeostasis during late anther development. Plant Cell 2011, 23, 515–533. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Fan, M.; Qin, Z.; Lv, H.; Wang, M.; Zhang, Z.; Zhou, W.; Zhao, N.; Li, X.; Han, C.; et al. Hydrogen peroxide positively regulates brassinosteroid signaling through oxidation of the BRASSINAZOLE-RESISTANT1 transcription factor. Nat. Commun. 2018, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Jiangang, L.; Minchong, S.; Chunlei, Z.; Yuanhua, D. Cold plasma treatment enhances oilseed rape seed germination under drought stress. Sci. Rep. 2015, 5, 13033. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Lu, Y.; Li, J.; Li, L.; He, X.; Shao, H.; Dong, Y. Effect of seed treatment by cold plasma on the resistance of tomato to Ralstonia solanacearum (Bacterial Wilt). PLoS ONE 2014, 9, e97753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buonopane, G.J.; Antonacci, C.; Lopez, J. Effect of cold plasma processing on botanicals and their essential oils. Plasma Med. 2016, 6, 315–324. [Google Scholar] [CrossRef]
- Pérez Pizá, M.C.; Prevosto, L.; Zilli, C.; Cejas, E.; Kelly, H.; Balestrasse, K. Effects of non-thermal plasmas on seed-borne Diaporthe/Phomopsis complex and germination parameters of soybean seeds. Innov. Food Sci. Emerg. Technol. 2018, 49, 82–91. [Google Scholar] [CrossRef]
- Perez, S.M.; Biondi, E.; Laurita, R.; Proto, M.; Sarti, F.; Gherardi, M.; Bertaccini, A.; Colombo, V. Plasma activated water as resistance inducer against bacterial leaf spot of tomato. PLoS ONE 2019, 14, e0217788. [Google Scholar] [CrossRef]
- Kyzek, S.; Holubová, Ľ.; Medvecká, V.; Tomeková, J.; Gálová, E.; Zahoranová, A. Cold atmospheric pressure plasma can induce adaptive response in pea seeds. Plasma Chem. Plasma Process. 2018, 39, 475–486. [Google Scholar] [CrossRef]
- Bafoil, M.; Le Ru, A.; Merbahi, N.; Eichwald, O.; Dunand, C.; Yousfi, M. New insights of low-temperature plasma effects on germination of three genotypes of Arabidopsis thaliana seeds under osmotic and saline stresses. Sci. Rep. 2019, 9, 8649. [Google Scholar] [CrossRef] [Green Version]
- Kabir, A.H.; Rahman, M.M.; Das, U.; Sarkar, U.; Roy, N.C.; Reza, M.A.; Talukdar, M.R.; Uddin, M.A. Reduction of cadmium toxicity in wheat through plasma technology. PLoS ONE 2019, 14, e0214509. [Google Scholar] [CrossRef] [Green Version]
- Gierczik, K.; Vukušić, T.; Kovács, L.; Székely, A.; Szalai, G.; Milošević, S.; Kocsy, G.; Kutasi, K.; Galiba, G. Plasma-activated water to improve the stress tolerance of barley. Plasma Process. Polym. 2020, 17, e1900123. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Chandra Adhikari, B.; Park, G.; Choi, E.H. Cold plasma seed priming modulates growth, redox homeostasis and stress response by inducing reactive species in tomato (Solanum lycopersicum). Free Radic. Biol. Med. 2020, 156, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Srey, S.; Park, S.Y.; Jahid, I.K.; Ha, S.-D. Reduction effect of the selected chemical and physical treatments to reduce L. monocytogenes biofilms formed on lettuce and cabbage. Food Res. Int. 2014, 62, 484–491. [Google Scholar] [CrossRef]
- Lacombe, A.; Niemira, B.A.; Gurtler, J.B.; Fan, X.; Sites, J.; Boyd, G.; Chen, H. Atmospheric cold plasma inactivation of aerobic microorganisms on blueberries and effects on quality attributes. Food Microbiol. 2015, 46, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wang, G.; Tian, Y.; Wang, K.; Zhang, J.; Fang, J. Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J. Hazard. Mater. 2015, 300, 643–651. [Google Scholar] [CrossRef]
