Influence of NOM on the Stability of Zinc Oxide Nanoparticles in Ecotoxicity Tests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.1.1. Nanomaterials
2.1.2. NOMs
2.2. Measurement of Dispersibility
2.3. Analysis of Ion Concentration
2.4. Characterization of ZnO Nanoparticles
2.5. Ecotoxicity Study
2.5.1. Test Media of Nanomaterials Dispersed with NOM
2.5.2. Conditions for Algae Growth Inhibition Tests
2.5.3. Conditions for Water Flea Acute Toxicity Test
2.5.4. Conditions for Fish Acute Toxicity Test
2.5.5. Statistical Analysis
3. Results and Discussion
3.1. Determination of Dispersibility
3.2. Measurement of Properties
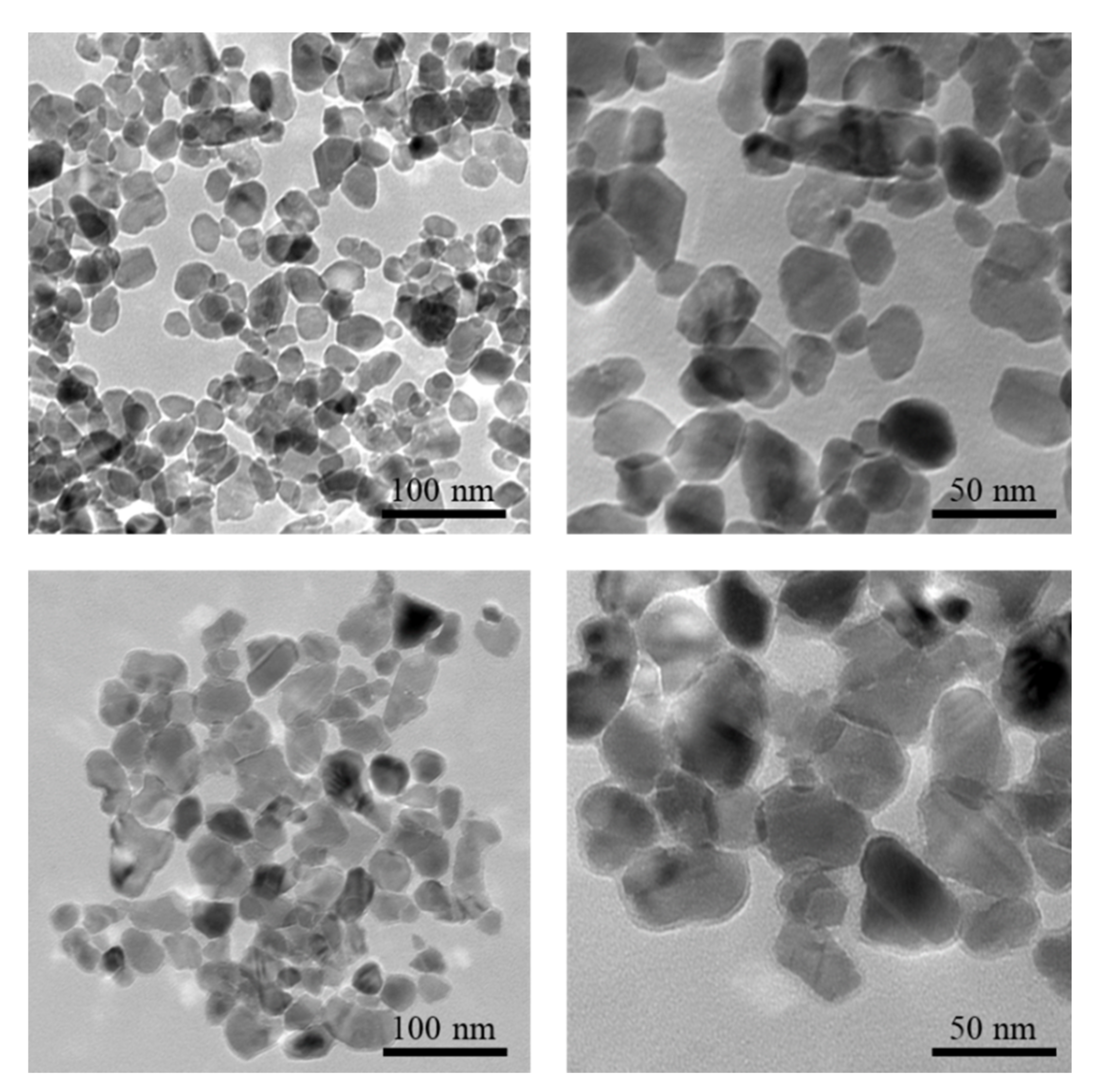
3.2.1. Particle-Size Determination via TEM
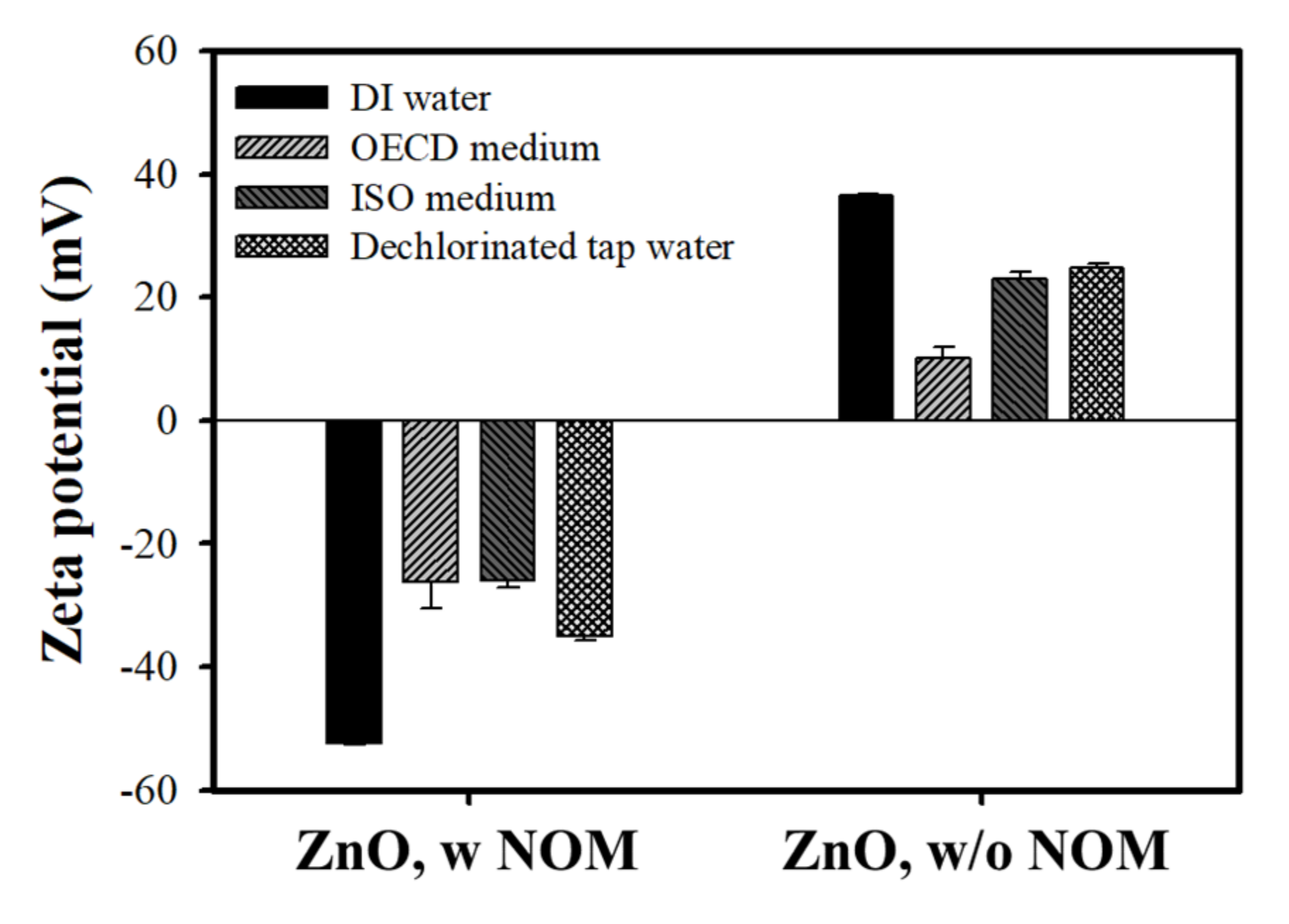
3.2.2. Determination of Surface Charge of Nanoparticles in Test Medium
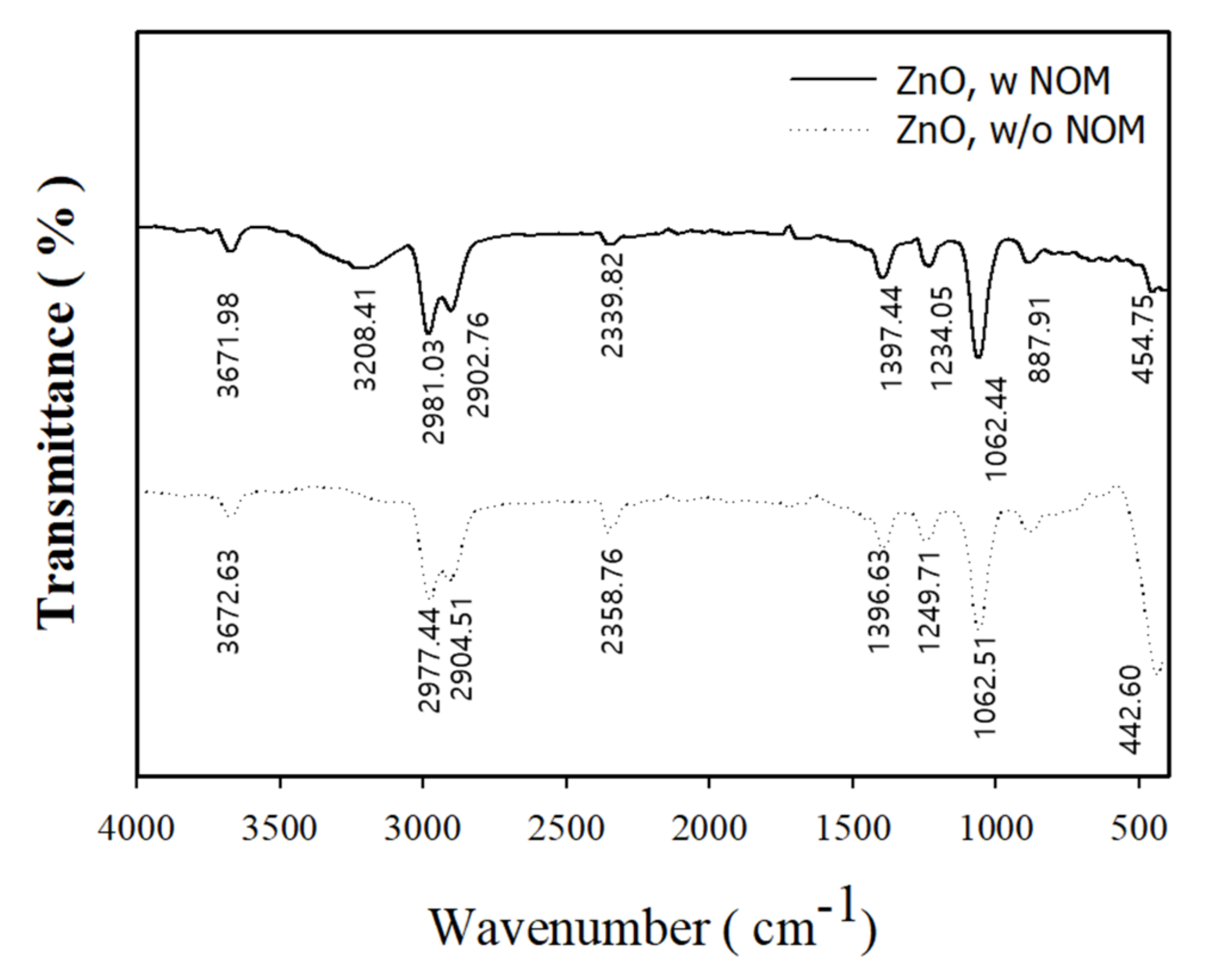
3.2.3. Measurement of Surface Reactor
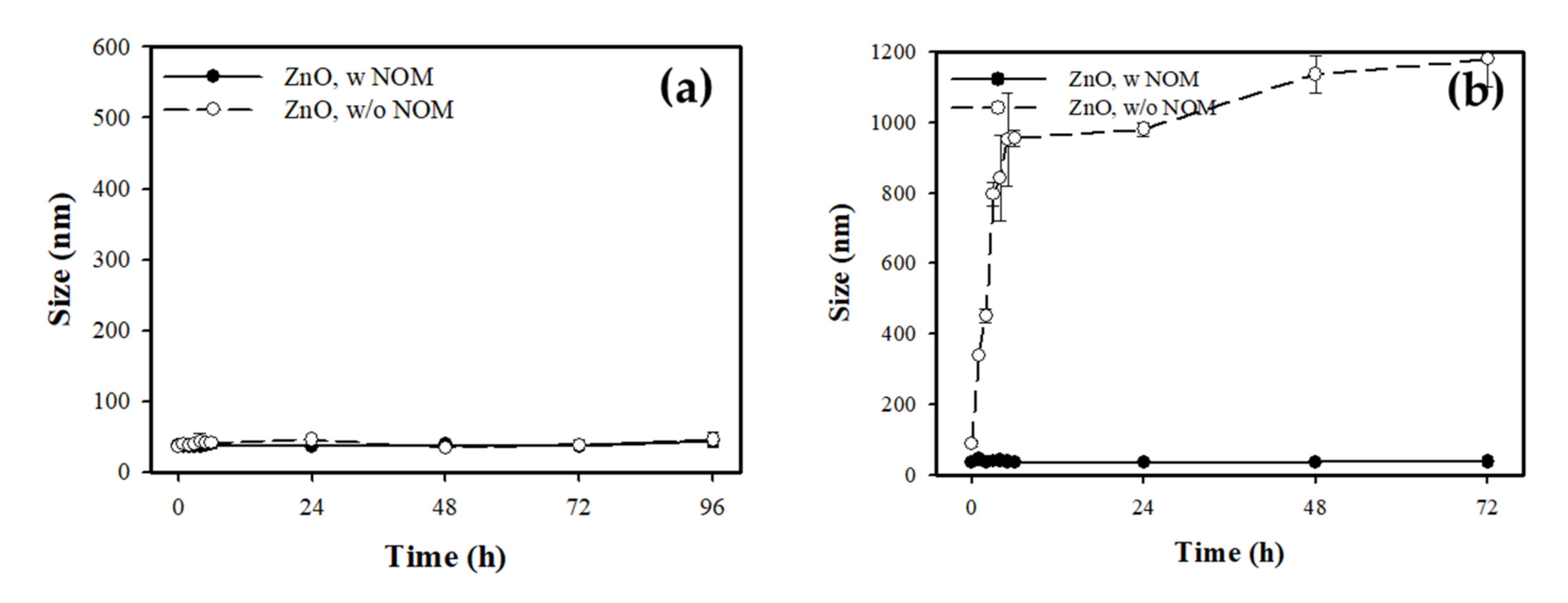
3.2.4. Determination of Particle Size and Distribution in Test Medium
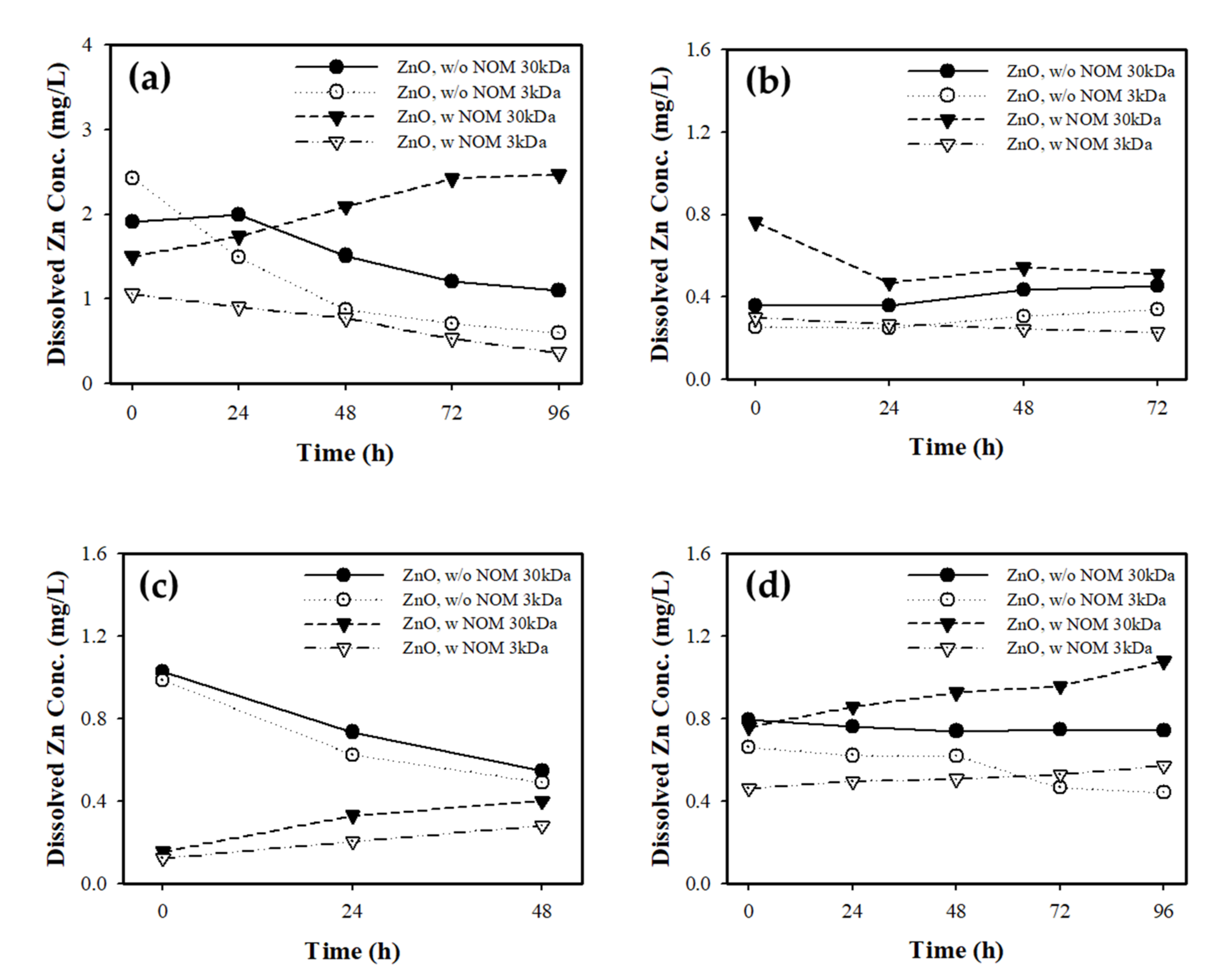
3.2.5. Analysis of Ionic Effect
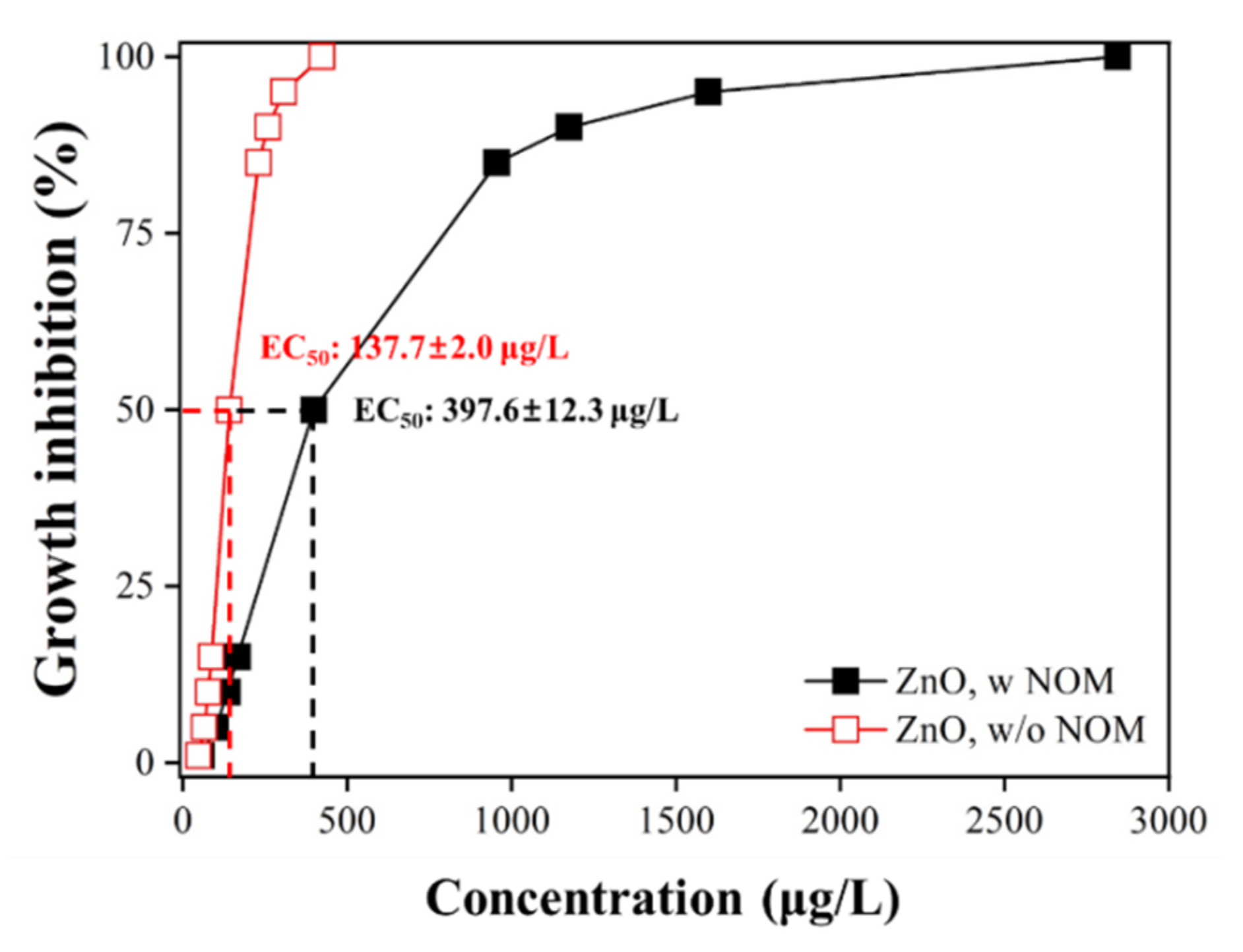
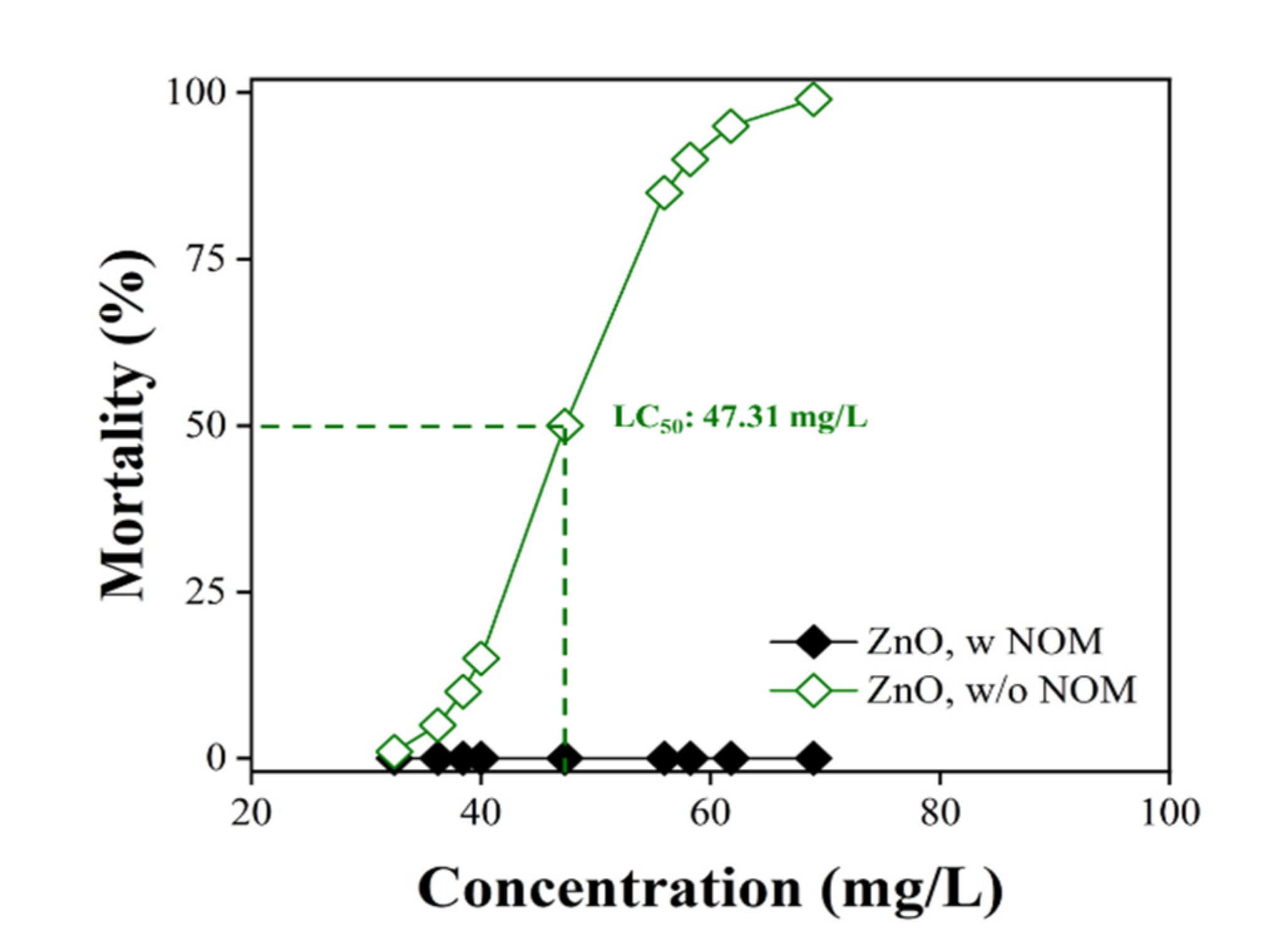
3.3. Ecotoxicological Effect of NOM on ZnO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hao, R.; Xing, R.; Xu, Z.; Hou, Y.; Gao, S.; Sun, S. Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles. Adv. Mater. 2010, 22, 2729–2742. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.L.; Kwon, N.; Yan, K.C.; Sedgwick, A.C.; Chen, G.R.; He, X.P.; James, T.D.; Yoon, J. Bio-Conjugated Advanced Materials for Targeted Disease Theranostics. Adv. Funct. Mater. 2020, 30, 1907906. [Google Scholar] [CrossRef]
