A Critical Review of SCWG in the Context of Available Gasification Technologies for Plastic Waste
Abstract
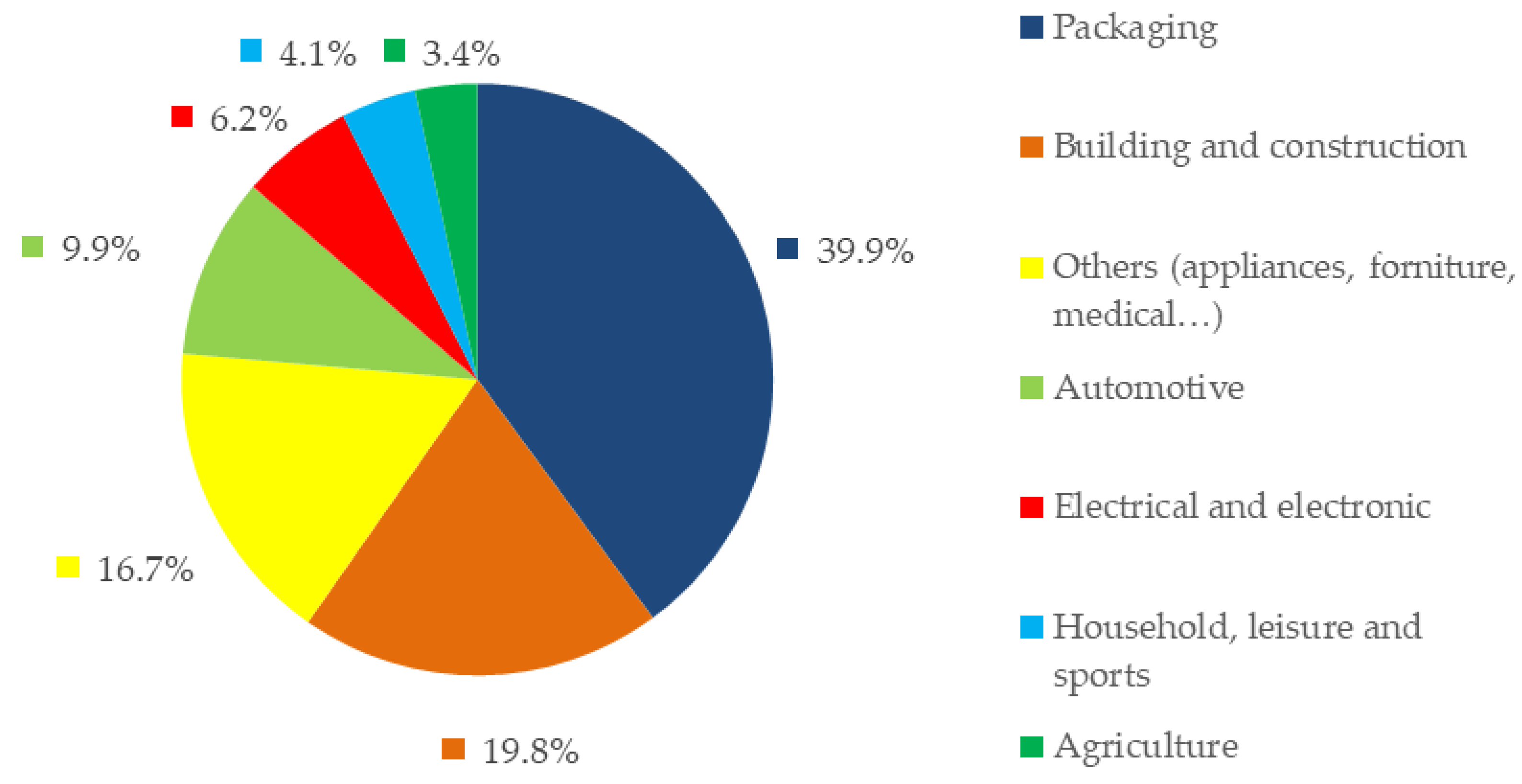
1. Introduction
- Primary Recycling: Primary recycling, also called re-extrusion, consists of recycling a single type of polymer, with properties close to the virgin material and free of contaminations. This process uses processing wastes, which are regenerated as they are or added to virgin polymer. This process is characterized by simplicity and low cost [15,16].
- Secondary Recycling (Mechanical Recycling/Waste to materials): Secondary recycling consists in the mechanical transformation of plastic waste, with the aim of obtaining raw materials for the creation of new objects [17]. Secondary recycling can only be done on thermoplastic polymers as they can be remelted and reprocessed. This process consists of a physical method, in which the plastic waste is shredded, washed, and then melted to make the new product by extrusion. The disadvantages of this method are related to the heterogeneity of plastic waste and the deterioration of products’ properties occurring cycle after cycle, due to the decrease in the molecular weight of the recycled polymers in comparison with originals. To partially obviate this last problem, the material can be blended with virgin polymer or specific additives [18].
- Tertiary recycling (Waste to energy/Waste to chemicals): This recycling consists of obtaining, by chemical or physical methods, monomers, oligomers, or other compounds from plastic waste. [15]. In chemical recycling, polymers are chemically depolymerized through chemical reactions. The resulting monomers can be used for new polymerization reactions, to reproduce the original polymer or other products [17,18]. Physical methods, refer to processes that consist of thermal degradation of polymers, that can be carried out in the complete absence of oxygen (pyrolysis) [19] or in the presence of sub-stoichiometric oxygen (gasification) [20]. Pyrolysis products are a gas, an oil, and a char, instead syngas (syngas is an abbreviation for synthesis gas) is the main product from gasification.
- Quaternary recycling (Energy recovery): In quaternary recycling, the waste material is treated to recover energy through incineration [15]. With this method a considerable energy is obtained from the polymers (the calorific value of different plastic polymers is comparable to oil and petroleum), but it is not considered a good solution at an ecological level because it leads to the formation of toxic substances for humans and the environment, such as dioxins [18]. Furthermore, with incineration all the energy used to form the object itself is lost. Recycling plastic waste by the energy recovery method is only logical when the recycling of waste is not possible due to other constraints [21].
2. Gasification Process
2.1. Fundamentals of Gasification
2.2. Gasification Mechanism
- Drying: Feedstock with variable moisture content are dried in a drying process in a range of temperatures between 100–150 °C. In this step no chemical reactions take place and the heat supplied is spent in the phase change between liquid water and water steam [33]. Unlike biomass or coal, plastics have a very low moisture content. Since it is external humidity, drying is a very rapid process, not being subject to diffusion processes [20].
- Pyrolysis: During this process complex chemical reactions of endothermic nature take place. Volatile substances and a solid or carbonized residue are formed. The proportions of these products are influenced by the process conditions such as the heating rate (°C/s) and the temperature. Furthermore, products distribution is affected by feedstock composition and size [33]. Pyrolysis is a “delicate” step, as the melted plastic particles tend to stick together. This causes the formation of agglomerates and defluidization in fluidized bed reactors [48]. An interesting aspect, is that some polymers such as PP, PE, and PS can be almost totally converted into volatile substances when fast pyrolysis is conducted [19,49]. This usually occurs in common gasification technologies.
- Oxidation: At elevated temperatures and in the presence of sub-stoichiometric oxygen, heterogeneous reactions occur between oxidant and raw material, forming carbon monoxide and water steam as shown in Table 2. Oxidation depends on the chemical composition of the feedstock, the nature of oxidant used, and the operating conditions. This step is mainly exothermic and the thermal energy released provides the heat needed for the process [47].
- Reduction: It is an endothermic step in which high-temperature chemical reactions take place in the absence of oxygen [47]. These reactions are shown in Table 2. Steam promotes two reactions: The steam reforming of char and tar (endothermic) and the water-gas shift reaction (exothermic) [50]. Both reactions lead to the formation of H2. In particular, the reduction of water in steam gasification is the most effective way of increasing H2 production [51]. CO2 reacts with char to produce CO. This reaction is known as the Boudouard reaction and it is endothermic in nature [52]. CO2 may also be recirculated with O2 within oxy-fuel combustion/gasification [53].
