Effect of Urea-Formaldehyde (UF) with Waterborne Emulsion Microcapsules on Properties of Waterborne Acrylic Coatings Based on Coating Process for American Lime
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Method
- (1)
- Preparation of microcapsules
- (2)
- Preparation of the paint film
2.3. Testing and Characterization
3. Results and Discussion
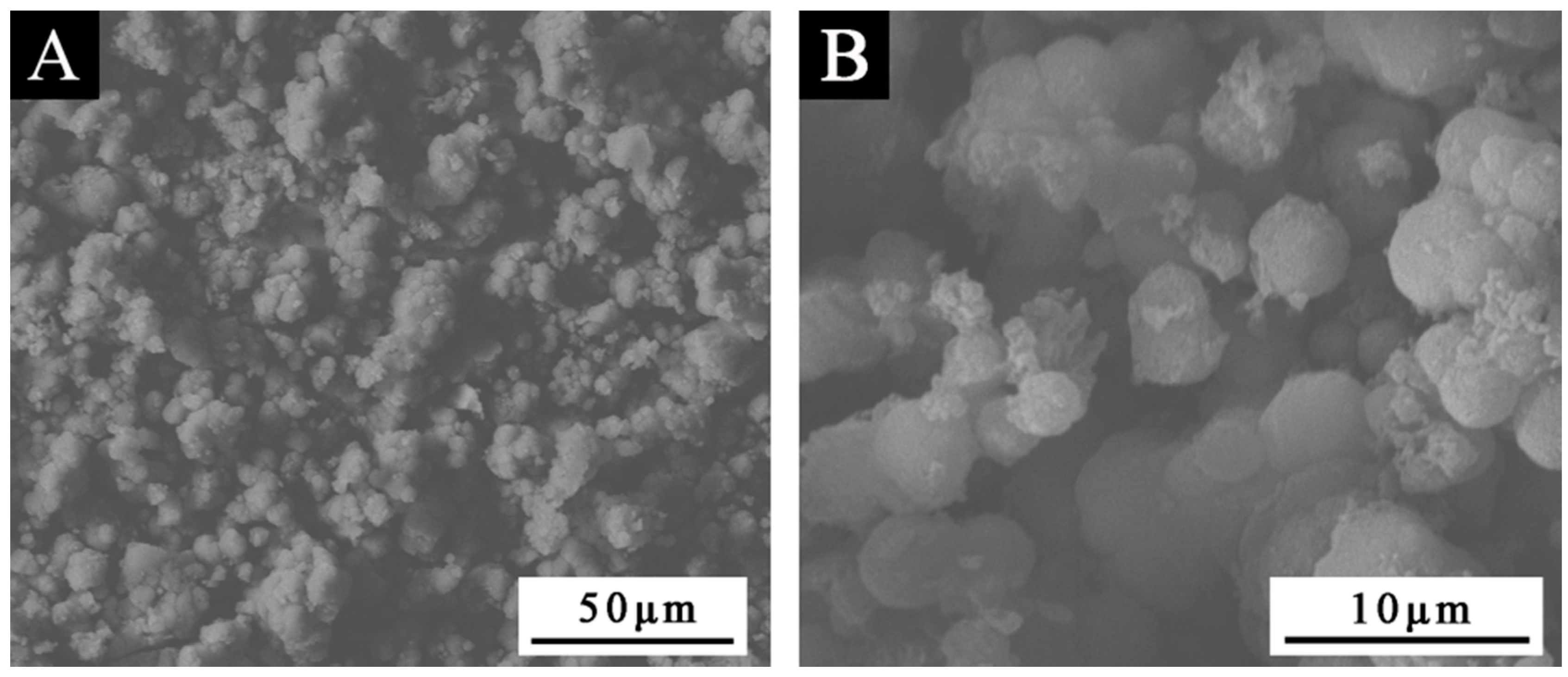
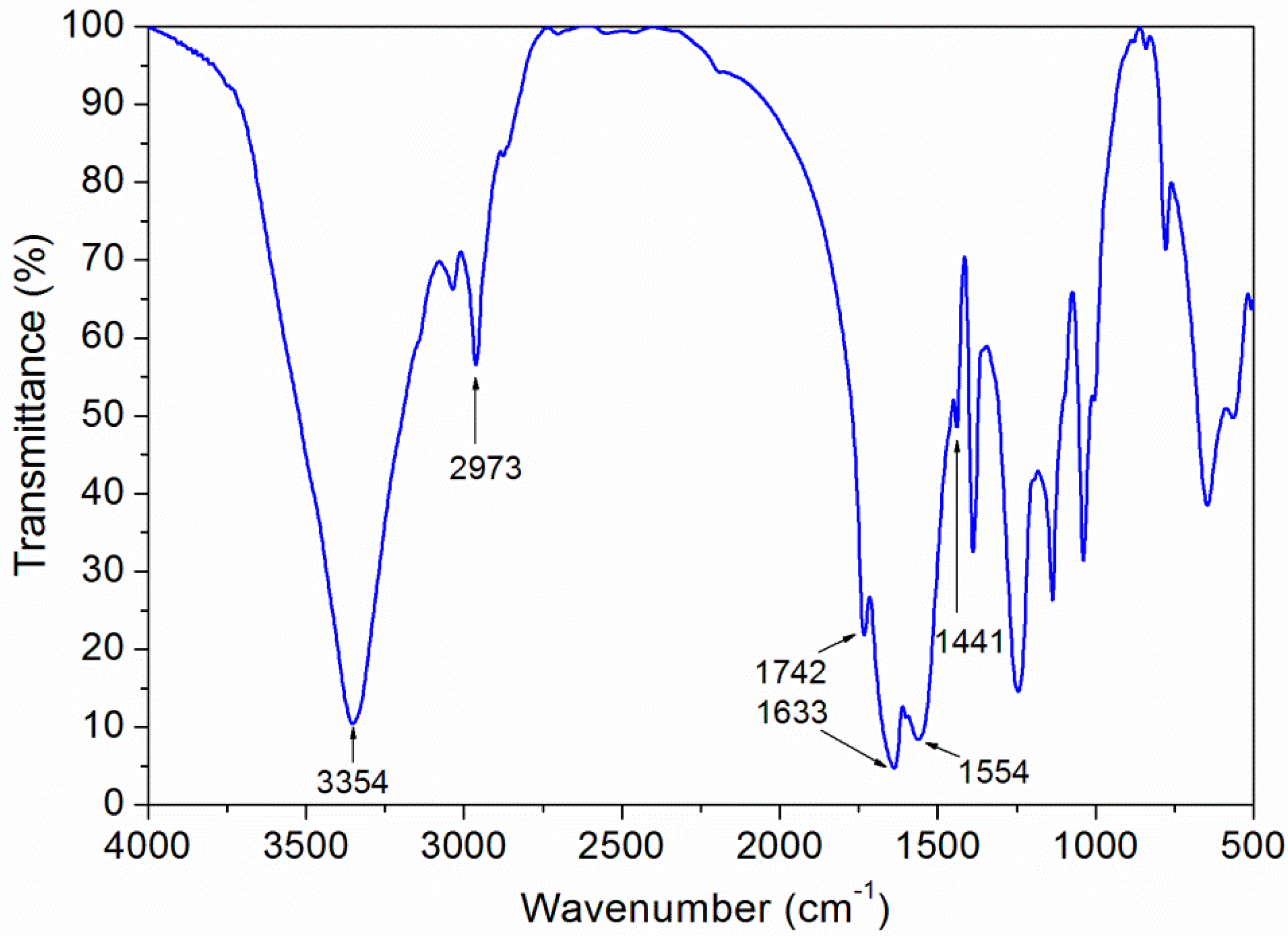
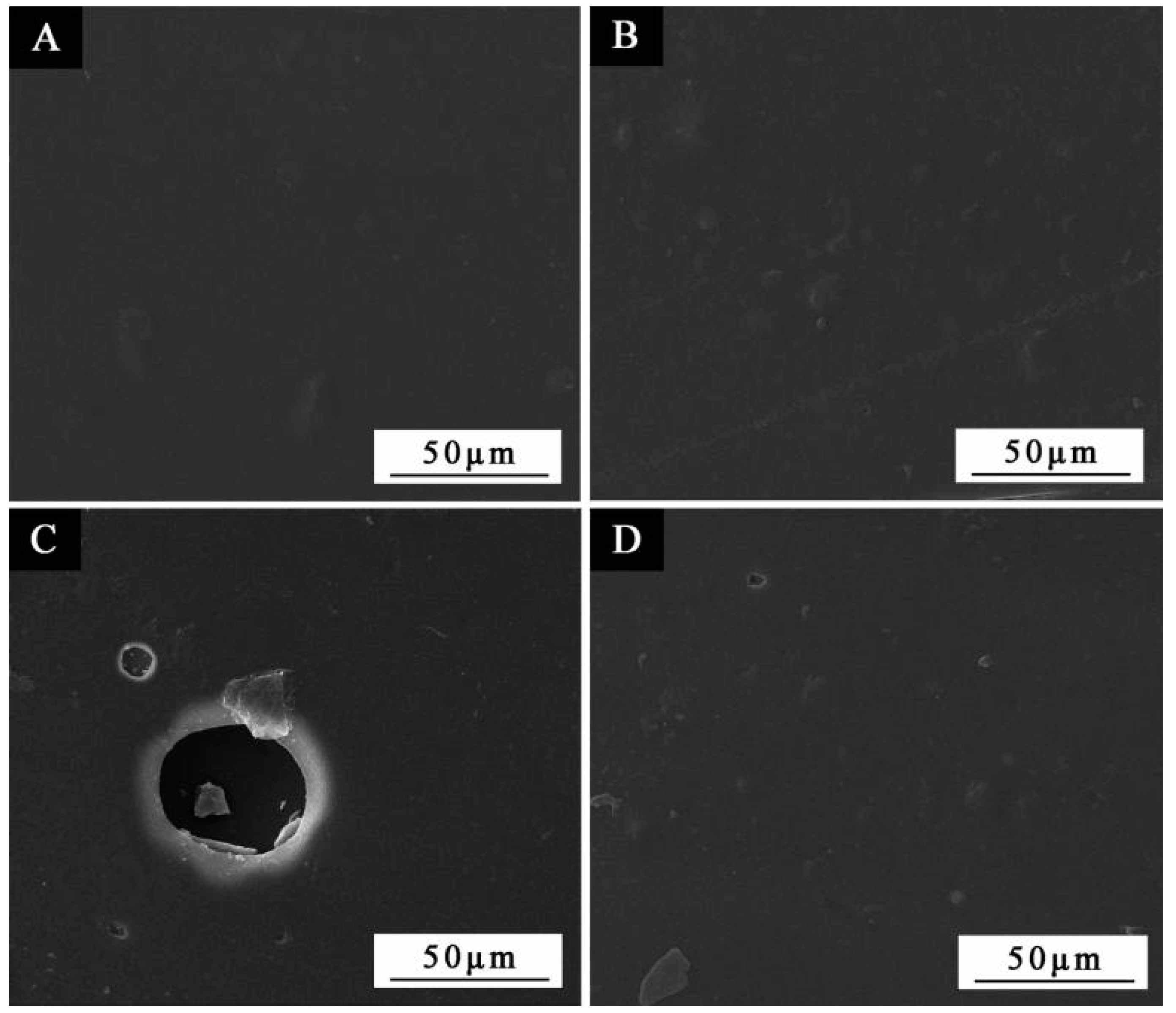
3.1. SEM and FTIR of the Microcapsules
3.2. Effect of Coating Process on Optical Properties
3.3. Effect of Coating Process on Mechanical Properties
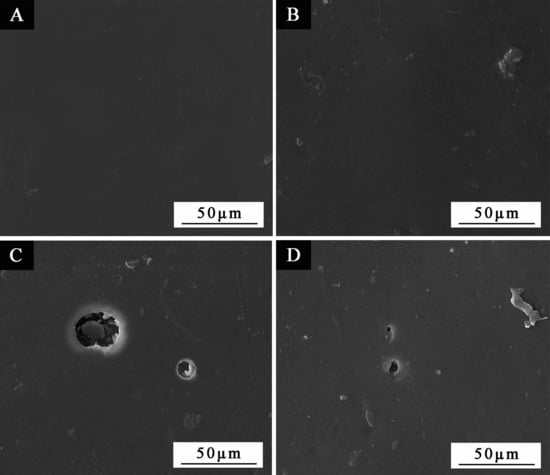
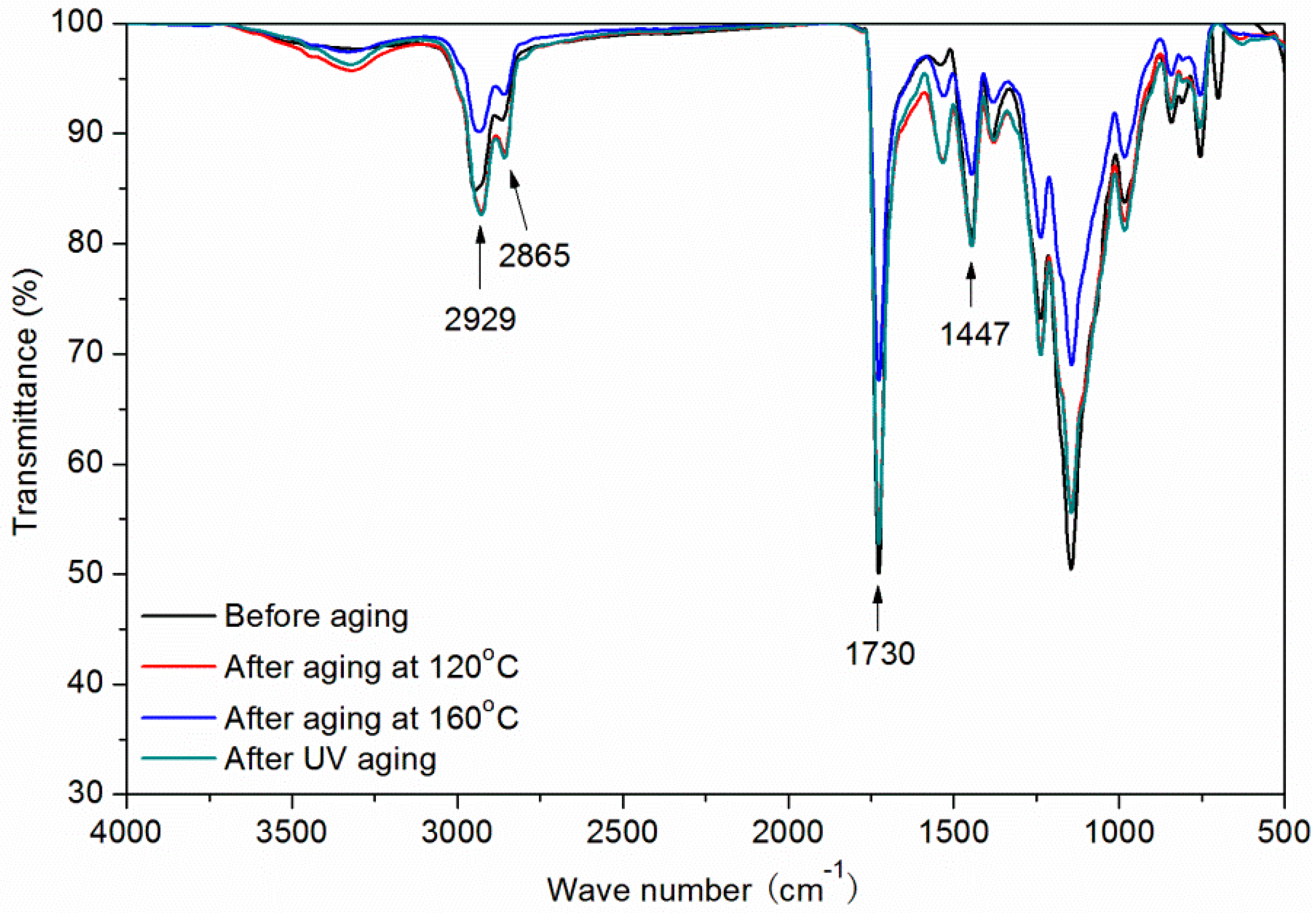
3.4. SEM and FTIR of the Paint Film
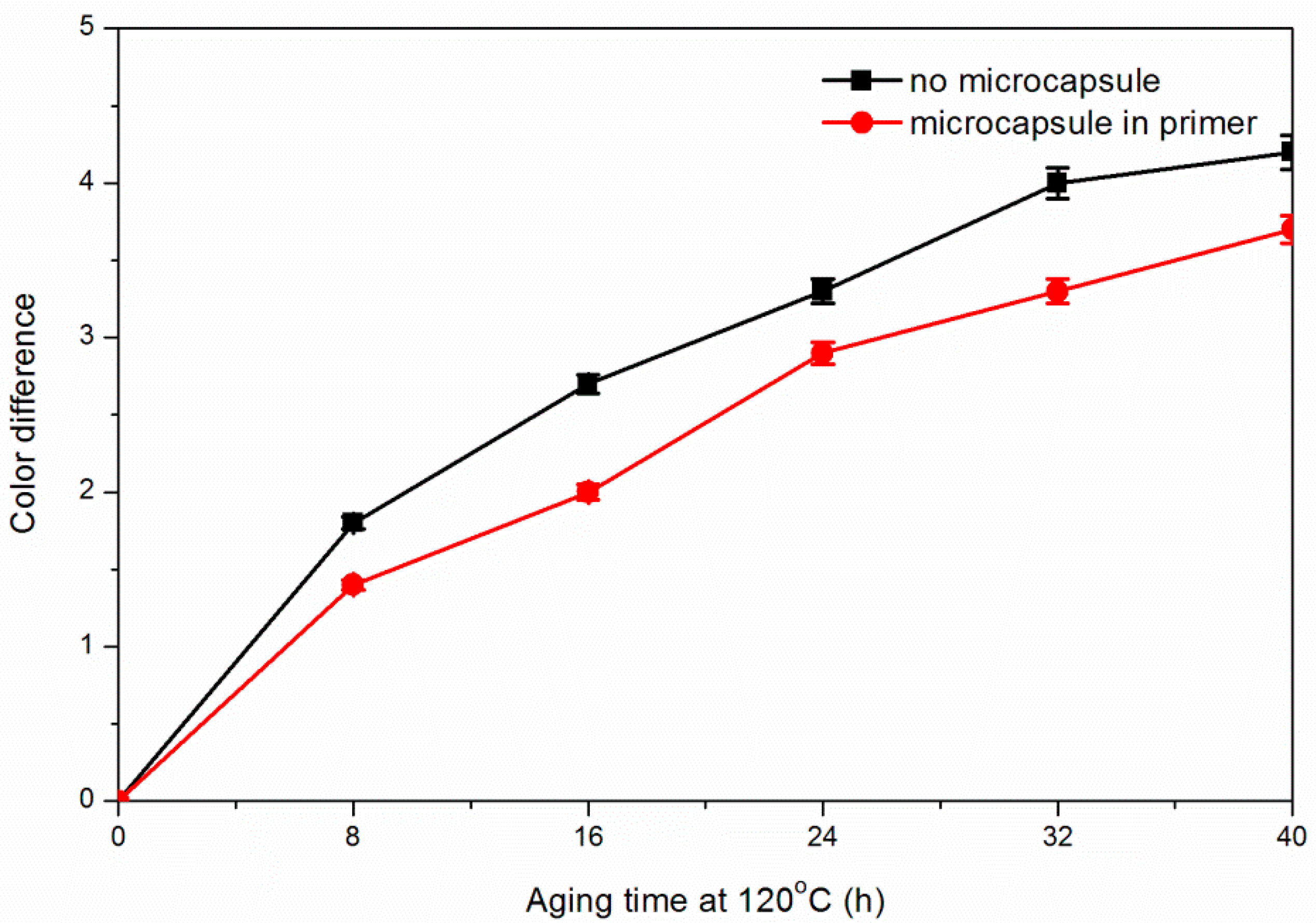
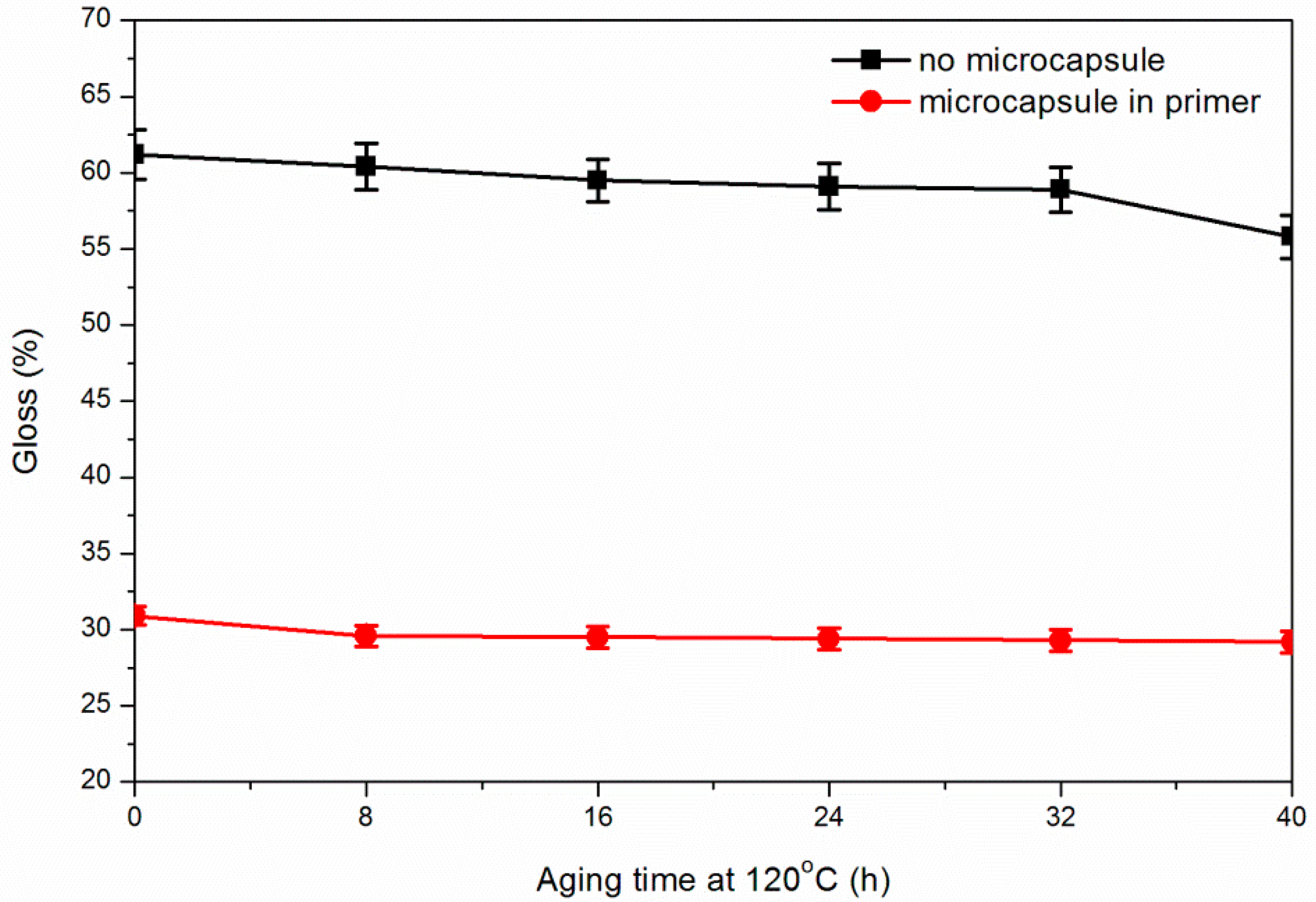
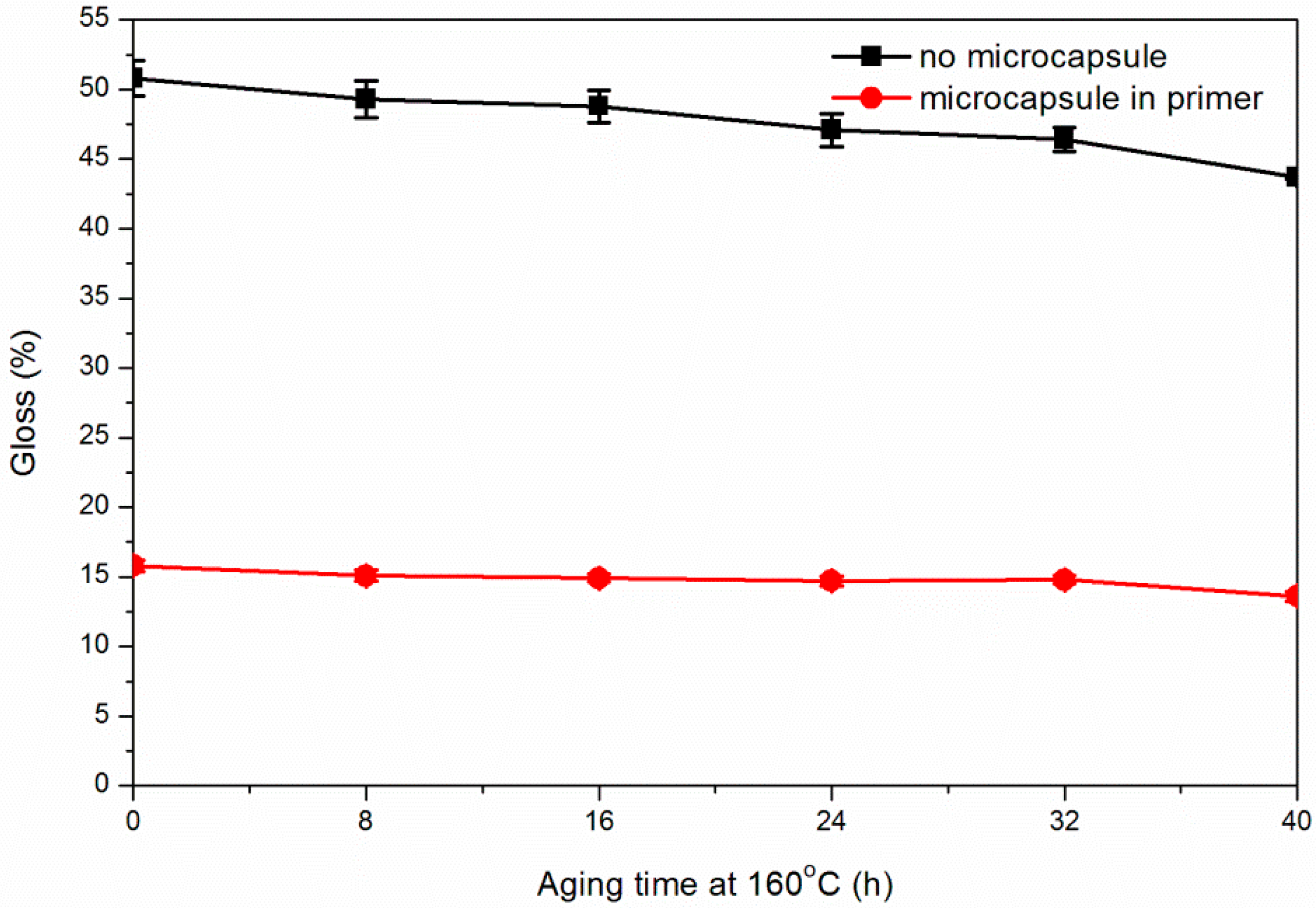
3.5. Effect of Coating Process on Aging Resistance Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Athawale, V.D.; Nimbalkar, R.V. Waterborne coatings based on renewable oil resources: An overview. J. Am. Oil. Chem. Soc. 2011, 88, 159–185. [Google Scholar] [CrossRef]
- Omel’chenko, S.I.; Tryhub, S.O.; Laskovenko, N.M. Prospects of investigation and production of waterborne paintwork materials. Mater. Sci. 2001, 37, 790–801. [Google Scholar] [CrossRef]