- Bayram, S.S.; Blum, A.S. 12 Directing the Self-Assembly of Nanoparticles for Advanced Materials. In Advanced Materials; Ven, T., Soldera., A., Eds.; Walter de Gruyter: Berlin, Germany, 2020; Chapter 12; pp. 207–326. ISBN 978-3-11-053765-9. [Google Scholar]
- Jahan, S.; Yusoff, I.B.; Alias, Y.B.; Bakar, A.F.B.A. Reviews of the toxicity behavior of five potential engineered nanomaterials (ENMs) into the aquatic ecosystem. Toxicol. Rep. 2017, 4, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Kahru, A.; Dubourguier, H.C.; Blinova, I.; Ivask, A.; Kasemets, K. Biotests and biosensors for ecotoxicology of metal oxide nanoparticles: A minireview. Sensors 2008, 8, 5153–5170. [Google Scholar] [CrossRef]
- French, R.A.; Jacobson, A.R.; Kim, B.; Isley, S.L.; Penn, R.L.; Baveye, P.C. Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles. Environ. Sci. Technol. 2009, 43, 1354–1359. [Google Scholar] [CrossRef]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle aggregation: Challenges to understanding transport and reactivity in the environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.S.; Peng, Y.H.; Shiung, C.E.; Shih, Y.H. The effect of cations on the aggregation of commercial ZnO nanoparticle suspension. J. Nanopart. Res. 2012, 14, 1259. [Google Scholar] [CrossRef]
- Wong, S.W.; Leung, P.T.; Djurišić, A.B.; Leung, K.M. Toxicities of nano zinc oxide to five marine organisms: Influences of aggregate size and ion solubility. Anal. Bioanal. Chem. 2010, 396, 609–618. [Google Scholar] [CrossRef]