- Tappi, S.; Gozzi, G.; Vannini, L.; Berardinelli, A.; Romani, S.; Ragni, L.; Rocculi, P. Cold plasma treatment for fresh-cut melon stabilization. Innov. Food Sci. Emerg. Technol. 2016, 33, 225–233. [Google Scholar] [CrossRef]
- Trevisani, M.; Berardinelli, A.; Cevoli, C.; Cecchini, M.; Ragni, L.; Pasquali, F. Effects of sanitizing treatments with atmospheric cold plasma, SDS and lactic acid on verotoxin-producing Escherichia coli and Listeria monocytogenes in red chicory (radicchio). Food Control 2017, 78, 138–143. [Google Scholar] [CrossRef]
- Lee, T.; Puligundla, P.; Mok, C. Intermittent corona discharge plasma jet for improving tomato quality. J. Food Eng. 2018, 223, 168–174. [Google Scholar] [CrossRef]
- Segura-Ponce, L.; Reyes, J.; Troncoso-Contreras, G.; Valenzuela-Tapia, G. Effect of low-pressure cold plasma (LPCP) on the wettability and the inactivation of Escherichia coli and Listeria innocua on fresh-cut apple (Granny Smith) skin. Food Bioprocess Technol. 2018, 11, 1075–1086. [Google Scholar] [CrossRef]
- Ramazzina, I.; Berardinelli, A.; Rizzi, F.; Tappi, S.; Ragni, L.; Sacchetti, G.; Rocculi, P. Effect of cold plasma treatment on physico-chemical parameters and antioxidant activity of minimally processed kiwifruit. Postharvest Biol. Technol. 2015, 107, 55–65. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, Y.; Ma, R.; Liu, Q.; Zhang, J. Effect of plasma activated water on the postharvest quality of button mushrooms, Agaricus bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef]
- Oh, Y.J.; Song, A.Y.; Min, S.C. Inhibition of Salmonella typhimurium on radish sprouts using nitrogen-cold plasma. Int. J. Food Microbiol. 2017, 249, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Won, M.Y.; Lee, S.J.; Min, S.C. Mandarin preservation by microwave-powered cold plasma treatment. Innov. Food Sci. Emerg. Technol. 2017, 39, 25–32. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, X.; Liu, S.; Ma, Y.; Xu, C.; Bai, Y. Effect of plasma-activated water on microbial quality and physicochemical characteristics of mung bean sprouts. Innov. Food Sci. Emerg. Technol. 2018, 52, 49–56. [Google Scholar] [CrossRef]
- Iranbakhsh, A.; Oraghi Ardebili, Z.; Molaei, H.; Oraghi Ardebili, N.; Amini, M. Cold plasma up-regulated expressions of WRKY1 transcription factor and genes involved in biosynthesis of cannabinoids in hemp (Cannabis sativa L.). Plasma Chem. Plasma Process. 2020, 40, 527–537. [Google Scholar] [CrossRef]
- Ochi, A.; Konishi, H.; Ando, S.; Sato, K.; Yokoyama, K.; Tsushima, S.; Yoshida, S.; Morikawa, T.; Kaneko, T.; Takahashi, H. Management of bakanae and bacterial seedling blight diseases in nurseries by irradiating rice seeds with atmospheric plasma. Plant Pathol. 2016, 66, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Bourke, P.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Keener, K. The potential of cold plasma for safe and sustainable food production. Trends Biotechnol. 2018, 36, 615–626. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, B.; Pangomm, K.; Veerana, M.; Mitra, S.; Park, G. Plant disease control by non-thermal atmospheric-pressure plasma. Front. Plant Sci. 2020, 11, 77. [Google Scholar] [CrossRef]
- Ziuzina, D.; Misra, N.; Cullen, P.; Keener, K.; Mosnier, J.; Vilaró, I.; Gaston, E.; Bourke, P. Demonstrating the potential of industrial scale in-package atmospheric cold plasma for decontamination of cherry tomatoes. Plasma Med. 2016, 6, 397–412. [Google Scholar] [CrossRef] [Green Version]