- Cheng, L.S.; Ren, S.B.; Lu, X.N. Application of eco-friendly waterborne polyurethane composite coating incorporated with nano cellulose crystalline and silver nano particles on wood antibacterial board. Polymers 2020, 12, 407. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Cai, M.; Li, W.; Fan, X.Q.; Zhu, M.H. Amino-functionalized Ti3C2Tx with anti-corrosive/wear function for waterborne epoxy coating. J. Mater. Sci. Technol. 2020, 54, 144–159. [Google Scholar] [CrossRef]
- Yan, X.X.; Wang, L.; Qian, X.Y. Effect of coating process on performance of reversible thermochromic waterborne coatings for Chinese fir. Coatings 2020, 10, 223. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.L.; Xu, D.D.; He, Z.X.; Wang, F.Q.; Gui, S.H.; Fan, J.L.; Pan, X.Y.; Dai, X.H.; Dong, X.Y.; Liu, B.X.; et al. Nanocellulose-reinforced polyurethane for waterborne wood coating. Molecules 2019, 24, 3151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, C.D.; Zhao, M.; Sun, D.; Yang, L.; Zhang, L.; Guo, R.W.; Yao, F.L.; An, Y. Preparation and properties of few-layer graphene modified waterborne epoxy coatings. J. Appl. Polym. Sci. 2018, 135, 46743. [Google Scholar] [CrossRef]
- Xiong, X.Q.; Niu, Y.T.; Yuan, Y.Y.; Zhang, L.T. Study on dimensional stability of veneer rice straw particleboard. Coatings 2020, 10, 558. [Google Scholar] [CrossRef]