- Khan, R.; Inam, M.A.; Zam, S.Z.; Park, D.R.; Yeom, I.T. Assessment of key environmental factors influencing the sedimentation and aggregation behavior of zinc oxide nanoparticles in aquatic environment. Water 2018, 10, 660. [Google Scholar] [CrossRef] [Green Version]
- OECD. Test No. 318: Dispersion Stability of Nanomaterials in Simulated Environmental Media; OECD Guidelines for the Testing of Chemicals, Section 3; Organization for Economic Co-Operation and Development: Paris, France, 2017. [Google Scholar]
- Kaur, I.; Ellis, L.-J.; Romer, I.; Tantra, R.; Carriere, M.; Allard, S.; Mayne-L’Hermite, M.; Minelli, C.; Unger, W.; Potthoff, A. Dispersion of nanomaterials in aqueous media: Towards protocol optimization. J. Vis. Exp. 2017, e56074. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, A.J.; Chappell, M.A.; Bednar, A.J.; Ryan, A.C.; Laird, J.G.; Stanley, J.K.; Steevens, J.A. Impact of organic carbon on the stability and toxicity of fresh and stored silver nanoparticles. Environ. Sci. Technol. 2012, 46, 10772–10780. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Powers, K.; Wang, Y.; Zhou, H.; Roberts, S.M.; Moudgil, B.M.; Koopman, B.; Barber, D.S. Influence of Suwannee River humic acid on particle properties and toxicity of silver nanoparticles. Chemosphere 2012, 89, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, K.-T.; Lee, B.G.; Lim, B.J.; Kim, S.D. Developmental toxicity of Japanese medaka embryos by silver nanoparticles and released ions in the presence of humic acid. Ecotoxicol. Environ. Saf. 2013, 92, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Aruoja, V.; Dubourguier, H.-C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef]