- Schnabel, U.; Schmidt, C.; Stachowiak, J.; Bösel, A.; Andrasch, M.; Ehlbeck, J. Plasma processed air for biological decontamination of PET and fresh plant tissue. Plasma Process. Polym. 2018, 15, 1600057. [Google Scholar] [CrossRef]
- Pan, Y.; Cheng, J.; Sun, D. Cold plasma-mediated treatments for shelf life extension of fresh produce: A review of recent research developments. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1312–1326. [Google Scholar] [CrossRef]
- Mehta, D.; Sharma, N.; Bansal, V.; Sangwan, R.S.; Yadav, S.K. Impact of ultrasonication, ultraviolet and atmospheric cold plasma processing on quality parameters of tomato-based beverage in comparison with thermal processing. Innov. Food Sci. Emerg. Technol. 2019, 52, 343–349. [Google Scholar] [CrossRef]
- Kovačević Bursać, D.; Gajdoš Kljusurić, J.; Putnik, P.; Vukušić, T.; Herceg, Z.; Dragović-Uzelac, V. Stability of polyphenols in chokeberry juice treated with gas phase plasma. Food Chem. 2016, 212, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.; Patil, S.; Moiseev, T.; Bourke, P.; Mosnier, J.; Keener, K.; Cullen, P. In-package atmospheric pressure cold plasma treatment of strawberries. J. Food Eng. 2014, 125, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Ramazzina, I.; Tappi, S.; Rocculi, P.; Sacchetti, G.; Berardinelli, A.; Marseglia, A.; Rizzi, F. Effect of cold plasma treatment on the functional properties of fresh-cut apples. J. Agric. Food Chem. 2016, 64, 8010–8018. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, S.K.; Wan, Z.; Keener, K.M. Effects of cold plasma on food quality: A review. Foods 2018, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, F.D.L.; Cavalcante, R.S.; Cullen, P.J.; Frias, J.M.; Bourke, P.; Fernandes, F.A.; Rodrigues, S. Effects of atmospheric cold plasma and ozone on prebiotic orange juice. Innov. Food Sci. Emerg. Technol. 2015, 32, 127–135. [Google Scholar] [CrossRef]
- Rodríguez, Ó.; Gomes, W.F.; Rodrigues, S.; Fernandes, F.A. Effect of indirect cold plasma treatment on cashew apple juice (Anacardium occidentale L.). LWT Food Sci. Technol. 2017, 84, 457–463. [Google Scholar] [CrossRef]
- Ben’ko, E.M.; Manisova, O.R.; Lunin, V.V. Effect of ozonation on the reactivity of lignocellulose substrates in enzymatic hydrolyses to sugars. Russ. J. Phys. Chem. A 2013, 87, 1108–1113. [Google Scholar] [CrossRef]

| Plant Species | Plasma Source | Feeder Gas | Treated Stage | Enhanced Effects | Reference |
|---|---|---|---|---|---|
| Avena sativa Hordeum vulgare | Glow discharge air plasma | Air | Seed | Germination | [8] |
| Hordeum vulgare Raphanus sativus Pisum sativum Glycine max L. Merr. Zea mays L. Phaseolus vulgaris L. | Low-pressure RF (radio frequency) rotating plasma | carbon tetrafluoride (CF4)/octadecafluorodeca -lin (ODFD) | Seed | Germination | [9] |
| Lycopersicon esculentum L. Mill. cv. Zhongshu No. 6 | Magnetized plasma | Seedling | Growth and productivity | [10] | |
| Chenopodium album agg. | Low-pressure microwave plasma | Mixture of Argon (Ar), Nitrogen (N2), and Oxygen (O2) | Seed | Germination | [11,12] |
| Avena sativa Triticum aestivum | Plasma plant Plasonic AR-550-M | Air | Seed | Germination and early growth | [13] |
| Lupinus angustifolius Galega virginiana Melilotus albus | RF air Plasma | Air | Seed | Germination and productivity | [14] |
| Solanum lycopersicum | DBD (dielectric barrier discharge) air plasma | Air | Seed | Growth and yield | [15] |
