Features of Electrophoretic Deposition of a Ba-Containing Thin-Film Proton-Conducting Electrolyte on a Porous Cathode Substrate
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of the Electrolyte and Electrode Materials
2.2. Porous Cathode Substrates’ Preparation and Characterization
2.3. Preparation of Suspensions Based on the Electrode and Electrolyte Powders and Their Characterization
2.4. Electrophoretic Deposition of the Films and Their Characterization
2.5. Conductivity Measurements
3. Results and Discussion
3.1. Formation of the LBNOnc Functional Layer on the Porous LNFOss and LNFOP Cathode Substrates by the EPD Method
3.2. Determination of the Optimal Deposition Mode of the BCGCuO Electrolyte Film (Test Deposition)
3.3. Formation of the BCGCuO Electrolyte Films on the LNFO Substrates with the Deposited LBNOnc Sublayer
3.4. Formation of the BCGCuO Electrolyte Film on a LNFOP Substrate
3.5. Formation of the BCGCuO Electrolyte Films on LBNOss Substrates
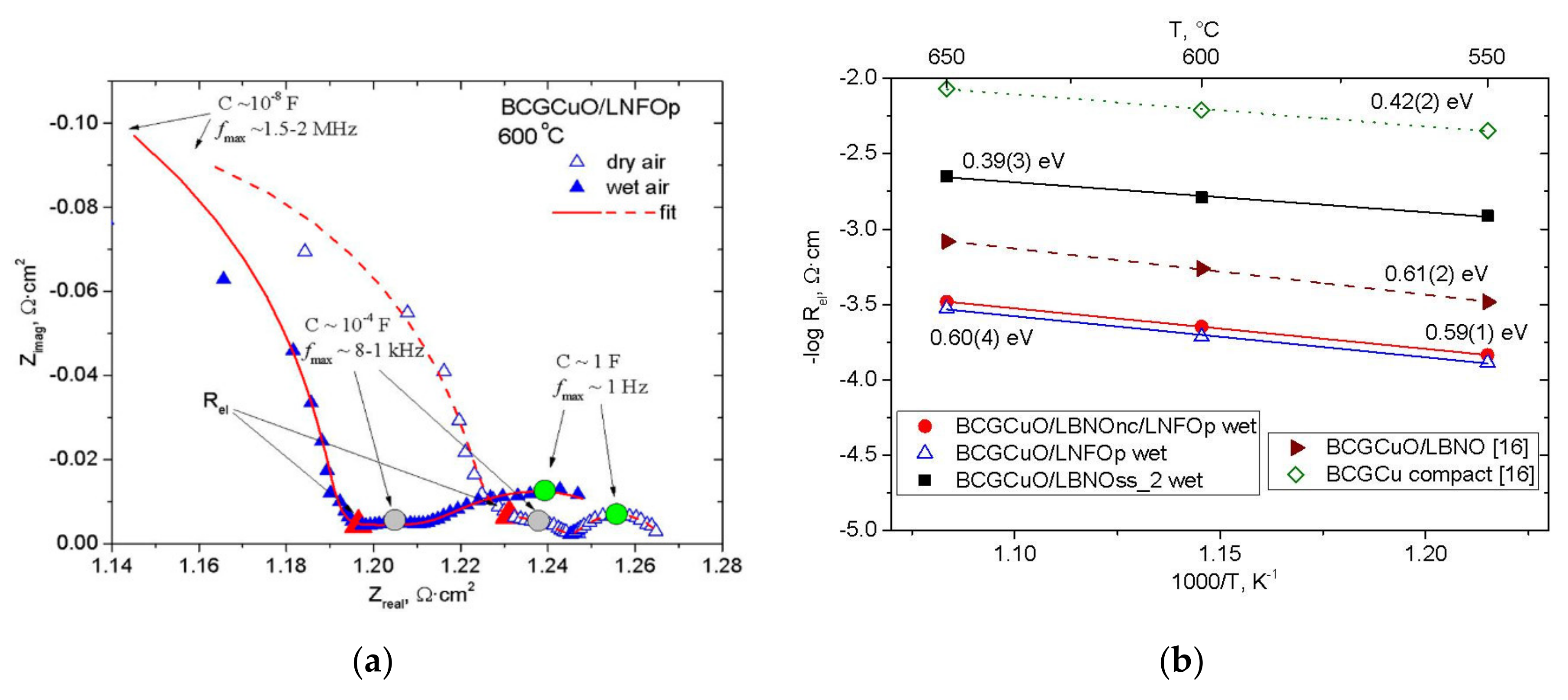
3.6. Electrical Properties of the Electrolyte BCGCuO Film Formed on Various Cathode Substrates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hossain, S.; Abdalla, A.M.; Jamain, S.N.B.; Zaini, J.H.; Azad, A.K. A review on proton conducting electrolytes for clean energy and intermediate temperature-solid oxide fuel cells. Renew. Sustain. Energy Rev. 2017, 79, 750–764. [Google Scholar] [CrossRef]
- Irshad, M.; Siraj, K.; Raza, R.; Ali, A.; Tiwari, P.; Zhu, B.; Rafique, A.; Ali, A.; Ullah, M.K.; Usman, A. A Brief Description of High Temperature Solid Oxide Fuel Cell’s Operation, Materials, Design, Fabrication Technologies and Performance. Appl. Sci. 2016, 6, 75. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wei, H.-H.; Xu, Q. A Solid Oxide Fuel Cell (SOFC)-Based Biogas-from-Waste Generation System for Residential Buildings in China: A Feasibility Study. Sustainability 2018, 10, 2395. [Google Scholar] [CrossRef] [Green Version]