- Peng, G.J.; Dou, G.J.; Hu, Y.H.; Sun, Y.H.; Chen, Z.T. Phase change material (PCM) microcapsules for thermal energy storage. Adv. Polym. Tech. 2020, 2020, 9490873. [Google Scholar] [CrossRef]
- Ullah, H.; Azizli, K.A.M.; Man, Z.B.; Ismail, M.B.C.; Khan, M.I. The potential of microencapsulated self-healing materials for microcracks recovery in self-healing composite systems: A review. Polym. Rev. 2016, 56, 429–485. [Google Scholar] [CrossRef]
- Gray, A.; Egan, S.; Bakalis, S.; Zhang, Z.B. Determination of microcapsule physicochemical, structural, and mechanical properties. Particuology 2016, 24, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, Z.W.; Feng, Q.K.; Qiu, H.X.; Yang, G.Z.; Zheng, S.Y.; Yang, J.H. Encapsulation of linseed oil in graphene oxide shells for preparation of self-healing composite coatings. Prog. Org. Coat. 2019, 129, 285–291. [Google Scholar] [CrossRef]
- Bao, Y.; Yan, Y.; Chen, Y.; Ma, J.Z.; Zhang, W.B.; Liu, C. Facile fabrication of BTA@ZnO microcapsules and their corrosion protective application in waterborne polyacrylate coatings. Prog. Org. Coat. 2019, 136, 105233. [Google Scholar] [CrossRef]
- Aruna, S.T.; Arunima, S.; Latha, S.; Grips, V.K.W. Preparation of oil-encapsulated microcapsules and tribological property of Ni composite coating. Mater. Manuf. Process. 2016, 31, 107–111. [Google Scholar] [CrossRef]