| Lens culinaris Phaseolus vulgaris Triticum | Cold radiofrequency Air plasma | Air | Seed | Germination | [16] |
| Fagopyrum aeseulentum | GlidArc plasma Surface DBD plasma Downstream microwave plasma Planar rotating electrode plasma | Air and mixture of air with water vapors | Seed | Germination (depending on plasma sources) | [17] |
| Glycine max L. Merr. cv. Zhongdou 40 | Low-pressure RF helium plasma | Vacuum | Seed and seedling | Germination and growth | [18] |
| Raphanus sativum var. Icicle | Surface discharge plasma | Air | Seed | Early growth | [19] |
| Andrographis paniculata | DBD air plasma | Air | Seed | Germination and growth | [20] |
| Pisum sativum | Surface DBD plasma | Air | Seed, sprout, and seedling | Germination and flavonol glycoside | [21] |
| Triticum aestivum | Surface discharge plasma | Air | Seed and vegetative stage | Germination and growth | [22] |
| Raphanus sativus var. longipinnatus | DBD plasma | Pure Oxygen (O2) | Seed and vegetative stage | Germination and growth | [23] |
| Coriander sativum | DBD N2 (nitrogen) plasma Microwave plasma generated gas | Nitrogen (N2) | Seed | Germination | [24] |
| Brassicaceae | Low-pressure RF O2 (oxygen) plasma | Oxygen (O2) | Seed | Antioxidant activity | [25] |
| Arabidopsis thaliana | Gliding arc air plasma | Air | Seed and reproductive stage | Germination and growth | [26] |
| Pisum sativum L. var. Prophet | Diffuse coplanar surface DBD plasma | Air | Seed and seedling | Germination and growth | [27] |
| Spinacia oleracea L. | High voltage nanosecond pulsed plasma Micro DBD plasma | Air and nitrogen (N2) gas | Seed | Germination and growth | [28] |
| Erythrina velutina | DBD He plasma | Helium (He) gas | Seed | Germination | [29] |
| Hordeum vulgare | Surface DBD plasma | Nitrogen (N2) with bubble air | Seed | Germination, growth, and GABA content | [30] |
| Raphanus sativus L. | DBD plasma with various feeding gases | Air, oxygen (O2), nitrogen (N2), helium(He), argon (Ar), and NO(10%)+nitrogen | Seed | Growth (depending on feeding gas and moisture) | [31] |
| Mung bean | DBD plasma generated in water using various gas | Air, oxygen (O2), nitrogen (N2), and helium (He) | Seed | Germination and growth | [32] |
| Brassica juncea L. | Nanosecond microspark plasma | Air | Seed | Germination | [33] |
| Chenopodium quinoa | DBD RF air plasma under atmospheric and low pressure | Air | Seed | Germination | [34] |
| Wheat | DBD plasma with various feeding gases | Air, oxygen (O2), nitrogen (N2), and argon (Ar) | Seed | Germination and growth | [35] |
| Lavatera thuringiaca L. | Gliding arc discharge N2 plasma | Nitrogen (N2) | Seed | Germination | [36] |
| Capsicum annuum | DBD Ar (argon) plasma | Argon (Ar) | Seed | Growth | [37] |
| Cannabis sativa L. | Gliding arc plasma Microwave plasma | Oxygen (O2) and argon (Ar) | Seed and vegetative stage | Germination and growth | [38] |
| Mimosa caesalpiniafolia | DBD plasma | Air | Seed | Germination | [39] |
| Glycine max L. Merrill | DBD Ar plasma | Argon (Ar) | Seed | Germination and growth | [40] |
| Sunflower | Ar/O2 plasma | Oxygen (O2) and argon (Ar) | Seed | Growth | [41] |
| Lavatera thuringiaca L. | DBD plasma jet with N2/He gas | Nitrogen (N2), and helium (He) | Seed | Germination | [42] |
| Wheat | Low-pressure DBD plasma with Ar/O2 and Ar/air gases | Air, oxygen (O2) and argon (Ar) | Seed | Germination and growth | [43] |
| Corn | Microwave plasma jet DBD He plasma Low-pressure RF N2 plasma | Nitrogen (N2), and helium (He) | Seed | Growth and yield (field) | [44] |