- Tarancón, A. Strategies for Lowering Solid Oxide Fuel Cells Operating. Energies 2009, 2, 1130–1150. [Google Scholar] [CrossRef]
- Matsumoto, H.; Kawasaki, Y.; Ito, N.; Enoki, M.; Ishihara, T. Relation between electrical conductivity and chemical stability of BaCeO3–based proton conductors with different trivalent dopants. Electrochem. Solid State Lett. 2007, 10, B77–B80. [Google Scholar] [CrossRef]
- Danilov, N.; Pikalova, E.; Lyagaeva, J.; Antonov, B.; Medvedev, D.; Demin, A.; Tsiakaras, P. Grain and grain boundary transport in BaCe0.5Zr0.3Ln0.2O3−δ (Ln–Y or lanthanide) electrolytes attractive for protonic ceramic fuel cells application. J. Power Sources 2017, 366, 161–168. [Google Scholar] [CrossRef]
- Pikalova, E.; Medvedev, D. Effect of anode gas mixture humidification on the electrochemical performance of the BaCeO3-based protonic ceramic fuel cell. Int. J. Hydrogen Energy 2016, 41, 4016–4025. [Google Scholar] [CrossRef]
- Amsif, M.; Marrero-Lopez, D.; Ruiz-Morales, J.C.; Savvin, S.N.; Gabás, M.; Nunez, P. Influence of rare-earth doping on the microstructure and conductivity of BaCe0.9Ln0.1O3−δ proton conductors. J. Power Sources 2011, 196, 3461–3469. [Google Scholar] [CrossRef]
- Medvedev, D.A.; Gorbova, E.V.; Demin, A.K.; Tsiakaras, P. Conductivity of Gd-doped BaCeO3 protonic conductor in H2–H2O–O2 atmospheres. Int. J. Hydrogen Energy 2014, 39, 21547–21552. [Google Scholar] [CrossRef]
- Lyagaeva, J.G.; Vdovin, G.K.; Medvedev, D.A. Distinguishing Bulk and Grain Boundary Transport of a Proton-Conducting Electrolyte by Combining Equivalent Circuit Scheme and Distribution of Relaxation Times Analyses. J. Phys. Chem. C 2019, 123, 21993–21997. [Google Scholar] [CrossRef]