2.3. The Role of Gasifying Agent
2.4. Variables Affecting the Gasification Process
- Size: The smaller the feedstock size, the better would be the heat transfer. The temperature would be uniform resulting in a reaction taking place throughout the particle. In the case of plastic waste due to the great variety of shapes and sizes, shredding is necessary to create a feed material of less than 5 cm in diameter. Some forms of waste plastics such as thin films may require a simple agglomeration step to produce particles of higher bulk density to facilitate feeding [71].
- Temperature: Temperature influences the yield and composition of the syngas [72,73] and the production of tar and ash. The increase in gasification temperature gives rise to an increase in gas formation and a subsequent decrease in tar and char yields [74]. The greater amount of gas produced at higher temperatures is explained by the larger release of gases during the initial devolatilization stage, and the secondary reactions undergone by the char and tar (char gasification and tar cracking/reforming) [74]. Many studies have shown that the H2 content in syngas increases as the gasification temperature increases. This phenomenon is due to the chemical breakdown (thermal cracking) of heavy hydrocarbons which favors molar fraction of the permanent gases such as H2 and CO at high temperature [75]. Qin et al. [76] examined the effects of reaction temperature on biomass gasification in a laboratory scale atmospheric pressure entrained flow reactor. They observed that by increasing the temperature from 1000 to 1350 °C, the yield of producer gas (defined as the sum of H2, CO, CO2, and hydrocarbons up to C3 species) increased dramatically by 72%. Moreover, a higher temperature was beneficial to decrease the amount of tar. In general, operating temperatures higher than 800 °C are recommended to minimize tar formation [74]. However, these high temperatures favor the formation of slag from ash agglomeration. Furthermore, higher temperatures are due to a greater oxidation of the feedstock that causes a decrease of the chemical energy of the syngas.
- Heating rate: Heating rate is one of the main parameters that affect the decomposition of waste [77]. In particular, it determines the yield and composition of the derived products of pyrolysis step. Pyrolysis involves a process of cracking polymeric structures to convert the feedstock into charcoal and volatile matter [78]. In general, the char yield is expected to increase at lower heating rates. Hence, the slow pyrolysis processes are always preferred for producing charcoal. Higher pyrolysis temperatures, high heating rates, and long residence times lead to the formation of gas products.
- Environment and reactor design: Generally, it is observed that the reactive environment (air/oxygen) results in complete gasification of feedstock while inert environment (nitrogen/argon) aids devolatilization (pyrolysis) yielding more char [70]. The equivalence ratio is commonly used to indicate quantitatively whether a fuel oxidizer mixture is rich, lean, or stoichiometric. Therefore, for fuel rich mixtures, the ER > 1, for fuel lean, ER < 1, and for stoichiometric mixture, ER = 1 [79,80]. Commercial gasifiers employ air at a sub stoichiometric quantity to generate producer gas. Plastic material has some particular characteristics given by the low thermal conductivity, the sticky nature when heated, the high volatile content, and the remarkable tar formation. Therefore, a suitable gasifier design for plastic handling must take these aspects into account [20]. Fluidized beds reactors are widely used in the gasification of plastics waste. Plastic gasification studies are often carried out in bubbling reactors [48,81,82,83,84,85,86,87]. In this gasifier, gas flows upward through a bed of free-flowing granular material at a gas velocity sufficient to agitate the material into a churning emulsion of levitated particles and gas bubbles. The fluidized bed itself resembles a boiling liquid and has many of the same physical properties as a fluid. Typical bed materials used in this kind of gasifier are sand, olivine, limestone, dolomite, or alumina. Beds can be fluidized with the gasification agent, typically air, oxygen, and/or steam [34].
- Other types of reactors are used in the gasification of plastic material, such as fixed beds ([88,89,90]) and spouted beds reactors ([67,91]). Fixed bed reactors represent the oldest and most proven technology for gasification. They are chosen for their simple design and low cost, but they are characterized by a poor heat transfer rate and a limited gas-solid contact. Fixed bed reactors are commonly used on small scale units and have many different designs. Spouted beds are gas-solid contactors in which the gas is introduced through a single orifice from the center of a flat [92]. They are characterized by their high heat and mass transfer rates, good solid mixing, and suitable gas-solid contact. A disadvantage for their application deals with the short residence of the volatiles, which hinders tar cracking reactions [93].
3. Supercritical Water Gasification (SCWG)
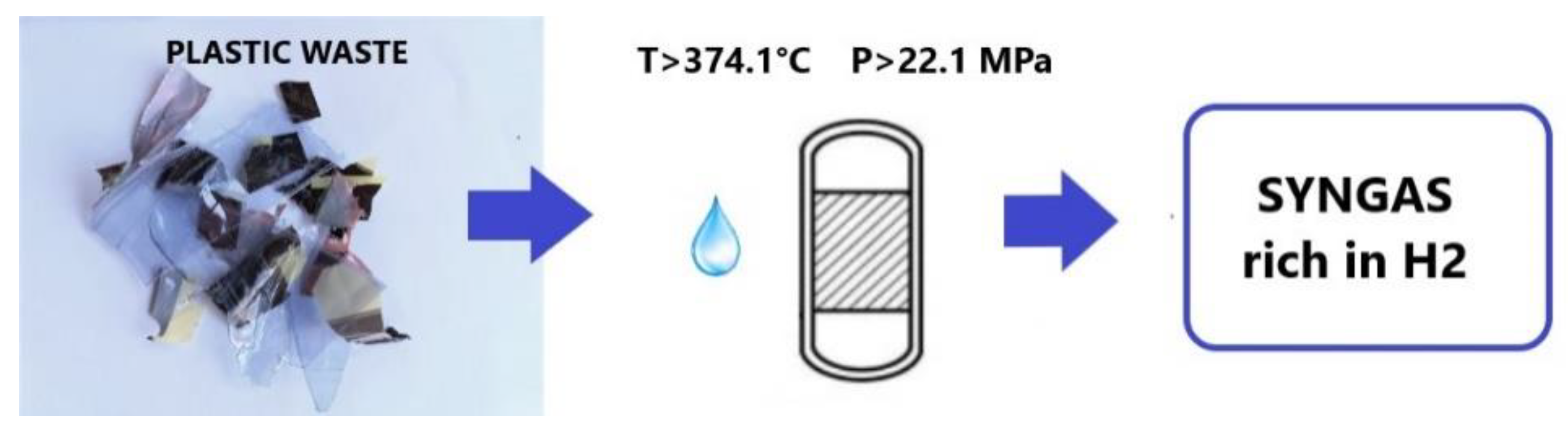
3.1. Properties of Supercritical Water
3.2. Biomass SCWG Process
- Steam reformationCHxOy + (1−y)H2O → CO + (1−y + x/2) H2
- Methanation for COCO + 3H2 ↔ CH4 + H2O
- Methanation for CO2CO2 + 3H2 ↔ CH4 + H2O
- Water–gas shit reactionCO + H2O ↔ CO2 + H2
- Water and feedstock must be pressurized to the supercritical pressure required for the process. The biomass is then ground and mixed with water using an emulsifying agent, in order to obtain a pumpable liquid.
- The resulting slurry is pumped into the reactor. The pressurized water is preheated by passing it through a heat exchanger, which exploits the heat of the product leaving the reactor. The pressurized water also passes through an externally heated preheater before entering the reactor.
- The syngas obtained in the process is cooled and passed through a gas-liquid separator. The gas mixture passes through further purification equipment such as the scrubber or pressure-swing adsorption unit.
3.3. Advantages and Disadvantages in The Use of SCW
- Supercritical water is an active reactant which results in a high hydrogen yield [99].
- In the SCWG, the drying step of feedstock is not required. This leads to greater energy efficiency especially for biomass with a high moisture content. Therefore, there is a considerable economic saving, since drying and pre-treatment of biomass add extra cost to the process economics [100].
- High pressure of the gaseous product enables the transportation, usage, carbon capture, and further purification of the product gas through steam reforming or PSA (pressure swing adsorption).
- The reaction temperature is much lower than that used in conventional gasification and pyrolysis processes.
- Tar and coke formation is inhibited by a fast solution of the formed gas components in the supercritical water [99].
- The gaseous product is very clean, as NOx and SOx are generated in very small quantities and the CO concentration is very low, especially in the presence of a catalyst to improve the water-gas shift reaction [101].