- Li, H.Y.; Cui, Y.X.; Li, Z.K.; Zhu, Y.J.; Wang, H.Y. Fabrication of microcapsules containing dual-functional tung oil and properties suitable for self-healing and self-lubricating coatings. Prog. Org. Coat. 2017, 115, 164–171. [Google Scholar] [CrossRef]
- Siva, T.; Sathiyanarayanan, S. Self healing coatings containing dual active agent loaded urea formaldehyde (UF) microcapsules. Prog. Org. Coat. 2015, 82, 57–67. [Google Scholar] [CrossRef]
- Hsieh, T.L.; Li, C.C.; Lin, P.C.; Hsu, Y.C. Encapsulating well-dispersed carbon nanoparticles for applications in the autonomous restoration of electronic circuits. ACS Appl. Mater. Inter. 2020, 12, 38690–38699. [Google Scholar] [CrossRef]
- Chen, S.W.; Lu, X.C.; Wang, T.Z.; Zhang, Z.M. Preparation and characterization of urea-formaldehyde resin/reactive kaolinite composites. Particuology 2016, 24, 203–209. [Google Scholar] [CrossRef]
- Yan, X.X.; Peng, W.W. Preparation of microcapsules of urea formaldehyde resin coated waterborne coatings and their effect on properties of wood crackle coating. Coatings 2020, 10, 764. [Google Scholar] [CrossRef]