- Dunyushkina, L.A. Solid Oxide Fuel Cells with film electrolyte: Problems and perspectives. Electrochem. Energetics 2016, 16, 196–206. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G. Place of Electrophoretic Deposition Among Thin-Film Methods Adapted to the Solid Oxide Fuel Cell Technology: A Short Review. Int. J. Energy Prod. Manag. 2019, 4, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, V.; Liu, M. Colloidal Processing of BaCeO3-Based Electrolyte Films. J. Electrochem. Soc. 1996, 143, 3239–3244. [Google Scholar] [CrossRef]
- Dubal, S.U.; Bhosale, C.H.; Jadhav, L.D. Performance of spray deposited Gd-doped barium cerate thin films for proton conducting SOFCs. Ceram. Int. 2015, 41, 5607–5613. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Pikalova, E.Y.; Farlenkov, A.S. Electrophoretic Deposition of Thin-Film Coatings of Solid Electrolyte Based on Microsize BaCeO3 Powders. Russ. J. Appl. Chem. 2018, 91, 934–941. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Pikalova, E.Y.; Kolchugin, A.A.; Pikalova, N.S.; Farlenkov, A.S. Comparative Study of Electrophoretic Deposition of Doped BaCeO3-Based Films on La2NiO4+δ and La1.7Ba0.3NiO4+δ Cathode Substrates. Materials 2019, 12, 2545. [Google Scholar] [CrossRef] [Green Version]
- Kalinina, E.G.; Pikalova, E.Y. New trends in the development of electrophoretic deposition method in the solid oxide fuel cell technology: Theoretical approaches, experimental solutions and development prospects. Russ. Chem. Rev. 2019, 88, 1179. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Progr. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G. Electrophoretic deposition in the solid oxide fuel cell technology: Fundamentals and recent advances. Renew. Sustain. Energy Rev. 2019, 116, 109440. [Google Scholar] [CrossRef]