3.4. SCWG of Plastic
4. Co-Gasification
5. The Problem of Tar: Formation and Removal
- Modification of the gasifier.
- The use of catalysts in the bed.
- Naturally occurring catalysts such as dolomite and olivine.
- Alkali metals such as KOH, K2CO3, KHCO3, Na2CO3, CaCO3, CsCO3, KCl, ZnCl2, and NaCl.
- Nickel-based catalysts, which have been evaluated for tar reduction in syngas.
6. Gasification of Plastic Waste: An Overview
6.1. Polyolefin
6.1.1. Polypropylene
6.1.2. Polyethylene
- (a)
- At bed bottom without any splitting of the flow rate;
- (b)
- At bed bottom and in the splashing zone, that is the volume just above the primary zone, by
- (c)
- splitting into two streams the necessary air flow rate;
- (d)
- at bed bottom, in the splashing zone and in the freeboard region, by splitting into three
- (e)
- streams the air flow rate.
- A high H2 yield was obtained.
- The CH4 conversion was almost full.
- A high H2/CO ratio was obtained in the product stream.
- A low coke yield was obtained.
6.2. Plastics Mix
6.3. Supercritical Water Gasification (SCWG)
6.4. Co-Gasification
7. Conclusions and Final Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Thompson, R.C.; Swan, S.H.; Moore, C.J.; vom Saal, F.S. Our plastic age. Philos. Trans. R. Soc. B 2009, 364, 1973–1976. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.L.; Ngadi, N.; Abdullah, T.A.; Inuwa, I.M. Current state and future prospects of plastic waste as source of fuel: A review. Renew. Sustain. Energy Rev. 2015, 50, 1167–1180. [Google Scholar] [CrossRef]
- Parlamento Europeo e Consiglio. Direttiva 2008/98/CE. Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/PDF/?uri=CELEX:02008L0098-20150731&from=ET (accessed on 4 April 2020).
- Plastics—The Facts-2019. Available online: https://www.plasticseurope.org/it/resources/publications/1804-plastics-facts-2019 (accessed on 4 April 2020).
- Polymers for Innovative Food Packaging. Available online: https://web.wpi.edu/Pubs/E-project/Available/E-project-050610124822/unrestricted/Polymers_for_Innovative_Food_Packaging.pdf (accessed on 4 April 2020).
- Ghosh, A. Basics of polymer packaging. Adv. Polym. Technol. 2015, 1–18. [Google Scholar] [CrossRef]
- Wikström, F.; Williams, H.; Trischler, J.; Rowe, Z. The importance of packaging functions for food waste of different products in households. Sustainability 2019, 11, 2641. [Google Scholar] [CrossRef]
- Goel, V.; Sharma, A.; Nautiyal, H. Environmental impacts of packaging materials. In Environmental Footprints of Packaging, 1st ed.; Subramanian, S.M., Ed.; Springer: Singapore, 2016; pp. 115–137. [Google Scholar]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef]
- Pongrácz, E. The environmental impacts of packaging. In Environmentally Conscious Materials and Chemicals Processing; Kutz, M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 237–278. [Google Scholar]
- Lavers, J.L.; Dicks, L.; Dicks, M.R.; Finger, A. Significant plastic accumulation on the Cocos (Keeling) Islands, Australia. Sci. Rep. 2019, 9, 7102. [Google Scholar] [CrossRef]
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro(nano)plastics: A threat to human health? Curr. Opin. Environ. Sci. Heal. 2018, 1, 17–23. [Google Scholar] [CrossRef]
- A European Strategy for Plastics in a Circular Economy. Brussels. Available online: https://ec.europa.eu/environment/circular-economy/pdf/plastics-strategy-brochure.pdf (accessed on 20 April 2020).
- Azeez, T.O. Thermoplastic Recycling: Properties, Modifications, and Applications. In Thermosoftening Plastics; Evingür, G.A., Ed.; Intech Open: London, UK, 2018. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. J. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Kumar, S.; Panda, A.K.; Singh, R.K. A review on tertiary recycling of high-density polyethylene to fuel. Resour. Conserv. Recycl. 2011, 55, 893–910. [Google Scholar] [CrossRef]
- Grigore, M.E. Methods of recycling, properties and applications of recycled thermoplastic polymers. Recycling 2017, 2, 24. [Google Scholar] [CrossRef]
- Sharuddin, A.S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.J.; Bilbao, A.J.; Olazar, M. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. The valorization of plastic solid waste (PSW) by primary to quaternary routes: From re-use to energy and chemicals. Prog. Energy Combust. 2010, 36, 103–129. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Geem, K.V. Mechanical and chemical recycling of solid plastic waste. Waste Manag. Pages 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Okolie, J.A.; Ajay, S.N.; Dalai, K.; Berruti, F.; Kozinski, J.A. A review on subcritical and supercritical water gasification of biogenic, polymeric and petroleum wastes to hydrogen-rich synthesis gas. Renew. Sustain. Energy Rev. 2020, 119, 109–546. [Google Scholar] [CrossRef]
- Speight, J.G. Feedstocks. Gasification of Unconventional Feedstocks; Gulf Professional Publishing: Houston, TX, USA, 2014. [Google Scholar]
- Brems, A.; Dewil, R.; Baeyens, J.; Zhang, R. Gasification of plastic waste as waste-to-energy or waste-to-syngas recovery route. Nat. Sci. 2013, 5, 695–704. [Google Scholar] [CrossRef]
- Albanese, J.; Ruiz, M.P. Solid Waste as a Renewable Resource: Methodologies, 1st ed.; Faria, J., Ruiz-Ramiro, M.P., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2015. [Google Scholar]
- Narobe, M.; Golob, J.; Klinar, D.; Francetič, V.; Likozar, B. Co-gasification of biomass and plastics: Pyrolysis kinetics studies, experiments on 100 kW dual fluidized bed pilot plant and development of thermodynamic equilibrium model and balances. Bioresour. Technol. 2014, 162, 21–29. [Google Scholar] [CrossRef]
- Quina, M.G.; Bordado, J.; Quinta-Ferreira, R. Air pollution control in municipal solid waste incinerators. In The Impact of Air Pollution on Health, Economy, Environment and Agricultural Sources; Khallaf, M., Ed.; Intech Open: London, UK, 2011. [Google Scholar]
- Quina, M.J.; Bordado, J.C.; Quinta-Ferreira, R.M. Treatment and use of air pollution control residues from MSW incineration: An overview. J. Waste Manag. 2008, 28, 2097–2121. [Google Scholar] [CrossRef]
- Kanan, S.; Samara, F. Dioxins and furans: A review from chemical and environmental perspectives. Trends Environ. Anal. 2018, 17, 1–13. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Speight, J.G. Gasifier types. Gasif. Unconv. Feedstocks 2014, 54–90. [Google Scholar] [CrossRef]
- Saghir, M.; Rehan, M.; Nizami, A.S. Recent trends in gasification based waste-to-energy. In Gasification for Low-Grade Feedstock; InTech: London, UK, 2018. [Google Scholar]
- Brown, R.C. Thermochemical Processing of Biomass: Conversion into Fuels, Chemicals and Power, 2nd ed.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- El-Nagar, R.A.; Ghanem, A.A. Syngas production, properties, and its importance. In Sustainable Alternative Syngas Fuel; Ghenai, C., Ed.; Intech Open: London, UK, 2019. [Google Scholar] [CrossRef]
- Syngas & Derivatives Market. Available online: https://www.marketsandmarkets.com/Market-Reports/syngas-market-1178.html (accessed on 26 April 2020).