- Nakano, Y.; Katakuse, Y.; Azechi, Y. An application of X-ray fluorescence as process analytical technology (PAT) to monitor particle coating processes. Chem. Pharm. Bull. 2018, 66, 596–601. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.Q.; Gao, D.; Xu, W. Effect of sanding processes on the surface properties of modified Poplar coated by primer compared with Mahogany. Coatings 2020, 10, 856. [Google Scholar] [CrossRef]
- Xu, W.; Fang, X.Y.; Han, J.T.; Wu, Z.H.; Zhang, J.L. Effect of coating thickness on sound absorption property of four wood species commonly used for piano soundboards. Wood Fiber Sci. 2020, 52, 28–43. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.X.; Wang, L.; Qian, X.Y. Effect of urea-formaldehyde-coated epoxy microcapsule modification on gloss, toughness and chromatic distortion of acrylic copolymers waterborne coating. Coatings 2019, 9, 239. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhou, J.C.; Huang, Q.T.; Yang, F.; Wang, Y.J.; Liang, X.M.; Li, J.Z. Study on the colorimetry properties of transparent wood prepared from six wood species. ACS Omega 2020, 5, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- GB/T 4893.6-2013. Test of Surface Coatings of Furniture-Part 6: Determination of Gloss Value; Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- GB/T 6739-2006. Paints and Varnishes-Determination of Film Hardness by Pencil Test; Standardization Administration of the People’s Republic of China: Beijing, China, 2006.