- Medvedev, D.; Brouzgou, A.; Demin, A.; Tsiakaras, P. Proton-Conducting Electrolytes for Solid Oxide Fuel Cell Applications. In Advances in Medium and High Temperature Solid Oxide Fuel Cell Technology; Boaro, M., Salvatore, A.A., Eds.; Springer Nature: Cham, Switzerland, 2016; Volume 574, pp. 77–119. [Google Scholar] [CrossRef]
- Mercadelli, E.; Montaleone, D.; Gondolini, A.; Pinasco, P.; Sanson, A. Tape-cast asymmetric membranes for hydrogen separation. Ceram. Int. 2017, 43, 8010–8017. [Google Scholar] [CrossRef]
- Park, I.; Kim, J.; Lee, H.; Park, J.; Shin, D. BaCeO3–BaCe0.8Sm0.2O3−δ bi-layer electrolyte-based protonic ceramic fuel cell. Solid State Ion. 2013, 252, 152–156. [Google Scholar] [CrossRef]
- Lyagaeva, J.; Danilov, N.; Pikalova, E.Y.; Plaksin, S.; Brouzgou, A.; Demin, A.; Tsiakaras, P. A detailed analysis of thermal and chemical compatibility of cathode materials suitable for BaCe0.8Y0.2O3-δ and BaZr0.8Y0.2O3−δ proton electrolytes for solid oxide fuel cell application. Int. J. Hydrogen Energy 2017, 42, 1715–1723. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Bogdanovich, N.M.; Kolchugin, A.A.; Osinkin, D.A.; Bronin, D.I. Electrical and Electrochemical Properties of La2NiO4+δ-Based Cathodes in Contact with Ce0.8Sm0.2O2 − δ Electrolyte. Procedia Eng. 2014, 98, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Pikalova, E.Y.; Kolchugin, A.A. The Influence of the Substituting Element (M = Ca, Sr, Ba) in La1.7M0.3NiO4+δ on the Electrochemical Performance of the Composite Electrodes. Eurasian Chem. Technol. J. 2016, 18, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Kalinina, E.G.; Pikalova, E.Y.; Kolchugin, A.A.; Pikalov, S.M.; Kaigorodov, A.S. Cyclic electrophoretic deposition of electrolyte thin-films on the porous cathode substrate utilizing stable suspensions of nanopowder. Solid State Ion. 2017, 302, 126–132. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Bogdanovich, N.M.; Bronin, D.I.; Pikalova, E.Y.; Pankratov, A.A. Formation of Thin-Film Electrolyte by Electrophoretic Deposition onto Modified Multilayer Cathode. Russ. J. Appl. Chem. 2019, 92, 191–198. [Google Scholar] [CrossRef]
- Dusoulier, L.; Cloots, R.; Vertruyen, B.; Moreno, R.; Burgos-Montes, O.; Ferrari, B. YBa2Cu3O7−x dispersion in iodine acetone for electrophoretic deposition: Surface charging mechanism in a halogenated organic media. J. Eur. Ceram. Soc. 2011, 31, 1075–1086. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Liu, M. Preparation of yttria-stabilized zirconia (YSZ) films on La0.85Sr0.15MnO3 (LSM) and LSM–YSZ substrates using an electrophoretic deposition (EPD) process. J. Eur. Ceram. Soc. 2001, 21, 127–134. [Google Scholar] [CrossRef]
- Bartolomeo, E.D.; Zunic, M.; Chevallier, L.; D’Epifanio, A.; Licoccia, S.; Traversa, E. Fabrication of proton conducting solid oxide fuel cells by using electrophoretic deposition. ECS Trans. 2009, 25, 577–684. [Google Scholar] [CrossRef]
- Besra, L.; Uchikoshi, T.; Suzuki, T.; Sakka, Y. Experimental verification of pH localization mechanism of particle consolidation at the electrode/solution interface and its application to pulsed DC electrophoretic deposition (EPD). J. Eur. Ceram. Soc. 2010, 30, 1187–1193. [Google Scholar] [CrossRef]
- Mishra, M.; Sakka, Y.; Uchikoshi, T.; Besra, L. pH localization: A case study during electrophoretic deposition of ternary max phase carbide-Ti3SiC2. J. Ceram. Soc. Jpn. 2013, 121, 348–354. [Google Scholar] [CrossRef] [Green Version]
- Lacz, A. Effect of microstructure on chemical stability and electrical properties of BaCe0.9Y0.1O3 − δ. Ionics 2016, 22, 1405–1414. [Google Scholar] [CrossRef] [Green Version]