- Santo, N.; Fascio, U.; Torres, F.; Guazzoni, N.; Tremolada, P.; Bettinetti, R.; Mantecca, P.; Bacchetta, R. Toxic effects and ultrastructural damages to Daphnia magna of two differently sized ZnO nanoparticles: Does size matter? Water Res. 2014, 53, 339–350. [Google Scholar] [CrossRef]
- Lopes, S.; Ribeiro, F.; Wojnarowicz, J.; Łojkowski, W.; Jurkschat, K.; Crossley, A.; Soares, A.M.; Loureiro, S. Zinc oxide nanoparticles toxicity to Daphnia magna: Size-dependent effects and dissolution. Environ. Toxicol. Chem. 2014, 33, 190–198. [Google Scholar] [CrossRef]
- Cupi, D.; Hartmann, N.B.; Baun, A. The influence of natural organic matter and aging on suspension stability in guideline toxicity testing of silver, zinc oxide, and titanium dioxide nanoparticles with Daphnia magna. Environ. Toxicol. Chem. 2015, 34, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.-C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef]
- Wiench, K.; Wohlleben, W.; Hisgen, V.; Radke, K.; Salinas, E.; Zok, S.; Landsiedel, R. Acute and chronic effects of nano-and non-nano-scale TiO2 and ZnO particles on mobility and reproduction of the freshwater invertebrate Daphnia magna. Chemosphere 2009, 76, 1356–1365. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, L.; Chen, Y.; Tian, S. Acute toxicities of six manufactured nanomaterial suspensions to Daphnia magna. J. Nanopart. Res. 2009, 11, 67–75. [Google Scholar] [CrossRef]