- GB/T 4893.4-2013. Test of Surface Coatings of Furniture-Part 4: Determination of Adhesion-Cross Cut; Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- GB/T 1732-1993. Determination of Impact Resistance of Film; Standardization Administration of the People’s Republic of China: Beijing, China, 1993.
- GB/T 4893.1-2005. Furniture-Asssessment of Surface Resistance to Cold Liquids; Standardization Administration of the People’s Republic of China: Beijing, China, 2005.
- Ataei, S.; Khorasani, S.N.; Torkaman, R.; Neisiany, R.E.; Koochaki, M.S. Self-healing performance of an epoxy coating containing microencapsulated alkyd resin based on coconut oil. Prog. Org. Coat. 2018, 120, 160–166. [Google Scholar] [CrossRef]
- Es-haghi, H.; Mirabedini, S.M.; Imani, M.; Farnood, R.R. Mechanical and self-healing properties of a water-based acrylic latex containing linseed oil filled microcapsules: Effect of pre-silanization of microcapsules’ shell compound. Compos. Part B Eng. 2015, 85, 305–314. [Google Scholar] [CrossRef]
- Pak, A.R.; Park, J.H.; Lee, S.G. Blowing properties and functionality of thermoplastic polyester film using thermally expandable microcapsules. Polymers 2019, 11, 1652. [Google Scholar] [CrossRef] [Green Version]
- Feng, G.Y.; Wang, X.Y.; Zhang, D.T.; Xiao, X.L.; Qian, K. Fabrication of bilayer antioxidant microcapsule and evaluation of its efficiency in stabilization of polypropylene. Mater. Res. Express 2019, 6, 125327. [Google Scholar] [CrossRef]
- Li, L.; Qian, Y.; Yang, D.J.; Qiu, X.Q. Preparation of lignin/silica nanoparticle based microcapsules and their application in self-healing coatings. Chem. J. Chin. Univ. 2019, 40, 1293–1300. [Google Scholar]
- Yan, X.; Ning, G.T.; Wang, X.F.; Ai, T.; Zhao, P.; Wang, Z.J. Preparation and short-term aging properties of asphalt modified by novel sustained-release microcapsules containing rejuvenator. Materials 2019, 12, 1122. [Google Scholar] [CrossRef] [Green Version]
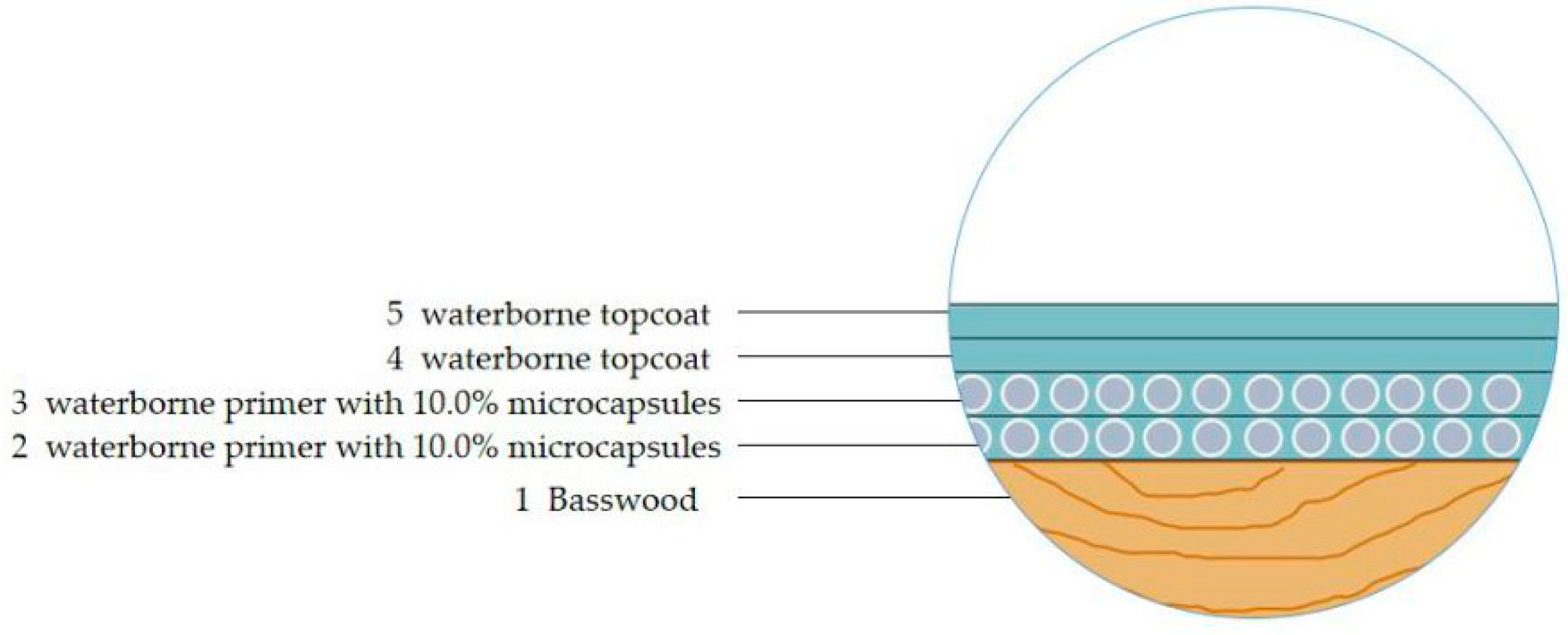






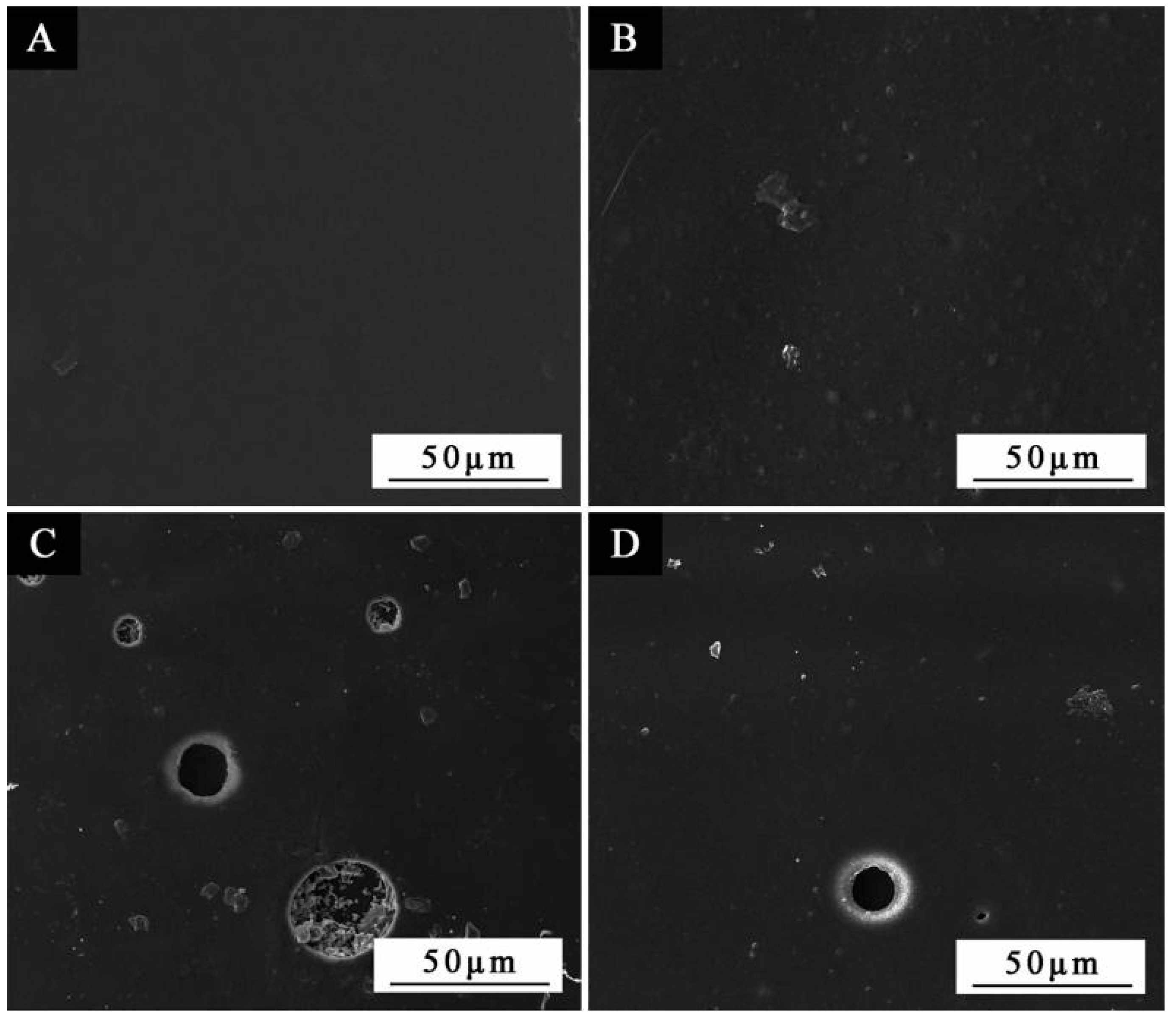

| Experiment Number | Times of Coated Primer | Times of Coated Topcoat | Microcapsule Adding Method |
|---|---|---|---|
| 1# | 2 | 2 | primer addition |
| 2# | 2 | 3 | primer addition |
| 3# | 3 | 2 | primer addition |
| 4# | 3 | 3 | primer addition |
| 5# | 2 | 2 | topcoat addition |
| 6# | 2 | 3 | topcoat addition |
| 7# | 3 | 2 | topcoat addition |
| 8# | 3 | 3 | topcoat addition |
| Experiment Number | Microcapsule Weight (g) | Weight of Waterborne Primer (g) | Weight of Waterborne Topcoat (g) | Weight of Waterborne Coating (g) |
|---|---|---|---|---|