- Lyagaeva, J.; Antonov, B.; Dunyushkina, L.; Kuimov, V.; Medvedev, D.; Demin, A.; Tsiakaras, P. Acceptor doping effects on microstructure, thermal and electrical properties of proton-conducting BaCe0.5 Zr0.3Ln0.2O3−δ (Ln = Yb, Gd, Sm, Nd, La or Y) ceramics for solid oxide fuel cell applications. Electrochim. Acta 2016, 192, 80–88. [Google Scholar] [CrossRef]
- Strandbakke, R.; Cherepanov, V.A.; Zuev, A.Y.; Tsvetkov, D.S.; Argirusis, C.; Sourkouni, G.; Prünte, S.; Norby, T. Gd- and Pr-based double perovskite cobaltites as oxygen electrodes for proton ceramic fuel cells and electrolyser cells. Solid State Ion. 2015, 278, 120–132. [Google Scholar] [CrossRef]
- Tarutin, A.P.; Gorshkov, M.Y.; Bainov, I.N.; Vdovin, G.K.; Vylkov, A.I.; Lyagaeva, J.G.; Medvedev, D.A. Barium-doped nickelates Nd2-xBaxNiO4+δ as promising electrode materials for protonic ceramic electrochemical cells. Ceram. Int. 2020, in press. [Google Scholar] [CrossRef]
- Beresnev, S.M.; Bobrenok, O.F.; Kuzin, B.L.; Bogdanovich, N.M.; Kurteeva, A.A.; Osinkin, D.A.; Vdovin, G.K.; Bronin, D.I. Single Fuel Cell with Supported LSM Cathode. Russ. J. Electrochem. 2012, 48, 969–975. [Google Scholar] [CrossRef]
- Osinkin, D.A. Complementary effect of ceria on the hydrogen oxidation kinetics on Ni-Ce0.8Sm0.2O2-δ anode. Electrochim. Acta 2020, 330, 135257. [Google Scholar] [CrossRef]
- Yang, L.; Wang, S.; Blinn, K.; Liu, M.; Liu, Z.; Cheng, Z.; Liu, M. Enhanced Sulfur and Coking Tolerance of a Mixed Ion Conductor for SOFCs: BaZr0.1Ce0.7Y0.2–xYbxO3−δ. Science 2009, 326, 126–129. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhao, F.; Zhang, L.; Chen, F. Synthesis of BaCe0.7Zr0.1Y0.1Yb0.1O3-δ proton conducting ceramic by a modified Pechini method. Solid State Ion. 2012, 213, 29–35. [Google Scholar] [CrossRef]