- Blinova, I.; Ivask, A.; Heinlaan, M.; Mortimer, M.; Kahru, A. Ecotoxicity of nanoparticles of CuO and ZnO in natural water. Environ. Pollut. 2010, 158, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Vijver, M.G.; Chen, G.; Peijnenburg, W.J. Toxicity and accumulation of Cu and ZnO nanoparticles in Daphnia magna. Environ. Sci. Technol. 2015, 49, 4657–4664. [Google Scholar] [CrossRef] [PubMed]
- Cupi, D.; Hartmann, N.B.; Baun, A. Influence of pH and media composition on suspension stability of silver, zinc oxide, and titanium dioxide nanoparticles and immobilization of Daphnia magna under guideline testing conditions. Ecotoxicol. Environ. Saf. 2016, 127, 144–152. [Google Scholar] [CrossRef]
- Yu, L.-P.; Fang, T.; Xiong, D.-W.; Zhu, W.-T.; Sima, X.-F. Comparative toxicity of nano-ZnO and bulk ZnO suspensions to zebrafish and the effects of sedimentation, OH production and particle dissolution in distilled water. J. Environ. Monit. 2011, 13, 1975–1982. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, L.; Duan, Z.; Qi, R.; Li, Y.; Lang, Y. Comparative toxicity of several metal oxide nanoparticle aqueous suspensions to Zebrafish (Danio rerio) early developmental stage. J. Environ. Sci. Health Part A 2008, 43, 278–284. [Google Scholar] [CrossRef]
- Han, Y.; Kim, D.; Hwang, G.; Lee, B.; Eom, I.; Kim, P.J.; Tong, M.; Kim, H. Aggregation and dissolution of ZnO nanoparticles synthesized by different methods: Influence of ionic strength and humic acid. Colloids Surf. A Physicochem. Eng. Asp. 2014, 451, 7–15. [Google Scholar] [CrossRef]