| 1–4# | 2.0 | 18.0 | 20.0 | 40.0 |
| 5–8# | 2.0 | 20.0 | 18.0 | 40.0 |
| Sample | L1 | a1 | b1 | c1 | H1 | L2 | a2 | b2 | c2 | H2 | ΔE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No microcapsules | 71.8 ± 1.7 | 13.7 ± 0.3 | 31.6 ± 0.7 | 34.4 ± 0.8 | 66.5 ± 1.6 | 72.1 ± 1.8 | 13.4 ± 0.3 | 31.5 ± 0.7 | 34.1 ± 0.8 | 66.3 ± 1.6 | 0.4 ± 0 |
| 1# | 71.8 ± 1.7 | 12.0 ± 0.3 | 28.0 ± 0.7 | 30.5 ± 0.7 | 66.8 ± 1.6 | 72.3 ± 1.8 | 11.9 ± 0.2 | 28.0 ± 0.7 | 30.4 ± 0.7 | 66.8 ± 1.6 | 0.5 ± 0 |
| 2# | 75.5 ± 1.8 | 10.7 ± 0.2 | 25.6 ± 0.6 | 27.8 ± 0.6 | 67.2 ± 1.6 | 76.0 ± 1.9 | 10.1 ± 0.2 | 25.9 ± 0.6 | 28.6 ± 0.7 | 69.2 ± 1.7 | 0.8 ± 0 |
| 3# | 69.2 ± 1.7 | 13.9 ± 0.3 | 31.5 ± 0.7 | 34.4 ± 0.8 | 66.1 ± 1.6 | 69.4 ± 1.7 | 14.2 ± 0.3 | 30.7 ± 0.7 | 33.8 ± 0.8 | 65.1 ± 1.6 | 0.9 ± 0 |
| 4# | 77.1 ± 1.9 | 10.4 ± 0.2 | 32.0 ± 0.8 | 33.7 ± 0.8 | 71.9 ± 1.7 | 77.8 ± 1.9 | 10.8 ± 0.2 | 31.7 ± 0.7 | 32.7 ± 0.8 | 70.5 ± 1.8 | 0.9 ± 0 |
| 5# | 70.2 ± 1.7 | 15.7 ± 0.3 | 32.3 ± 0.8 | 35.9 ± 0.9 | 64.0 ± 1.6 | 70.7 ± 1.7 | 14.8 ± 0.3 | 31.8 ± 0.7 | 35.1 ± 0.9 | 64.9 ± 1.6 | 1.1 ± 0 |
| 6# | 72.2 ± 1.8 | 11.9 ± 0.2 | 32.5 ± 0.8 | 34.7 ± 0.8 | 69.9 ± 1.7 | 71.3 ± 1.7 | 12.2 ± 0.3 | 32.6 ± 0.8 | 34.8 ± 0.8 | 69.5 ± 1.7 | 1.0 ± 0 |
| 7# | 73.7 ± 1.8 | 12.6 ± 0.3 | 31.5 ± 0.7 | 33.9 ± 0.8 | 68.2 ± 1.7 | 72.9 ± 1.8 | 13.0 ± 0.3 | 31.6 ± 0.7 | 34.2 ± 0.8 | 67.5 ± 1.6 | 0.9 ± 0 |
| 8# | 69.6 ± 1.7 | 14.2 ± 0.3 | 31.6 ± 0.7 | 34.7 ± 0.8 | 65.7 ± 1.6 | 69.1 ± 1.7 | 14.5 ± 0.4 | 31.8 ± 0.7 | 35.0 ± 0.9 | 65.4 ± 1.6 | 0.6 ± 0 |
| Sample | 20° Gloss (%) | 60° Gloss (%) | 85° Gloss (%) |
|---|---|---|---|
| No microcapsules | 7.3 ± 0.1 | 27.9 ± 0.6 | 30.7 ± 0.7 |
| 1# | 5.6 ± 0.1 | 22.9 ± 0.5 | 39.0 ± 0.9 |
| 2# | 4.1 ± 0.1 | 17.1 ± 0.4 | 49.7 ± 1.2 |
| 3# | 8.3 ± 0.2 | 29.1 ± 0.7 | 53.3 ± 1.3 |
| 4# | 7.1 ± 0.1 | 24.5 ± 0.6 | 40.7 ± 1.0 |
| 5# | 1.9 ± 0 | 7.3 ± 0.1 | 22.1 ± 0.5 |
| 6# | 2.5 ± 0 | 10.0 ± 0.2 | 16.1 ± 0.4 |
| 7# | 2.8 ± 0 | 10.8 ± 0.2 | 19.3 ± 0.4 |
| 8# | 1.8 ± 0 | 7.2 ± 0.1 | 21.6 ± 0.5 |
| Sample | Hardness | Adhesion (Grade) | Impact Resistance (N·cm−2) | Elongation at Break (%) |
|---|---|---|---|---|
| No microcapsules | HB ± 0 | 0 ± 0 | 50.0 ± 0 | 5.2 ± 0.1 |
| 1# | 2H ± 0 | 0 ± 0 | 100.0 ± 0 | 20.1 ± 0.4 |
| 2# | 3H ± 0 | 0 ± 0 | 100.0 ± 0 | 29.4 ± 0.7 |
| 3# | 3H ± 0 | 0 ± 0 | 110.0 ± 0 | 29.7 ± 0.7 |
| 4# | 2H ± 0 | 0 ± 0 | 100.0 ± 0 | 23.7 ± 0.5 |
| 5# | 2H ± 0 | 0 ± 0 | 100.0 ± 0 | 29.8 ± 0.7 |
| 6# | 3H ± 0 | 1 ± 0 | 100.0 ± 0 | 24.7 ± 0.6 |
| 7# | 2H ± 0 | 0 ± 0 | 100.0 ± 0 | 26.6 ± 0.6 |
| 8# | 3H ± 0 | 1 ± 0 | 110.0 ± 0 | 25.6 ± 0.6 |