- Danilov, N.A.; Lyagaeva, J.G.; Medvedev, D.A.; Demin, A.K.; Tsiakaras, P. Transport properties of highly dense proton-conducting BaCe0.8–xZrxDy0.2O3−δ materials in low- and high-temperature ranges. Electrochim. Acta 2018, 284, 551–559. [Google Scholar] [CrossRef]
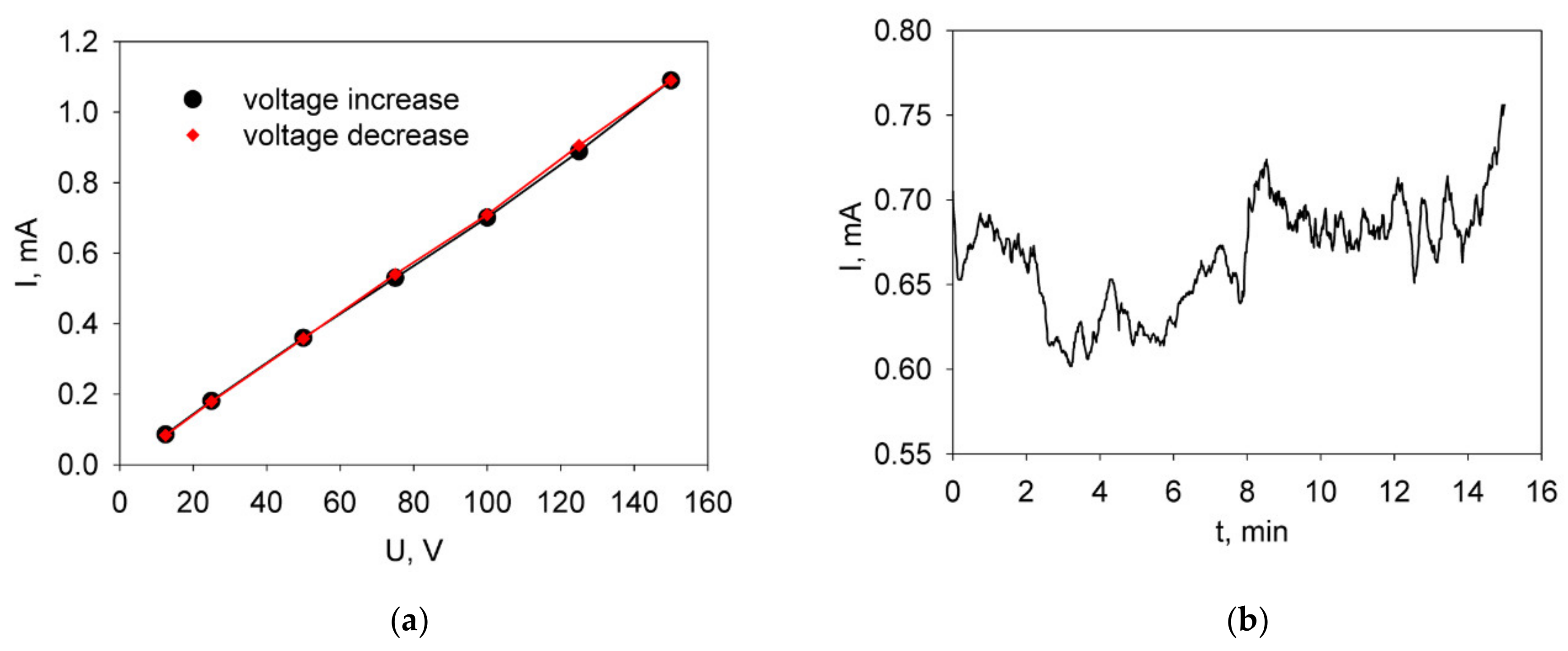

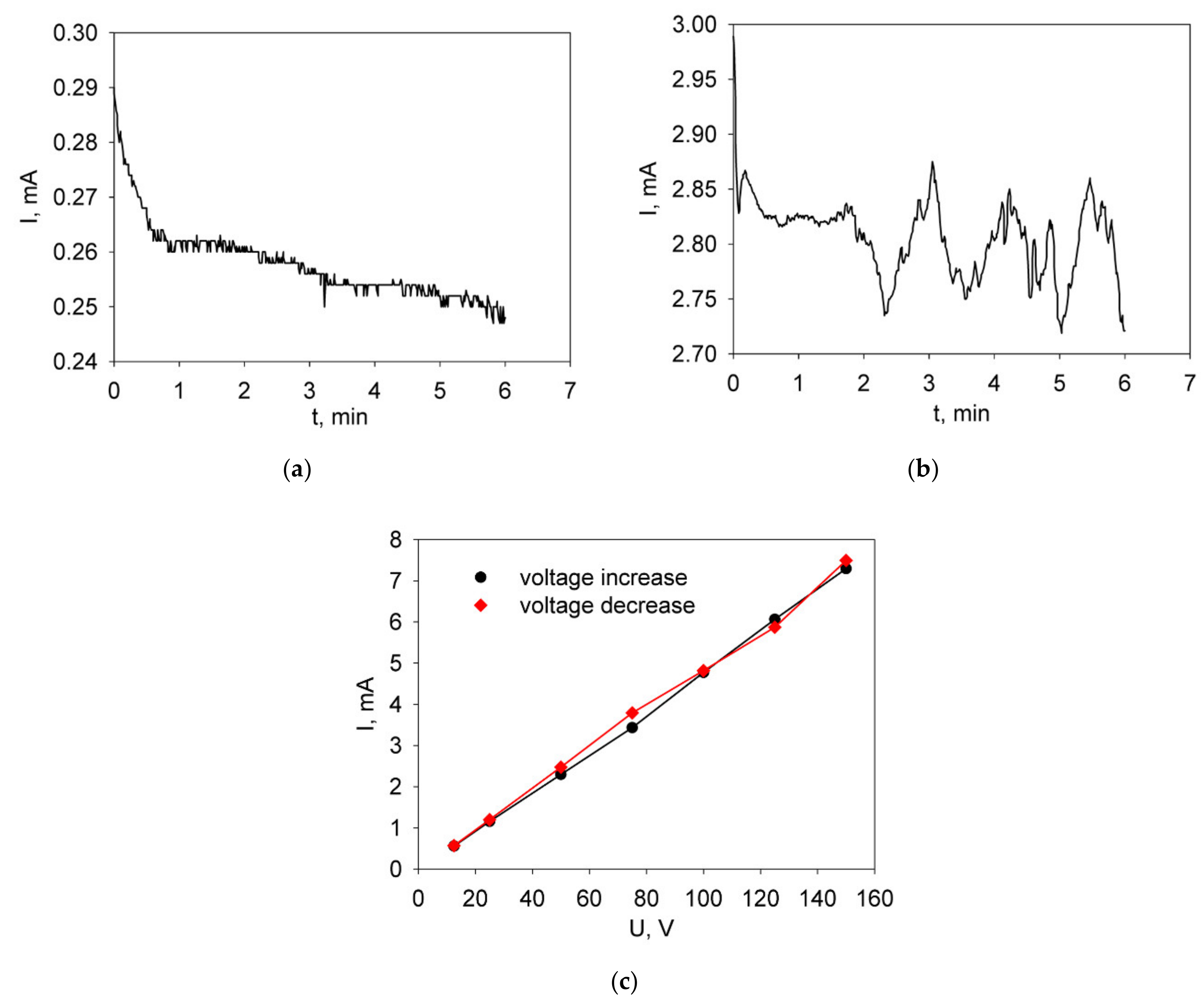




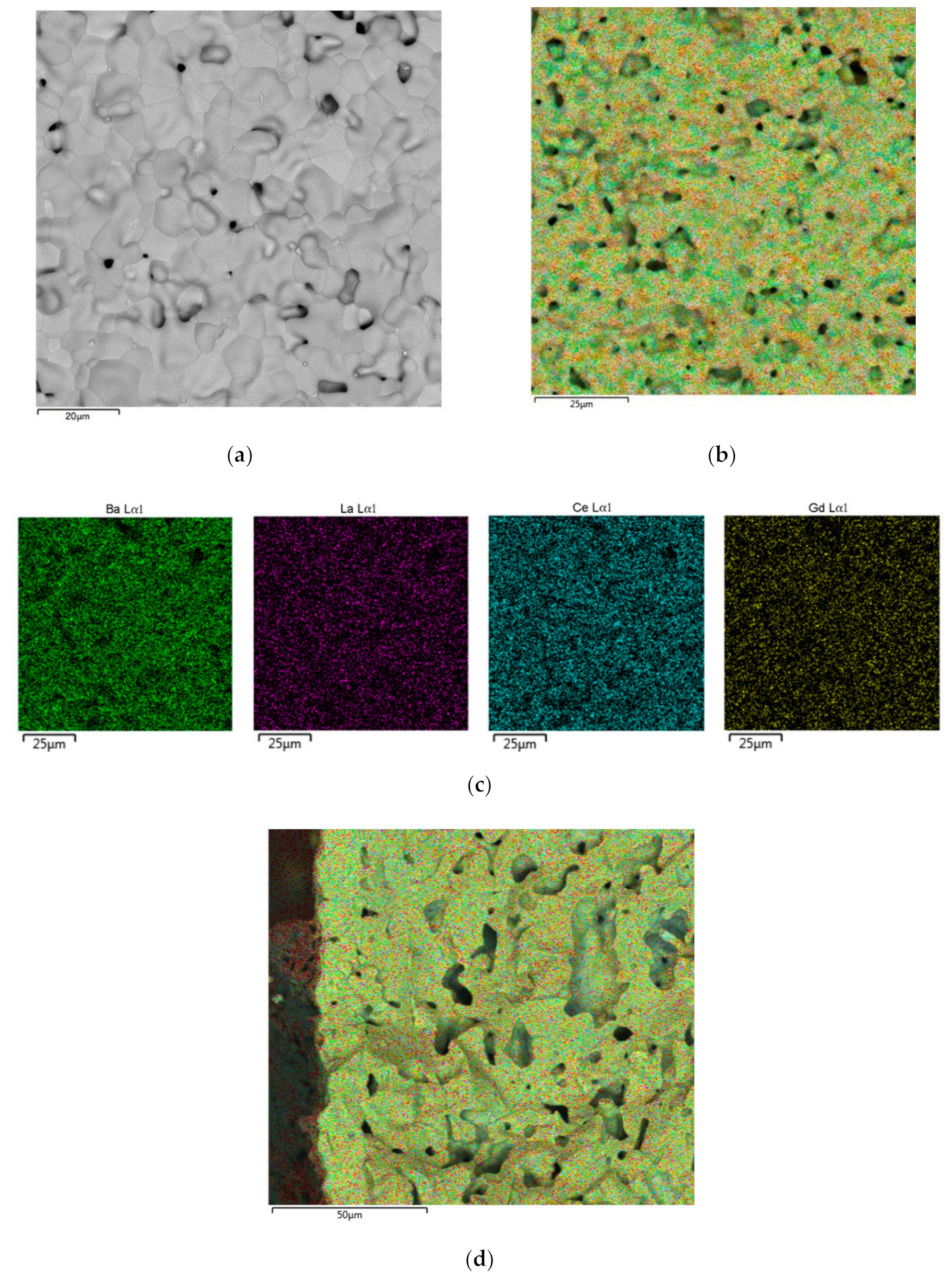
| Material | Synthesis Method | Designation | XRD Data | SBET, m2/g (After Final Ball-Milling) |
|---|---|---|---|---|
| Electrolyte BaCe0.89Gd0.1Cu0.01O3-δ | nitrate combustion method | BCGCuO | orthorhombic structure (sp. gr. Pmcn) a = 8.7929(21) Å b = 6.2333(13) Å c = 6.2197(9) Å | 3.01(7) |
| Electrode La1.7Ba0.3NiO4+δ | two-stage solid state reaction method | LBNOss | tetragonal structure (sp. gr. I4/mmm) a = 3.8466(5) Å, c = 12.7985(21) Å | 1.60(2) |
| nitrate combustion method | LBNOnc | tetragonal structure (sp. gr. I4/mmm) a = 3.8582(4) Å, c = 12.8378(37) Å | 2.21(6) | |
| Electrode LaNi0.6Fe0.4O3-δ | modified Pechini method | LNFOp | rhombohedral structure (sp. gr. R-3c) a = 5.5053(13) Å b = 5.5053(13) Å, c = 13.2727(31) Å | 6.50(13) (as-prepared) 1.06(2) (calcinated at 1180 °C) |
| two-stage solid state reaction method | LNFOss | rhombohedral structure (sp. gr. R-3c) a = 5.5062(12) Å b = 5.5062(12) Å c = 13.2501(31) Å | 1.50(3) |
| Half-Cell | Stage | Total Number of Cycles//Number of Cycles on the Stage, Sintering Conditions | BCGCuO Film, µm | Electrolyte Film Content (at.%) |
|---|---|---|---|---|
| BCGCuO/ LBNOnc/LNFOP | 1 | 2//2 LBNO (1350 °C, 1 h) BCGCuO (1450 °C, 2 h) | 7 | Ba is not detected |
| 2 | 3//1 BCGCuO (1450 °C, 2 h) | 12 | Ba is not detected | |
| 3 | 6//3 BCGCuO (1200 °C, 2 h) BCGCuO (1350 °C, 2 h) BCGCuO (1450 °C, 2 h) sintering with BaCO3 | 21 | O–66 Ni–0 Ba–17.1 La–1.2 Ce–15 Gd–0.7 | |