- Bremaud, M.; Fongarland, P.; Anfray, J.; Jallais, S.; Schweich, D.; Khodakov, A.Y. Influence of syngas composition on the transient behavior of a Fischer-Tropsch continuous slurry reactor. Catal. Today 2005, 106, 137–142. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Peregryn, B.; Lermontov, A.S.; Girardon, J.S.; Pietrzyk, S. Transient studies of the elementary steps of Fischer-Tropsch synthesis. Catal. Today 2005, 106, 132–136. [Google Scholar] [CrossRef]
- Ma, W.; Jacobs, G.; Sparks, D.E.; Pendyala, V.R.R.; Hopps, S.G.; Thomas, G.A.; Hamdeh, H.H.; MacLennan, A.; Hu, Y.; Davis, B.H. Fischer-Tropsch synthesis: Effect of ammonia in syngas on the Fischer-Tropsch synthesis performance of a precipitated iron catalyst. J. Catal. 2015, 326, 149–160. [Google Scholar] [CrossRef]
- Tsiamis, D.A.; Castaldi, M.J. The Effects of Non-Recycled Plastic (NRP) on Gasification: A Quantitative Assessment; Earth Engineering Center City University of New York: New York, NY, USA, 2018. [Google Scholar]
- Enerkem. Available online: https://enerkem.com/company/facilities-projects/ (accessed on 26 April 2020).
- Kumar, A.; Jones, D.D.; Hanna, M. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Xiao, R.; Jin, B.; Zhou, H.; Zhong, Z.; Zhang, M. Air gasification of polypropylene plastic waste in fluidized bed gasifier. Energy Convers. Manag. 2006, 48, 778–786. [Google Scholar] [CrossRef]
- Ruan, R. Biofuels: Introduction. In Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–43. [Google Scholar]
- Kirsanovs, V.; Žandeckis, A.; Rochas, C. Biomass gasification thermodynamic model including tar and char. Agron. Res. 2016, 14, 1321–1331. [Google Scholar]
- Pereira, E.G.; Da Silva, J.N.; De Oliveira, J.L.; MacHado, C.S. Sustainable energy: A review of gasification technologies. Renew. Sustain. Energy Rev. 2012, 16, 4753–4762. [Google Scholar] [CrossRef]
- Mahinpey, N.; Gomez, A. Review of gasification fundamentals and new findings: Reactors, feedstock, and kinetic studies. Chem. Eng. Sci. 2016, 148, 14–31. [Google Scholar] [CrossRef]
- Aznar, M.P.; Caballero, M.A.; Sancho, J.A.; Francés, E. Plastic waste elimination by co-gasification with coal and biomass in fluidized bed with air in pilot plant. Fuel Process. Technol. 2006, 87, 409–420. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review. Renew. Sustain. Energy Rev. 2017, 73, 346–368. [Google Scholar] [CrossRef]
- Hernández, J.J.; Ballesteros, R.; Aranda, G. Characterization of tars from biomass gasification: Effect of the operating conditions. Energy 2013, 50, 333–342. [Google Scholar] [CrossRef]
- Kim, Y.K.; Il Park, J.; Jung, D.; Miyawaki, J.; Yoon, S.H.; Mochida, I. Low-temperature catalytic conversion of lignite: 1. Steam gasification using potassium carbonate supported on perovskite oxide. J. Ind. Eng. Chem. 2014, 20, 216–221. [Google Scholar] [CrossRef]
- Hernández, J.J.; Aranda, G.; Barba, J.; Mendoza, J.M. Effect of steam content in the air-steam flow on biomass entrained flow gasification. Fuel Process. Technol. 2012, 99, 43–55. [Google Scholar] [CrossRef]
- Gil, M.V.; Riaza, J.; Álvarez, L.; Pevida, C.; Pis, J.J.; Rubiera, F. Kinetic models for the oxy-fuel combustion of coal and coal/biomass blend chars obtained in N2 and CO2 atmospheres. Energy 2012, 48, 510–518. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Te Nijenhuis, K. Thermal decomposition. In Properties of Polymers, 4th ed.; Elsevier Science: Amsterdam, The Netherlands, 2009; pp. 763–777. [Google Scholar]
- Horton, S.R.; Woeckener, J.; Mohr, R.; Zhang, Y.; Petrocelli, F.; Klein, M.T. Molecular-Level Kinetic Modeling of the Gasification of Common Plastics. Energy Fuels 2016, 30, 1662–1674. [Google Scholar] [CrossRef]
- Arabiourrutia, M.; Elordi, G.; Lopez, G.; Borsella, E.; Bilbao, J.; Olazar, M. Characterization of the waxes obtained by the pyrolysis of polyolefin plastics in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis 2012, 94, 230–237. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.; San Miguel, G. European trends in the feedstock recycling of plastic wastes. Glob. NEST J. 2007, 9, 12–19. [Google Scholar]
- Ueno, T.; Nakashima, E.; Takeda, K. Quantitative analysis of random scission and chain-end scission in the thermal degradation of polyethylene. Polym. Degrad. Stab. 2010, 95, 1862–1869. [Google Scholar] [CrossRef]
- Artetxe, M.; Lopez, G.; Amutio, M.; Elordi, G.; Olazar, M.; Bilbao, J. Operating conditions for the pyrolysis of poly-(ethylene terephthalate) in a conical spouted-bed reactor. Ind. Eng. Chem. Res. 2010, 49, 2064–2069. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Li, B.; Gao, X.; Jiang, B. Energy and exergy characteristics of syngas produced from air gasification of walnut sawdust in an entrained flow reactor. Int. J. Exergy 2017, 23, 244–262. [Google Scholar] [CrossRef]
- Niu, M.; Huang, Y.; Jin, B.; Wang, X. Oxygen gasification of municipal solid waste in a fixed-bed gasifier. Chin. J. Chem. Eng. 2014, 22, 1021–1026. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Li, H.; Zhang, B. Exergy analysis of biomass utilization via steam gasification and partial oxidation. Thermochim. Acta 2012, 538, 21–28. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Li, B.; Zhao, Y.; Jiang, B. Determination of the intrinsic reactivities for carbon dioxide gasification of rice husk chars through using random pore model. Bioresour. Technol. 2016, 218, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Jin, H.; Lu, Y. Supercritical water gasification research and development in China. J. Supercrit. Fluids 2015, 96, 144–150. [Google Scholar] [CrossRef]
- Arena, U.; Di Gregorio, F. Energy generation by air gasification of two industrial plastic wastes in a pilot scale fluidized bed reactor. Energy 2014, 68, 735–743. [Google Scholar] [CrossRef]
- Saad, J.M.; Williams, P.T. Manipulating the H2/CO ratio from dry reforming of simulated mixed waste plastics by the addition of steam. Fuel Process. Technol. 2017, 156, 331–338. [Google Scholar] [CrossRef]
- Erkiaga, A.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Syngas from steam gasification of polyethylene in a conical spouted bed reactor. Fuel 2013, 109, 461–469. [Google Scholar] [CrossRef]
- Ravaghi, Z.; Manenti, F.; Pirola, C.; Ranzi, E. Influence of the effective parameters on H2:CO ratio of syngas at low-temperature gasification. Chem. Eng. Trans. 2014, 37, 253–258. [Google Scholar]
- Sahoo, B.B.; Sahoo, N.; Sahab, U.K. Effect of H2:CO ratio in syngas on the performance of a dual fuel diesel engine operation. Appl. Therm. Eng. 2012, 49, 139–146. [Google Scholar] [CrossRef]
- Kirubakaran, V.; Sivaramakrishnan, V.; Nalini, R.; Sekar, T.; Premalatha, M.; Subramanian, P. A review on gasification of biomass. Renew. Sustain. Energy Rev. 2009, 13, 179–186. [Google Scholar] [CrossRef]