| Trigonella foenum-graecum | Ar plasma jet | Argon (Ar) | Seed | Germination and growth | [45] |
| Allium sativum Ptujski spomladanski | Low-pressure RF O2 plasma | Oxygen (O2) | Seed and seedling | Germination and growth | [46] |
| Triticum spp. | Ar plasma Q-switched Nd:YAG (Quantel Brilliant) pulsed laser | Argon (Ar) | Seed | Germination and sterilization | [47] |
| Zoysia willd. | Low-vaccum He plasma | Helium (He) and air | Seedling | Growth | [48] |
| Glycine max L. Merrill | DBD plasma | Oxygen (O2) and nitrogen (N2) | Seed and seedling | Germination and growth | [49] |
| Cucurbita pepo L. cv. Cinderella Cucurbita maxima L. cv. Jarrahdale Cucurbita maxima L. cv. Warty Goblin | Cold atmospheric pressure plasma | Helium (He) and argon (Ar) | Seed | Germination | [50] |
| Cichorium intybus | DBD plasma (Model PS200) | Argon (Ar) | Seed and seedling | Germination, growth, and flowering | [51] |
| Ocimum basilicum | Volume barrier discharge plasma | Humid Air (40% RH) | Seed | Germination | [52] |
| Catharanthus roseus | DBD plasma | Argon (Ar) | Seed | Growth and physiology | [53] |
| Vitis vinifera | DBD Ar plasma | Argon (Ar) | Seed and seedling | Germination and growth | [54] |
| Plant Species | Plasma Source | Feeder Gas | Treated Stage | Enhanced Effects | Reference |
|---|---|---|---|---|---|
| Citrullus lanatus Zinnia peruviana Medicago sativa Phaseolus cocconeus | Plasma-treated water | Air | Vegetative stage | Growth | [55] |
| Janie marigold Better Boy tomato Early Scarlet radish | Plasma-treated water | Air | Seed and seedling | Growth | [56] |
| Raphanus sativus Solanum lycopersicum Capsicum annum | DBD air plasma and Plasma activated water | Air | Seed and vegetative stage | Germination and growth | [57] |
| Arabidopsis thaliana | DBD air and He (helium) plasma Plasma-treated water | Air and Helium (He) | Seed and seedling | Germination and growth | [58] |
| Coral lentils (Lens culinaris) | Plasma-treated tap water | Air | Seed | Growth | [59] |
| Glycine max L. Merrill | Plasma-treated water | Air | Seed | Growth and quality | [60] |
| Solanum lycopersicum | Plasma-treated water | Air | Seedling | Growth | [61] |
| Pisum sativum L. | DBD plasma Plasma-treated tap water | Air | Seed and seedling | Germination, growth, and flowering | [62] |
| Radish sprout | Plasma-treated organic solutions | Argon (Ar) and oxygen (O2) mixture | Seedling | Growth | [63] |
| Spinacia oleracea L. | Plasma-treated water | Mixture of oxygen (O2) and nitrogen (N2) | Seed | Growth | [64] |
| Tomato Lettuce Mung bean Sticky bean Radish Dianthus Mustard Wheat | DBD plasma Plasma-treated water | Air, oxygen (O2) and nitrogen (N2) | Seed | Germination and growth | [65] |
| Mung bean | Plasma-treated water | Air, oxygen (O2), nitrogen (N2), and helium (He) | Seed | Germination and disease tolerance | [66] |
| Plant Species | Plasma Source | Feeder Gas | Treated Stage | Improved Effects | Reference |
|---|---|---|---|---|---|
| Pre-harvest tolerance to biotic stresses | |||||
| Solanum lycopersicum | RF helium plasma | Helium (He) | Seed | Bacterial wilt resistance | [85] |
| Glycine max | DBD O2 and N2 plasma | Oxygen (O2) and nitrogen (N2) | Seed | Diaporthe/Phomopsis fungal resistance | [87] |
| Solanum lycopersicum cv. Moneymaker and VF010 | Plasma-activated water | Ambient air | Seedling | Bacterial leaf spot resistance | [88] |
| Pre-harvest tolerance to abiotic stresses | |||||