| BCGCuO/ LBNOnc/LNFOss | 1 | 3//3 LBNO (1350 °C, 1 h) BCGCuO (1350 °C, 1 h) BCGCuO (1450 °C, 2 h) | 7 | Ba is not detected |
| 2 | 4//1 BCGCuO (1450 °C, 2 h) | 10 | O–64 Ni, Cu, Ba–0 La–12 Ce–22 Gd–2 | |
| BCGCuO/ LNFOP | 1 | 3//3 BCGCuO (1200 °C, 2 h) BCGCuO (1350 °C, 2 h) BCGCuO (1450 °C, 2 h) sintering with BaCO3 | 10 | O–63.5 Ba–0 La–14 Ce–21 Gd–1.5 |
| 2 | 7//4 BCGCuO (1200 °C, 2 h) BCGCuO (1200 °C, 2 h) BCGCuO (1350 °C, 2 h) BCGCuO (1450 °C, 2 h) sintering with BaCO3 | 28 | O–61 Ba–19.3 La–1.3 Ce–17.4 Gd–1 | |
| BCGCuO/ LBNOss_1 | 1 | 3//3 BCGCuO (1200 °C, 2 h) BCGCuO (1350 °C, 2 h) BCGCuO (1450 °C, 2 h) sintering with BaCO3 | 10 | O–60.1 Ba–20.7 La–1.4 Ce–17 Gd–0.8 |
| 2 | 6//3 BCGCuO (1200 °C, 2 h) BCGCuO (1350 °C, 2 h) BCGCuO (1450 °C, 2 h) sintering with BaCO3 | 17 | O–60.4 Ba–20 La–1.5 Ce–17 Gd–1.1 | |
| BCGCuO/ LBNOss_2 | 1 | 3//3 BCGCuO (1200 °C, 2 h) BCGCuO (1350 °C, 2 h) BCGCuO (1450 °C, 2 h) sintering with BaCO3 | 10 | O–60 Ba–19.8 La–1.4 Ce–18 Gd–0.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinina, E.; Kolchugin, A.; Shubin, K.; Farlenkov, A.; Pikalova, E. Features of Electrophoretic Deposition of a Ba-Containing Thin-Film Proton-Conducting Electrolyte on a Porous Cathode Substrate. Appl. Sci. 2020, 10, 6535. https://doi.org/10.3390/app10186535
Kalinina E, Kolchugin A, Shubin K, Farlenkov A, Pikalova E. Features of Electrophoretic Deposition of a Ba-Containing Thin-Film Proton-Conducting Electrolyte on a Porous Cathode Substrate. Applied Sciences. 2020; 10(18):6535. https://doi.org/10.3390/app10186535
Chicago/Turabian StyleKalinina, Elena, Alexander Kolchugin, Kirill Shubin, Andrei Farlenkov, and Elena Pikalova. 2020. "Features of Electrophoretic Deposition of a Ba-Containing Thin-Film Proton-Conducting Electrolyte on a Porous Cathode Substrate" Applied Sciences 10, no. 18: 6535. https://doi.org/10.3390/app10186535