- The Environment and Industry Council (EPIC). The gasification of residual plastics derived from municipal recycling facilities. Can. Plast. Ind. Assoc. 2004, 1, 1–11. [Google Scholar]
- Muvhiiwa, R.F.; Sempuga, B.; Hildebrandt, D.; Van Der Walt, J. Study of the effects of temperature on syngas composition from pyrolysis of wood pellets using a nitrogen plasma torch reactor. J. Anal. Appl. Pyrolysis 2018, 130, 249–255. [Google Scholar] [CrossRef]
- Shehzad, A.; Bashir, M.J.K.; Horttanainen, M.; Manttari, M.; Havukainen, J.; Abbas, G. Modeling and comparative assessment of bubbling fluidized bed gasification system for syngas production–a gateway for a cleaner future in Pakistan. Environ. Technol. 2018, 39, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, J.M.; Fidalgo, B. Production of bio-syngas and bio-hydrogen via gasification. In Handbook of Biofuels Production, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 431–494. [Google Scholar]
- Guangul, F.M.; Sulaiman, S.A.; Raghavan, V.R. Gasification and effect of gasifying temperature on syngas quality and tar generation: A short review. AIP Conf. Proc. 2012, 1440, 491. [Google Scholar]
- Qin, K.; Lin, W.; Jensen, P.A.; Jensen, A.D. High-temperature entrained flow gasification of biomass. Fuel 2012, 93, 589–600. [Google Scholar] [CrossRef]
- Amini, E.; Safdari, M.S.; DeYoung, J.T.; Weise, D.R.; Fletcher, T.H. Characterization of pyrolysis products from slow pyrolysis of live and dead vegetation native to the southern United States. Fuel 2018, 235, 1475–1491. [Google Scholar] [CrossRef]
- Elorf, A.; Kandasamy, J.; Belandria, V.; Bostyn, S.; Sarh, B.; Gökalp, I. Heating rate effects on pyrolysis, gasification and combustion of olive waste. Biofuels 2019. [Google Scholar] [CrossRef]
- Jangsawang, W.; Laohalidanond, K.; Kerdsuwan, S. Optimum equivalence ratio of biomass gasification process based on thermodynamic equilibrium model. Energy Procedia 2015, 79, 520–527. [Google Scholar] [CrossRef]
- Yang, R.X.; Chuang, K.H.; Wey, M.Y. Effects of temperature and equivalence ratio on carbon nanotubes and hydrogen production from waste plastic gasification in fluidized bed. Energy Fuels 2018, 32, 5462–5470. [Google Scholar] [CrossRef]
- Rüdisüli, M.; Schildhauer, T.J.; Biollaza, S.M.A.; van Ommenb, J.R. Scale-up of bubbling fluidized bed reactors—A review. Powder Technol. 2012, 217, 21–38. [Google Scholar] [CrossRef]
- Toledo, J.M.; Aznar, M.P.; Sancho, J.A. Catalytic air gasification of plastic waste (polypropylene) in a fluidized bed. Part II: Effects of some operating variables on the quality of the raw gas produced using olivine as the in-bed material. Ind. Eng. Chem. Res. 2011, 50, 11815–11821. [Google Scholar] [CrossRef]
- Martínez-Lera, S.; Torrico, J.; Pallarés, J.; Gil, A. Thermal valorization of post-consumer film waste in a bubbling bed gasifier. Waste Manag. 2013, 33, 1640–1647. [Google Scholar] [CrossRef] [PubMed]
- Arena, U.; Zaccariello, L.; Mastellone, M.L. Tar removal during the fluidized bed gasification of plastic waste. Waste Manag. 2008, 29, 783–791. [Google Scholar] [CrossRef]
- Mastellone, M.L.; Zaccariello, L. Gasification of polyethylene in a bubbling fluidized bed operated with the air staging. Fuel 2013, 106, 226–233. [Google Scholar] [CrossRef]
- Mastellone, M.L.; Zaccariello, L.; Arena, U. Co-gasification of coal, plastic waste and wood in a bubbling fluidized bed reactor. Fuel 2010, 89, 2991–3000. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.; Miranda, M.; Neves, D.; Varela, F.; Santos, J. Effect of gasification agent on co-gasification of rice production wastes mixtures. Fuel 2016, 180, 407–416. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. A novel Ni-Mg-Al-CaO catalyst with the dual functions of catalysis and CO2 sorption for H2 production from the pyrolysis-gasification of polypropylene. Fuel 2010, 89, 1435–1441. [Google Scholar] [CrossRef]
- Cho, M.H.; Mun, T.Y.; Kim, J.S. Production of low-tar producer gas from air gasification of mixed plastic waste in a two-stage gasifier using olivine combined with activated carbon. Energy 2013, 58, 688–694. [Google Scholar] [CrossRef]
- Chai, Y.; Gao, N.; Wang, M.; Wu, C. H2 production from co-pyrolysis/gasification of waste plastics and biomass under novel catalyst Ni-CaO-C. Chem. Eng. J. 2020, 382, 122–947. [Google Scholar] [CrossRef]
- Lopez, G.; Erkiaga, A.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Hydrogen production by high density polyethylene steam gasification and in-line volatile reforming. Ind. Eng. Chem. Res. 2015, 54, 9536–9544. [Google Scholar] [CrossRef]
- Sahoo, P.; Sahoo, A. Fluidization and spouting of fine particles: A comparison. Adv. Mater. Sci. Eng. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Fernandez-Akarregia, A.R.; Makibara, J.; Lopez, G.; Amutio, M.; Olazar, M. Design and operation of a conical spouted bed reactor pilot plant (25 kg/h) for biomass fast pyrolysis. Fuel Process. Technol. 2013, 112, 48–56. [Google Scholar] [CrossRef]
- Wagner, W.; Pruß, A. The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific use. J. Phys. Chem. 2002, 31, 387–535. [Google Scholar] [CrossRef]
- Möller, M.; Nilges, P.; Harnisch, F.; Schröder, U. Subcritical water as reaction environment: Fundamentals of hydrothermal biomass transformation. ChemSusChem. 2011, 4, 566–579. [Google Scholar] [CrossRef]
- Basu, P.; Mettanant, V. Biomass gasification in supercritical water—A review. Int. J. Chem. React. Eng. 2009, 7. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Prado, J.M.; Mayanga, T.P.; Forster-Carneiro, T.; Angela, M.; Meireles, A. Supercritical water gasification of biomass for hydrogen production: Variable of the process. Food Public Heal. 2015, 6, 92–101. [Google Scholar] [CrossRef][Green Version]
- Hu, Y.; Gong, M.; Xing, X.; Wang, H.; Zeng, Y.; Xu, C.C. Supercritical water gasification of biomass model compounds: A review. Renew. Sustain. Energy Rev. 2020, 118, 109–529. [Google Scholar] [CrossRef]
- Heidenreich, S.; Müller, M.; Foscolo, P.U. New and improved gasification concepts. In Advanced Biomass Gasification; Elsevier: Amsterdam, The Netherlands, 2016; pp. 98–114. [Google Scholar]
- Okolie, J.A.; Rana, R.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of biomass: A state-of-the-art review of process parameters, reaction mechanisms and catalysis. Sustain. Energy Fuels 2019, 3, 578–598. [Google Scholar] [CrossRef]
- Guo, L.; Cao, C.; Lu, Y. Supercritical water gasification of biomass and organic wastes. Biomass 2010. [Google Scholar] [CrossRef]
- Li, K.; Xu, Z. A review of current progress of supercritical fluid technologies for e-waste treatment. J. Clean. Prod. 2019, 227, 794–809. [Google Scholar] [CrossRef]