- Jiang, C.; Aiken, G.R.; Hsu-Kim, H. Effects of natural organic matter properties on the dissolution kinetics of zinc oxide nanoparticles. Environ. Sci. Technol. 2015, 49, 11476–11484. [Google Scholar] [CrossRef]
- Yang, S.P.; Bar-Ilan, O.; Peterson, R.E.; Heideman, W.; Hamers, R.J.; Pedersen, J.A. Influence of humic acid on titanium dioxide nanoparticle toxicity to developing zebrafish. Environ. Sci. Technol. 2013, 47, 4718–4725. [Google Scholar] [CrossRef]
- Manzo, S.; Miglietta, M.L.; Rametta, G.; Buono, S.; Di Francia, G. Toxic effects of ZnO nanoparticles towards marine algae Dunaliella tertiolecta. Sci. Total Environ. 2013, 445, 371–376. [Google Scholar] [CrossRef]
- OECD. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test; Organization for Economic Co-Operation and Development: Paris, France, 2006. [Google Scholar]
- OECD. Test No. 202: Daphnia sp. Acute Immobilisation Test; OECD Guidelines for the Testing of Chemicals; Organization for Economic Co-Operation and Development: Paris, France, 2004. [Google Scholar]
- OECD. Test No. 203: Fish, Acute Toxicity Test; OECD Guidelines for the Testing of Chemicals, Section 2; Organization for Economic Co-Operation and Development: Paris, France, 1992. [Google Scholar]
- Hwang, G.; Gomez-Flores, A.; Bradford, S.A.; Choi, S.; Jo, E.; Kim, S.B.; Tong, M.; Kim, H. Analysis of stability behavior of carbon black nanoparticles in ecotoxicological media: Hydrophobic and steric effects. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 306–316. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, C.; Hou, J.; Wang, P.; Miao, L.; Lv, B.; Yang, Y.; You, G.; Xu, Y.; Zhang, M. Aggregation, sedimentation, and dissolution of CuO and ZnO nanoparticles in five waters. Environ. Sci. Pollut. Res. 2018, 25, 31240–31249. [Google Scholar] [CrossRef] [PubMed]