| Brassica napus | He plasma discharge | Helium (He) | Seed | Drought stress tolerance | [84] |
| Pisum sativum L. | Coplanar DBD plasma | Ambient Air | Seed | Tolerance to zeocinLess DNA damage | [89] |
| Arabidopsis thaliana | DBD air plasma | Air and helium (He) | Seed | Salt stress tolerance | [90] |
| Triticum aestivum | Low-pressure DBD plasma with Ar/O2 and Ar/air gases | Argon (Ar)/oxygen (O2) and argon (Ar)/air mixture | Seed | Tolerance to cadmium (Cd) | [91] |
| Hordeum vulgare | Plasma-activated water | Nitrogen (N2) | Seed | Tolerance to low temperature and hypoxia | [92] |
| Solanum lycopersicum | Air Plasma Jet | Air | Seed | Tolerance to PEG (polyethlene glycol)-mediated drought stress | [93] |
| Post-harvest sanitation | |||||
| Lactuca sativaBrassica oleracea sp. Capitata | Cold oxygen plasma lamp (Photoplasma, Model: Induct ID60) | Oxygen (O2) | Lettuce and cabbage vegetables | L. monocytogenes biofilm removal | [94] |
| Blueberries | AC plasma jet | Air | Blueberry fruits | Removed microbial contamination | [95] |
| Strawberries | Plasma-activated water | Argon (Ar)/oxygen (O2) mixture | Strawberry fruits | Removed microbial contamination | [96] |
| Cucumis melo L. var. Reticolatus cv. Raptor | DBD plasma | Air | Melon fruits | Removed microbial contamination | [97] |
| Red chicory | DBD plasma | Air | Chicory vegetables | Reduced microbial contamination | [98] |
| Lycopersicum esculentum Mill. | Intermittent corona discharge plasma jet | Air | Cherry tomato fruits | Reduced microbial contamination and increased shelf life | [99] |
| Apple cv. Granny Smith | Low-pressure plasma (expanded plasma cleaner PDC-001/002) | Argon (Ar), nitrogen (N2), oxygen (O2), and Argon–oxygen (Ar-O2) | Apple fruits | Removed microbial contamination | [100] |
| Post-harvest quality | |||||
| Actinidia deliciosa cv. Hayward | DBD plasma | Air | Kiwi fruits | Improved visual quality and extended storage life | [101] |
| Agaricus bisporus | Plasma jetPlasma-activated water | Argon–oxygen (Ar-O2) | Button mushrooms | Reduced microbial contamination and delayed softening | [102] |
| Radish sprouts | Microwave N2 plasma | Nitrogen (N2) | Radish sprout vegetables | Reduced moisture content during storage without changing antioxidant activity or ascorbic acid concentration. | [103] |
| Mandarins | Microwave N2, He, N2 + O2 plasma | Nitrogen (N2), helium (He) and nitrogen (N2)/oxygen (O2) mixture | Mandarin fruits | Increased antioxidant activity and phenolic content | [104] |
| Mung bean sprouts | Plasma-activated water | Air | Mung bean sprout vegetables | Reduced microbial contamination without changing polyphenolic and flavonoid contents. | [105] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, B.; Adhikari, M.; Park, G. The Effects of Plasma on Plant Growth, Development, and Sustainability. Appl. Sci. 2020, 10, 6045. https://doi.org/10.3390/app10176045
Adhikari B, Adhikari M, Park G. The Effects of Plasma on Plant Growth, Development, and Sustainability. Applied Sciences. 2020; 10(17):6045. https://doi.org/10.3390/app10176045
Chicago/Turabian StyleAdhikari, Bhawana, Manish Adhikari, and Gyungsoon Park. 2020. "The Effects of Plasma on Plant Growth, Development, and Sustainability" Applied Sciences 10, no. 17: 6045. https://doi.org/10.3390/app10176045
APA StyleAdhikari, B., Adhikari, M., & Park, G. (2020). The Effects of Plasma on Plant Growth, Development, and Sustainability. Applied Sciences, 10(17), 6045. https://doi.org/10.3390/app10176045