- Purkarová, E.; Ciahotný, K.; Šváb, M.; Skoblia, S.; Beňo, Z. Supercritical water gasification of wastes from the paper industry. J. Supercrit. Fluids 2018, 135, 130–136. [Google Scholar] [CrossRef]
- Hernández, J.J.; Aranda-Almansa, G.; Serrano, C. Co-gasification of biomass wastes and coal-coke blends in an entrained flow gasifier: An experimental study. Energy Fuels 2010, 24, 2479–2488. [Google Scholar] [CrossRef]
- Farzad, S.; Mandegari, M.A.; Görgens, J.F. A critical review on biomass gasification, co-gasification, and their environmental assessments. Biofuel Res. J. 2016, 3, 483–495. [Google Scholar] [CrossRef]
- Emami Taba, L.; Irfan, M.F.; Wan Daud, W.A.M.; Chakrabarti, M.H. The effect of temperature on various parameters in coal, biomass and CO-gasification: A review. Renew. Sustain. Energy Rev. 2012, 16, 5584–5596. [Google Scholar] [CrossRef]
- Aigner, I.; Pfeifer, C.; Hofbauer, H. Co-gasification of coal and wood in a dual fluidized bed gasifier. Fuel 2011, 90, 2404–2412. [Google Scholar] [CrossRef]
- Koukouzas, N.; Katsiadakis, A.; Karlopoulos, E.; Kakaras, E. Co-gasification of solid waste and lignite—A case study for Western Macedonia. Waste Manag. 2008, 28, 1263–1275. [Google Scholar] [CrossRef]
- Ramos, A.; Monteiro, E.; Silva, V.; Rouboa, A. Co-gasification and recent developments on waste-to-energy conversion: A review. Renew. Sustain. Energy Rev. 2018, 81, 380–398. [Google Scholar] [CrossRef]
- Wilk, V.; Hofbauer, H. Co-gasification of plastics and biomass in a dual fluidized-bed steam gasifier: Possible interactions of fuels. Energy Fuels 2013, 27, 3261–3273. [Google Scholar] [CrossRef]
- Block, C.; Ephraim, A.; Weiss-Hortala, E.; Minh, D.P.; Nzihou, A.; Vandecasteele, C. Co-pyrogasification of Plastics and Biomass. Waste Biomass Valorization 2019, 10, 483–509. [Google Scholar] [CrossRef]
- Jakab, E.; Várhegyi, G.; Faix, O. Thermal decomposition of polypropylene in the presence of wood-derived materials. J. Anal. Appl. Pyrolysis 2000, 56, 273–285. [Google Scholar] [CrossRef]
- Huang, B.S.; Chen, H.Y.; Chuang, K.H.; Yang, R.X.; Wey, M.Y. Hydrogen production by biomass gasification in a fluidized-bed reactor promoted by an Fe/CaO catalyst. Int. J. Hydrogen Energy 2012, 37, 6511–6518. [Google Scholar] [CrossRef]
- Balas, M.; Lisy, M.; Kubicek, J.; Pospisil, J. Syngas cleaning by wet scrubber. Trans. Heat Mass Transf. 2014, 9, 195–204. [Google Scholar]
- Wilk, V.; Hofbauer, H. Conversion of mixed plastic wastes in a dual fluidized bed steam gasifier. Fuel 2013, 107, 787–799. [Google Scholar] [CrossRef]
- Machina, E.B.; Pedroso, D.T.; Proenza, N.; Silveira, J.L.; Conti, L.; Bollini Braga, L.; Machina, A.B. Tar reduction in downdraft biomass gasifier using a primary method. Renew. Energy 2015, 78, 478–483. [Google Scholar] [CrossRef]
- Lis’y, M.L.; Baláš, M.; Moskalík, J.; Stelcl, O. Biomass gasification-primary methods for eliminating tar. Acta Polytech. 2012, 52, 66–70. [Google Scholar]
- Surjosatyo, A.; Anggriawan, M.B.; Hermawan, A.A.; Dafiqurrohman, H. Comparison between secondary thermal cracking methods and venturi scrubber filtering in order to reduce tar in biomass gasification. Energy Procedia 2019, 158, 749–754. [Google Scholar] [CrossRef]
- Chandrasekaran, J.; Nithyaprakash, D.; Ajjan, K.B.; Maruthamuthu, S.; Manoharan, D.; Kumar, S. Hybrid solar cell based on blending of organic and inorganic materials—An overview. Renew. Sustain. Energy Rev. 2011, 15, 1228–1238. [Google Scholar] [CrossRef]
- Di Sultan, A. Plastics to Energy: Fuel, Chemicals, and Sustainability Implications; Andrew, W., Ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Sun, S.; Zhao, Y.; Ling, F.; Su, F. Experimental research on air staged cyclone gasification of rice husk. Fuel Process. Technol. 2009, 90, 465–471. [Google Scholar] [CrossRef]
- Michel, R.; Rapagnà, S.; Burg, P.; Mazziotti di Celso, G.; Courson, C.; Zimny, T.; Gruber, R. Steam gasification of Miscanthus X Giganteus with olivine as catalyst production of syngas and analysis of tars (IR, NMR and GC/MS). Biomass Bioenergy 2011, 35, 2650–2658. [Google Scholar] [CrossRef]
- Mohammed, M.A.A.; Salmiaton, A.; Wan Azlina, W.A.K.G.; Mohammad Amran, M.S.; Fakhru’L-Razi, A.; Taufiq-Yap, Y.H. Hydrogen rich gas from oil palm biomass as a potential source of renewable energy in Malaysia. Renew. Sustain. Energy Rev. 2011, 15, 1258–1270. [Google Scholar] [CrossRef]
- De Andrés, J.M.; Narros, A.; Rodríguez, M.E. Behaviour of dolomite, olivine and alumina as primary catalysts in air-steam gasification of sewage sludge. Fuel 2011, 90, 521–527. [Google Scholar] [CrossRef]
- Baratieri, M.; Pieratti, E.; Nordgreen, T.; Grigiante, M. Biomass gasification with dolomite as catalyst in a small fluidized bed experimental and modelling analysis. Waste Biomass Valori 2010, 1, 283–291. [Google Scholar] [CrossRef]
- Correia, L.M.; Campelo, N.S.; Novaesa, D.S.; Cavalcante, C.L., Jr.; Cecilia, J.A.; Rodríguez-Castellón, E.; Vieira, R.S. Characterization and application of dolomite as catalytic precursor for canola and sunflower oils for biodiesel production. Chem. Eng. J. 2015, 269, 35–43. [Google Scholar] [CrossRef]
- Sancho, J.A.; Aznar, M.P.; Toledo, J.M. Catalytic Air Gasification of Plastic Waste (Polypropylene) in Fluidized Bed. Part I: Use of in-Gasifier Bed Additives. Ind. Eng. Chem. Res. 2008, 47, 1005–1010. [Google Scholar] [CrossRef]
- Fredriksson, H.O.A.; Lancee, R.J.; Thüne, P.C.; Veringa, H.J.; Niemantsverdriet, J.W.H. Olivine as tar removal catalyst in biomass gasification: Catalyst dynamics under model conditions. Appl. Catal. B Environ. 2013, 130–131, 168–177. [Google Scholar] [CrossRef]
- Soomro, A.; Chen, S.; Ma, S.; Xiang, W. Catalytic activities of nickel, dolomite, and olivine for tar removal and H2-enriched gas production in biomass gasification process. Energy Environ. 2018, 29, 839–867. [Google Scholar] [CrossRef]
- Arnold, R.A.; Hill, J.M. Catalysts for gasification: A review. Sustain. Energy Fuels 2019, 3, 656–672. [Google Scholar] [CrossRef]
- Chan, F.L.; Tanksale, A. Review of recent developments in Ni-based catalysts for biomass gasification. Renew. Sustain. Energy Rev. 2014, 38. [Google Scholar] [CrossRef]
- Posch, D.W. Polyolefins. In Applied Plastics Engineering Handbook: Processing, Materials, and Applications, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 27–52. [Google Scholar]
- Ishii, A.; Amagai, K.; Furuhata, T.; Arai, M. Thermal gasification behavior of plastics with flame retardant. Fuel 2007, 86, 2475–2484. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Hydrogen production by steam gasification of polypropylene with various nickel catalyst. Appl. Catal. B Environ. 2009, 87, 152–161. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Hydrogen from waste plastics by way of pyrolysis-gasification. Proc. Inst. Civ. Eng. Waste Resour. Manag. 2014, 167, 35–46. [Google Scholar] [CrossRef]