| Sample | Liquid Color Difference | Liquid Resistant Gloss (%) | Liquid Resistance (Grade) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NaCl | Ethanol | Detergent | Red Ink | NaCl | Ethanol | Detergent | Red Ink | NaCl | Ethanol | Detergent | Red Ink | |
| No microcapsules | 1.2 ± 0 | 0.3 ± 0 | 1.1 ± 0 | 1.7 ± 0 | 27.3 ± 0.5 | 27.9 ± 0.6 | 27.9 ± 0.6 | 25.6 ± 0.5 | 1.0 ± 0 | 1.0 ± 0 | 1.0 ± 0 | 1.0 ± 0 |
| 1# | 0.9 ± 0 | 1.0 ± 0 | 0.8 ± 0 | 2.5 ± 0 | 22.1 ± 0.5 | 22.7 ± 0.5 | 22.0 ± 0.5 | 22.5 ± 0.5 | 1.0 ± 0 | 1.0 ± 0 | 1.0 ± 0 | 2.0 ± 0 |
| 2# | 0.9 ± 0 | 0.7 ± 0 | 0.7 ± 0 | 2.7 ± 0 | 17.2 ± 0.4 | 17.1 ± 0.4 | 16.6 ± 0.4 | 16.4 ± 0.4 | 1.0 ± 0 | 1.0 ± 0 | 1.0 ± 0 | 2.0 ± 0 |
| 3# | 0.5 ± 0 | 1.2 ± 0 | 0.5 ± 0 | 1.9 ± 0 | 28.4 ± 0.7 | 28.7 ± 0.4 | 28.6 ± 0.7 | 28.3 ± 0.7 | 1.0 ± 0 | 1.0 ± 0 | 1.0 ± 0 | 1.0 ± 0 |
| 4# | 0.9 ± 0 | 0.9 ± 0 | 0.8 ± 0 | 2.2 ± 0 | 24.1 ± 0.6 | 24.3 ± 0.7 | 24.2 ± 0.6 | 23.7 ± 0.6 | 1.0 ± 0 | 1.0 ± 0 | 1.0 ± 0 | 2.0 ± 0 |
| 5# | 0.9 ± 0 | 0.9 ± 0 | 1.1 ± 0 | 5.5 ± 0.1 | 6.7 ± 0.1 | 6.9 ± 0.1 | 6.3 ± 0.1 | 5.6 ± 0.1 | 1.0 ± 0 | 1.0 ± 0 | 1.0 ± 0 | 3.0 ± 0 |
| 6# | 1.1 ± 0 | 0.7 ± 0 | 0.8 ± 0 | 3.7 ± 0 | 9.4 ± 0.2 | 9.1 ± 0.2 | 9.7 ± 0.2 | 9.0 ± 0.2 | 1.0 ± 0 | 1.0 ± 0 | 1.0 ± 0 | 3.0 ± 0 |
| 7# | 1.0 ± 0 | 0.7 ± 0 | 0.9 ± 0 | 4.0 ± 0.1 | 9.6 ± 0.2 | 10.1 ± 0.2 | 9.1 ± 0.2 | 8.7 ± 0.2 | 1.0 ± 0 | 1.0 ± 0 | 1.0 ± 0 | 3.0 ± 0 |
| 8# | 1.3 ± 0 | 1.1 ± 0 | 1.2 ± 0 | 1.7 ± 0 | 7.4 ± 0.1 | 7.0 ± 0.1 | 7.2 ± 0.1 | 6.5 ± 0.1 | 1.0 ± 0 | 1.0 ± 0 | 1.0 ± 0 | 2.0 ± 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Zhao, W.; Qian, X. Effect of Urea-Formaldehyde (UF) with Waterborne Emulsion Microcapsules on Properties of Waterborne Acrylic Coatings Based on Coating Process for American Lime. Appl. Sci. 2020, 10, 6341. https://doi.org/10.3390/app10186341
Yan X, Zhao W, Qian X. Effect of Urea-Formaldehyde (UF) with Waterborne Emulsion Microcapsules on Properties of Waterborne Acrylic Coatings Based on Coating Process for American Lime. Applied Sciences. 2020; 10(18):6341. https://doi.org/10.3390/app10186341
Chicago/Turabian StyleYan, Xiaoxing, Wenting Zhao, and Xingyu Qian. 2020. "Effect of Urea-Formaldehyde (UF) with Waterborne Emulsion Microcapsules on Properties of Waterborne Acrylic Coatings Based on Coating Process for American Lime" Applied Sciences 10, no. 18: 6341. https://doi.org/10.3390/app10186341
APA StyleYan, X., Zhao, W., & Qian, X. (2020). Effect of Urea-Formaldehyde (UF) with Waterborne Emulsion Microcapsules on Properties of Waterborne Acrylic Coatings Based on Coating Process for American Lime. Applied Sciences, 10(18), 6341. https://doi.org/10.3390/app10186341