- National Insititutes of Health (NIH). Available online: http://www.nih.gov (accessed on 8 July 2020).
- U.S. Department of Agriculture. Available online: http://www.ars.usda.gov (accessed on 8 July 2020).
- Zhong, L.; Hu, X.; Cao, Z.; Wang, H.; Chen, Y.; Lian, H.-Z. Aggregation and dissolution of engineering nano Ag and ZnO pretreated with natural organic matters in the simulated lung biological fluids. Chemosphere 2019, 225, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Shen, M.; Li, S.; Fu, Y.; Zhang, D.; Liu, H.; Liu, J. Aggregation kinetics of different surface-modified polystyrene nanoparticles in monovalent and divalent electrolytes. Environ. Pollut. 2019, 255, 113302. [Google Scholar] [CrossRef] [PubMed]
- Majedi, S.M.; Kelly, B.C.; Lee, H.K. Role of combinatorial environmental factors in the behavior and fate of ZnO nanoparticles in aqueous systems: A multiparametric analysis. J. Hazard. Mater. 2014, 264, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Maenosono, S. Amine-terminated water-dispersible FePt nanoparticles. J. Magn. Magn. Mater. 2008, 320, L121–L124. [Google Scholar] [CrossRef]
- Aboorvakani, R.; Vethanathan, S.J.K.; Madhu, K. Influence of Zn concentration on zinc oxide nanoparticles and their anti-corrosion property. J. Alloys Compd. 2020, 155078. [Google Scholar] [CrossRef]
- Chemistry LibreTexts: Home. Available online: http://chem.libretexts.org (accessed on 8 July 2020).