- Yin, S.; Rajarao, R.; Pahlevani, F.; Sahajwalla, V. Sustainable steel carburization by using snack packaging plastic waste as carbon resources. Metals 2018, 8, 78. [Google Scholar] [CrossRef]
- Cagnetta, G.; Zhang, K.; Zhang, Q.; Huang, J.; Yu, G. Augmented hydrogen production by gasification of ball milled polyethylene with Ca(OH)2 and Ni(OH)2. Front. Environ. Sci. Eng. 2019, 13, 1–9. [Google Scholar] [CrossRef]
- Cho, M.H.; Mun, T.Y.; Kim, J.S. Air gasification of mixed plastic wastes using calcined dolomite and activated carbon in a two-stage gasifier to reduce tar. Energy 2013, 53, 299–305. [Google Scholar] [CrossRef]
- Friengfung, P.; Jamkrajang, E.; Sunphorka, S.; Kuchonthara, P.; Mekasut, L. NiO/dolomite catalyzed steam/O2 gasification of different plastics and their mixtures. Ind. Eng. Chem. Res. 2014, 53, 1909–1915. [Google Scholar] [CrossRef]
- Okajima, I.; Shimoyama, D.; Sako, T. Hydrogen production from cross-linked polyethylene with water in high temperature and pressure. J. Chem. Eng. Jpn. 2004, 37, 1521–1527. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Williams, P.T. Catalytic supercritical water gasification of plastics with supported RuO2: A potential solution to hydrocarbons-water pollution problem. Process Saf. Environ. Prot. 2016, 102, 140–149. [Google Scholar] [CrossRef]
- Bai, B.; Liu, Y.; Wang, Q.; Zou, J.; Zhang, H.; Jina, H.; Lic, X. Experimental investigation on gasification characteristics of plastic wastes in supercritical water. Renew. Energy 2019, 135, 32–40. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, C.; Zhang, H.; Zou, J.; Zhou, F.; Jin, H. The resource utilization of ABS plastic waste with subcritical and supercritical water treatment. Int. J. Hydrogen Energy 2019, 15758–15765. [Google Scholar] [CrossRef]
- Bai, B.; Wang, W.; Jin, H. Experimental study on gasification performance of polypropylene (PP) plastics in supercritical water. Energy 2020, 191, 116–527. [Google Scholar] [CrossRef]
- Bai, B.; Jin, H.; Zhu, S.; Wu, P.; Fan, C.; Sun, J. Experimental investigation on in-situ hydrogenation induced gasification characteristics of acrylonitrile butadiene styrene (ABS)microplastics in supercritical water. Fuel Process. Technol. 2019, 192, 170–178. [Google Scholar] [CrossRef]
- Bai, B.; Liu, Y.; Zhang, H.; Zhou, F.; Han, X.; Wang, Q.; Jin, H. Experimental investigation on gasification characteristics of polyethylene terephthalate (PET) microplastics in supercritical water. Fuel 2020, 262, 116–630. [Google Scholar] [CrossRef]
- Su, H.; Kanchanatip, E.; Wang, D. Production of H2-rich syngas from gasification of unsorted food waste in supercritical water. Waste Manag. 2020, 102, 520–527. [Google Scholar] [CrossRef]
- Ponzio, A.; Kalisz, S.; Blasiak, W. Effect of operating conditions on tar and gas composition in high temperature air/steam gasification (HTAG) of plastic containing waste. Fuel Process. Technol. 2006, 87, 223–233. [Google Scholar] [CrossRef]
- Mastellone, M.L.; Zaccariello, L.; Santoro, D.; Arena, U. The O2-enriched air gasification of coal, plastics and wood in a fluidized bed reactor. Waste Manag. 2012, 32, 733–742. [Google Scholar] [CrossRef]
- Meng, Q.M.; Chen, X.P.; Zhuang, Y.M.; Liang, C. Effect of temperature on controlled air oxidation of plastic and biomass in a packed-bed reactor. Chem. Eng. Technol. 2013, 36, 220–227. [Google Scholar] [CrossRef]
- Ruoppolo, G.; Ammendola, P.; Chirone, R.; Miccio, F. H2-rich syngas production by fluidized bed gasification of biomass and plastic fuel. Waste Manag. 2012, 32, 724–732. [Google Scholar] [CrossRef]
- Parparita, E.; Uddin, M.A.; Watanabe, T.; Kato, Y.; Yanik, J.; Vasile, C. Gas production by steam gasification of polypropylene/biomass waste composites in a dual-bed reactor. J. Mater. Cycles Waste Manag. 2015, 17, 756–768. [Google Scholar] [CrossRef]
- Baloch, H.A.; Yang, T.; Li, R.; Nizamuddin, S.; Kai, X.; Bhutto, A.W. Parametric study of co-gasification of ternary blends of rice straw, polyethylene and polyvinylchloride. Clean Technol. Environ. Policy 2016, 18, 1031–1042. [Google Scholar] [CrossRef]
- Burra, K.G.; Gupta, A.K. Synergistic effects in steam gasification of combined biomass and plastic waste mixtures. Appl. Energy 2018, 211, 230–236. [Google Scholar] [CrossRef]
- Fan, H.; Gu, J.; Hu, S.; Yuan, H.; Chen, Y. Co-pyrolysis and co-gasification of biomass and polyethylene: Thermal behaviors, volatile products and characteristics of their residues. J. Energy Inst. 2019, 92, 1926–1935. [Google Scholar] [CrossRef]
- Cao, C.; Bian, C.; Wang, G.; Bai, B.; Xie, Y.; Jin, H. Co-gasification of plastic wastes and soda lignin in supercritical water. Chem. Eng. J. 2020, 388, 124–277. [Google Scholar] [CrossRef]



| Carbon | Hydrogen | Nitrogen | Sulfur | |
|---|---|---|---|---|
| Combustion | CO2 | H2O | NO, NO2 | SO2 or SO3 |
| Gasification | CO | H2 | HCN, NH3 or N2 | H2S or COS |
| Oxidation Reaction | Reduction Reaction | ||
|---|---|---|---|
| C + O2 ⇆ CO2 | ΔH = −393.5 kJ/mol | C + CO2 ⇆ 2CO | ΔHR1 = 172.5 kJ/mol (Boudouard reaction) |
| H2 + ½ O2 ⇆ H2O | ΔH = −285.9 kJ/mol | C + H2O → H2 + CO | ΔHR2 = 131.3 kJ/mol (Char steam reforming) |
| CO + H2O ⇆ H2 + CO2 | ΔHR3 = −41.2 kJ/mol (Water-gas shift reaction) | ||
| C + 2 H2 → CH4 | ΔHR4 = −74.5 kJ/mol (Carbon hydrogenation reaction) | ||
| CH4 + H2O ⇆ 3 H2+CO | ΔHR5 = 205.8 kJ/mol (Methane reforming) | ||
| Reactor | Feedstock | Bed Material | Gasification Temperature | Tar Content | Gasifying Agent | Heating Value | Reference | Year |
|---|---|---|---|---|---|---|---|---|
| Fluidized bed | Virgin PP | ash | 690–950 °C | 250 mg/Nm3 | air | LHV: 5.2–11.4 MJ/Nm3 | Xiao et al. [43] | 2006 |
| Image furnace | PP + Al(OH)3 and PP + (NH4PO3)n | - | 1227 °C | - | air | - | Ishii et al. [133] | 2007 |
| bubbling fluidized bed | Recycled PP | bed of silica sand with dolomite or olivine | ∼850 °C |
| air | LHV:
| Sancho et al. [127] | 2008 |
| two-stage fixed bed | Virgin PP. | PP in pyrolysis reactor nickel-catalyst supported on quartz wool in gasification reactor. | 800 °C | - | steam | LHV: from 10.9 to 13.1 MJ/Nm3 depending on the nature of the catalyst | Wu and Williams [134] | 2009 |
| Two-stage fixed bed | Virgin PP | PP in pyrolysis reactor nickel-catalyst in gasification reactor | 700–800 °C | - | steam | - | Wu and Williams [88] | 2010 |
| bubbling fluidized bed | Recycled PP | Silica sand-mixtures silica sand/olivine-olivine | 850 °C; | 2 g of tar/m3 | air | LHV: 9 MJ/Nm3 | Toledo et al. [82] | 2011 |
| bubbling fluidized bed | Pure PP Pure PE Film waste | Silica sand | 750 °C | Waste: 102 g/m3 Pure PE: 128 g/m3 | air | HHV: 3.6 to 5.6 MJ/Nm3 | Martinez–Lera [83] | 2013 |
| screw kiln reaction system (fixed bed) | Virgin PP | Ni/SiO2/Al2O3 catalyst | 850 °C | - | steam | - | Wu and Williams [135] | 2014 |
| Reactor | Feedstock | Bed Material | Gasification Temperature | Tar Content | Gasifying Agent | Heating Value | Reference | Year |
|---|---|---|---|---|---|---|---|---|
| bubbling fluidized bed | Recycled PE | quartz sand or olivine | 783–898 °C | 14.6–6.2 kg/h | Air, steam | LHV 5500–9200 kJ/Nm3 | Arena et al. [84] | 2008 |
| Conical spouted bed | Virgin HDPE | Sand or olivine or ɣ -Al2O3 | 800–900 °C | sand: 16.7g/Nm3 olivine: 15.0 g/Nm3 ɣ-Al2O3: 16.1 g/Nm3 | steam | LHV 15.5 MJ/kg at 900 °C | Erkiaga et al. [67] | 2013 |
| bubbling fluidized bed | Recycled PE | silica sand | - | 11–68 g/Nm3 | Air, steam | LHV 7737–10,349 kJ/Nm3 | Mastellone and Zacariello [85] | 2013 |
| conical spouted bed | Virgin HDPE | Olivine + Ni catalyst | 900 °C | - | steam | - | Lopez et al. [91] | 2015 |
| glass tube | Virgin PE + Ca(OH)2 + Ni(OH)2 | - | 350 °C | - | - | - | Cagnetta et al. [137] | 2019 |
| Reactor | Feedstock | Bed Material | Gasification Temperature | Tar Content | Gasifying Agent | Heating Value | Reference | Year |
|---|---|---|---|---|---|---|---|---|
| Two-stage gasifier | PP, PE, PS, PVC, PMMA, PET | Olivine and activated carbon | 800 °C | 2–2170 mg/Nm3 | air | LHV: From 3.9 to 8.2 MJ/Nm3. | Cho et al. [89] | 2013 |
| Two-stage gasifier | PP, PE, PS, PVC, PMMA, PET | Calcined dolomite and activated carbon | 800 °C | 20–3490 mg/Nm3 | air | LHV: 13.4 MJ/Nm3 | Cho et al. [138] | 2013 |
| Dual fluidized bed | PE, PP, and mixtures of PE + PS, PE + PET and PE + PP | olivine | 850 °C | 100 g/Nm3 | steam | LHV: From 27.2 to 41.2 MJ/Nm3 | Wilk and Hofbauer [115] | 2013 |
| drop-tube fixed bed | LDPE, HDPE, PP, and PS | Dolomite supported Ni catalyst, alumina balls over a quartz wool filter | 850 °C | - | steam and oxygen | - | Friengfung et al. [139] | 2020 |
| Reactor | Feedstock | Optimal Reaction Conditions | Catalyst | Reference | Year |
|---|---|---|---|---|---|
| Batch reactor | XPLE | 700 °C, 30 MPa, 30 min, 20 of molar ratio of water to carbon in XPLE | Nickel | Okajima et al. [140] | 2004 |
| batch reactor | LDPE, HDPE, PP, PS | 450 °C, 10–38 MPa, 60 min | RuO2 | Onwudili and Williams [141] | 2016 |
| quartz tube reactor | HIPS | 800 °C, 60 min, 23 MPa, feedstock concentration of 3 wt% | - | Bai et al. [142] | 2018 |
| quartz tube reactor | ABS | 450 °C, 60 min, 23 MPa, water/ABS (15:1) | - | Liu et al. [143] | 2018 |
| quartz tube reactor | PP | 750 °C, 60 min, 23 MPa, feedstock concentration was 5–25 wt% | inorganic salts in seawater | Bai, Wanga and Jina [144] | 2019 |
| quartz tube reactor | ABS | 800 °C, 60 min, 23 MPa, a feedstock concentration of 3 wt% and a solution of 1 wt% formic acid | formic acid, hydrochloric acid | Bai et al. [145] | 2019 |
| quartz tube reactor | PET | 800 °C, 10 min, 23 MPa | inorganic salts in seawater | Bai et al. [146] | 2020 |
| batch reactor | food waste and LDPE | 420–480 °C, 35 MPa, 30–75 min | food additives (NaCl, Na2CO3, and NaHCO3) | Su et al. [147] | 2020 |
| Reactor | Feedstock | Bed Material | Gasification Temperature | Tar Content | Gasifying Agent | Heating Value | Reference | Year |
|---|---|---|---|---|---|---|---|---|
| bubbling fluidized bed | Coal + biomass + plastic waste | silica sand mixed with dolomite | 850 °C | lower than 0.5 g/Nm3 | air | LHV: 4–8 MJ/Nm3 | Aznar et al. [48] | 2006 |
| fixed bed (KTH/Energy and Furnace Technology) | Rofire (paper fiber mixed with other substances such as fabric fiber, wood chips, and plastics) | Solid feedstock | 1400 °C | 1118–2164 μg | air-steam | LHV: 7.5–9.5 MJ/Nm3 | Ponzio et al. [148] | 2006 |
| pre-pilot scale fluidized bed gasifier | Coals + plastics + wood | Quartz sand | 850 °C | 25–45 g/Nm3 | air, oxygen, steam | LHV: 11100–45500 KJ/Nm3 | Mastellone et al. [86] | 2010 |
| bubbling fluidized bed | Coal + plastics + wood | Quartz sand | 850 °C | 13,500–21,800 mg/Nm3 | oxygen-enriched air | LHV: 5150–8950 kJ/Nm3 | Mastellone et al. [149] | 2011 |
| packed-bed | PP + poplar sawdust | 150 g sawdust and 250 g PP | 400–800 °C | - | air | LHV: 4334–9805 kJ/Nm3 | Meng et al. [150] | 2012 |
| catalytic fluidized bed gasifier | Wood +biomass + plastic + olive husks | inert quarzite sand or 5.5wt% Ni-based catalyst supported on ɣ-alumina | 780 °C | 60 g/Ncm3 | steam | LHV: 21.9 MJ/Kg | Ruoppolo et al. [151] | 2012 |
| dual fluidized bed | plastics + soft wood pellets | olivine | 850 °C | - | steam | - | Wilk and Hofbauer [110] | 2013 |
| dual-bed quartz reactor | isotactic PP and different types of lignocellulosic biomass waste | quartz wool bed + Fe2O3/CeO2 catalyst | 700 °C | - | steam | LHV: 14,599–32,594 kJ/kg | Parparita et al. [152] | 2015 |
| bubbling fluidized bed gasifier | rice husk + straw PE | Silica sand | 850 °C | 5–15 g/m3 | Mixtures of air, oxygen, steam and CO2 | HHV/10: 11–22 KJ/Ndm3 | Pinto et al. [87] | 2016 |
| glass tube | rice straw + PE+ PVC | - | 700–900 °C | - | electrically heated | LHV: 15.3 MJ/Nm3 | Baloch et al. [153] | 2016 |
| laboratory scale semi-batch | pine wood + PP+PET+PBC | - | 900 °C | - | steam | - | Burra and Gupta [154] | 2018 |
| Thermogravimetric analyzer | eucalyptus wood or rice straw + PE | - | 1000 °C | - | - | - | Fan et al. [155] | 2019 |
| autoclave | soda lignin (from black liquor) + PE, PC, PP and ABS | - | 750 °C, 23–26 MPa | - | SCW | - | Cao et al. [156] | 2020 |
| two-stage fixed bed | LDPE+ pine sawdust | quartz wool + Ni-CaO-C | 800 °C | - | steam | - | Chai et al. [90] | 2020 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciuffi, B.; Chiaramonti, D.; Rizzo, A.M.; Frediani, M.; Rosi, L. A Critical Review of SCWG in the Context of Available Gasification Technologies for Plastic Waste. Appl. Sci. 2020, 10, 6307. https://doi.org/10.3390/app10186307
Ciuffi B, Chiaramonti D, Rizzo AM, Frediani M, Rosi L. A Critical Review of SCWG in the Context of Available Gasification Technologies for Plastic Waste. Applied Sciences. 2020; 10(18):6307. https://doi.org/10.3390/app10186307
Chicago/Turabian StyleCiuffi, Benedetta, David Chiaramonti, Andrea Maria Rizzo, Marco Frediani, and Luca Rosi. 2020. "A Critical Review of SCWG in the Context of Available Gasification Technologies for Plastic Waste" Applied Sciences 10, no. 18: 6307. https://doi.org/10.3390/app10186307
APA StyleCiuffi, B., Chiaramonti, D., Rizzo, A. M., Frediani, M., & Rosi, L. (2020). A Critical Review of SCWG in the Context of Available Gasification Technologies for Plastic Waste. Applied Sciences, 10(18), 6307. https://doi.org/10.3390/app10186307