- Fatehah, M.O.; Aziz, H.A.; Stoll, S. Stability of ZnO nanoparticles in solution. Influence of pH, dissolution, aggregation and disaggregation effects. J. Colloid Sci. Biotechnol. 2014, 3, 75–84. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Chen, K.; Chen, H.; Wang, F.; Han, X.; Zhou, B.; Chen, H.; Yuan, R. NOM mitigates the phytotoxicity of AgNPs by regulating rice physiology, root cell wall components and root morphology. Environ. Pollut. 2020, 260, 113942. [Google Scholar] [CrossRef]
- Misra, S.K.; Dybowska, A.; Berhanu, D.; Luoma, S.N.; Valsami-Jones, E. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci. Total Environ. 2012, 438, 225–232. [Google Scholar] [CrossRef]
- Yang, L.; Wang, W.-X. Comparative contributions of copper nanoparticles and ions to copper bioaccumulation and toxicity in barnacle larvae. Environ. Pollut. 2019, 249, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pokhrel, S.; Jin, X.; Mädler, L.; Damoiseaux, R.; Hoek, E.M. Stability, bioavailability, and bacterial toxicity of ZnO and iron-doped ZnO nanoparticles in aquatic media. Environ. Sci. Technol. 2011, 45, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Carlos, L.; Cipollone, M.; Soria, D.B.; Moreno, M.S.; Ogilby, P.R.; Einschlag, F.S.G.; Mártire, D.O. The effect of humic acid binding to magnetite nanoparticles on the photogeneration of reactive oxygen species. Sep. Purif. Technol. 2012, 91, 23–29. [Google Scholar] [CrossRef]
- Lin, D.; Ji, J.; Long, Z.; Yang, K.; Wu, F. The influence of dissolved and surface-bound humic acid on the toxicity of TiO2 nanoparticles to Chlorella sp. Water Res. 2012, 46, 4477–4487. [Google Scholar] [CrossRef]



| Species | Exposure Period | Toxicity Values | Particle Size and Type | Exposure Media | pH | Reference |
|---|---|---|---|---|---|---|
| P. subcapitata | 72 h | EC50 42 µg/L | Ion (ZnSO4) | OECD test medium | 8.0 ± 0.4 | Aruoja et al., 2009 [16] |
| EC50 42 µg/L | 50–70 nm | |||||
| EC50 37 µg/L | Bulk | |||||
| EC50 49 µg/L | 30 nm (Dispersant) | EPA medium | 7.5 ± 0.1 | Franklin et al., 2007 [17] | ||
| EC50 68 µg/L | 30 nm (Powder) | |||||
| EC50 63 µg/L | Bulk | |||||
| Skeletonema costatum | 96 h | EC50 2.36 mg/L | 20 nm | Artificial seawater | 8.0 ± 0.1 | Wong et al., 2010 [9] |
| Dunaliella tertiolecta | 96 h | EC50 2.42 mg/L | 100 nm | Artificial seawater | - | Manzo et al., 2013 [32] |
| Daphnia magna | 48 h | LC50 3.2 mg/L | 50–70 nm | Synthetic freshwater | 7.3–7.8 | Heinlaan et al., 2008 [21] |
| LC50 7.5 mg/L | <200 nm | Elendt M4 medium | 7.7–8.4 | Wiench et al., 2009 [22] | ||
| EC50 0.62 mg/L | 20 nm | ISO test medium | - | Zhu et al., 2009 [23] | ||
| LC50 1.51 mg/L | ||||||
| EC50 2.6 mg/L | 50–70 nm | Natural water | 7.5–8.2 | Blinova et al., 2010 [24] | ||
| EC50 1.9 mg/L | <50 nm | Commercial mineral water (San Benedetto®) | 8.81 | Santo et al., 2014 [18] | ||
| EC50 3.1 mg/L | <100 nm | |||||
| LC50 0.99 mg/L | 43 nm | ISO test medium | 7.8 ± 0.2 | Xiao et al., 2015 [25] | ||
| LC50 1.15 mg/L | 43 nm | |||||
| LC50 1.01 mg/L | Ion (Zn(NO3)2) | |||||
| LC50 0.76 mg/L | Ion (ZnCl2) | ASTM hard water | 7.9 ± 0.3 | Lopes et al., 2013 [19] | ||
| LC50 1.02 mg/L | 30 nm | |||||
| LC50 1.10 mg/L | 80–100 nm | |||||
| LC50 0.89 mg/L | >200 nm | |||||
| EC50 4.7 mg/L | 151 nm | M7 medium | 8.2 ± 8.5 | Cupi et al., 2015 [20] | ||
| EC50 2.2 mg/L | 151 nm | M7 medium + NOM | ||||
| EC50 0.047 mg/L | 151 nm | VS EPA medium | 7 | Cupi et al., 2016 [26] | ||
| EC50 4.9 mg/L | 151 nm | M7 medium | 8.6 | |||
| Danio rerio | 96 h | LC50 3.97 mg/L | 30 nm | Distilled water | 6.9–7.3 | Yu et al., 2011 [27] |
| LC50 2.52 mg/L | <500 nm | |||||
| LC50 1.79 mg/L | 20 nm | Distilled water | - | Zhu et al., 2008 [28] | ||
| LC50 1.55 mg/L | 1000 nm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.-c.; Hong, G.; Lee, H.; Kim, P.; Seo, D.-Y.; Hwang, G.; Kim, G.; Kim, P. Influence of NOM on the Stability of Zinc Oxide Nanoparticles in Ecotoxicity Tests. Appl. Sci. 2020, 10, 6431. https://doi.org/10.3390/app10186431
Lee B-c, Hong G, Lee H, Kim P, Seo D-Y, Hwang G, Kim G, Kim P. Influence of NOM on the Stability of Zinc Oxide Nanoparticles in Ecotoxicity Tests. Applied Sciences. 2020; 10(18):6431. https://doi.org/10.3390/app10186431
Chicago/Turabian StyleLee, Byoung-cheun, Gilsang Hong, Hyejin Lee, Pyeongsoon Kim, Do-Yeon Seo, Gukhwa Hwang, Geunbae Kim, and Pilje Kim. 2020. "Influence of NOM on the Stability of Zinc Oxide Nanoparticles in Ecotoxicity Tests" Applied Sciences 10, no. 18: 6431. https://doi.org/10.3390/app10186431
APA StyleLee, B.-c., Hong, G., Lee, H., Kim, P., Seo, D.-Y., Hwang, G., Kim, G., & Kim, P. (2020). Influence of NOM on the Stability of Zinc Oxide Nanoparticles in Ecotoxicity Tests. Applied Sciences, 10(18), 6431. https://doi.org/10.3390/app10186431





