Application of k0-INAA Method in Preliminary Characterization of KRISS Urban Airborne Particulate Matter Certified Reference Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. k0-INAA Experiment
2.3. WD-XRF Experiment
3. Results and Discussion
3.1. Elemental Composition of Blank (Cellulose Paper) by k0-INAA
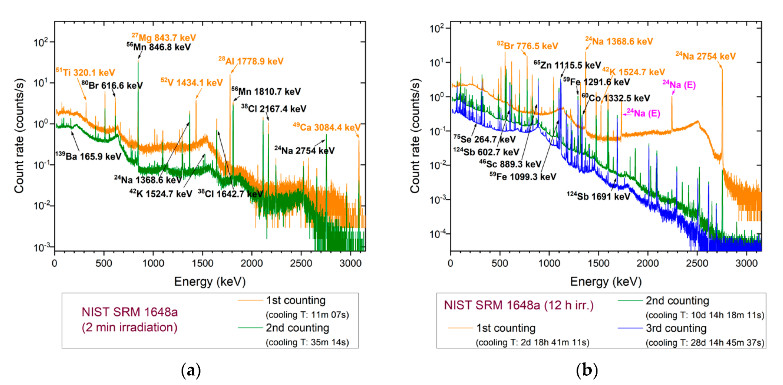
3.2. Validation of the k0-INAA Method Using NIST SRM 1648a Urban PM
3.3. Analysis of Elemental Compositions in Urban PMs and Incineration Ash
3.3.1. Analysis of Elements of Incineration Ash by the k0-INAA Method
3.3.2. Analysis of Elements of Incineration Ash and Urban PMs by the WD-XRF Method
3.4. Comparison of NIST SRM 1648a Urban PM and KRISS Urban Dust CRM Candidate: Element Composition and Source Contribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choi, J.-k.; Heo, J.-B.; Ban, S.-J.; Yi, S.-M.; Zoh, K.-D. Source apportionment of PM2.5 at the coastal area in Korea. Sci. Total Environ. 2013, 447, 370–380. [Google Scholar] [CrossRef]
- Jovanović, M.V.; Savić, J.; Kovačević, R.; Tasić, V.; Todorović, Ž.; Stevanović, S.; Manojlović, D.; Jovašević-Stojanović, M. Comparison of fine particulate matter level, chemical content and oxidative potential derived from two dissimilar urban environments. Sci. Total Environ. 2020, 708, 135209. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.D.; Riegel, R.P.; Klauck, C.R.; Ceratti, A.M.; Hansen, J.; Cansi, L.M.; Pozza, S.A.; Quevedo, D.M.d.; Osório, D.M.M. Source apportionment of metallic elements in urban atmospheric particulate matter and assessment of its water-soluble fraction toxicity. Environ. Sci. Pollut. Res. 2020, 27, 12202–12214. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; Hopke, P.K.; Dumanoglu, Y.; Altiok, H.; Elbir, T.; Odabasi, M.; Bayram, A. Characterization of PM Using Multiple Site Data in a Heavily Industrialized Region of Turkey. Aerosol Air Qual. Res. 2015, 15, 11–27. [Google Scholar] [CrossRef]
- Kim, M.-G.; Lee, S.-J.; Park, D.; Kim, C.-h.; Lee, K.-h.; Hwang, J.-m. Relationship between the actual fine dust concentration and media exposure that influenced the changes in outdoor activity behavior in South Korea. Sci. Rep. 2020, 10, 12006. [Google Scholar] [CrossRef]
- Itoh, N.; Inagaki, K.; Narukawa, T.; Aoyagi, Y.; Narushima, I.; Koguchi, M.; Numata, M. Certified reference material for quantification of polycyclic aromatic hydrocarbons and toxic elements in tunnel dust (NMIJ CRM 7308-a) from the National Metrology Institute of Japan. Anal. Bioanal. Chem. 2011, 401, 2909–2918. [Google Scholar] [CrossRef]
- Przyk, E.P.; Held, A.; Charoud-Got, J. Development of Particulate Matter Certified Reference Materials (PM10 CRMs); Technical Report; JRC Scientific and Technical Reports: Brussels, Belgium, 2008. [Google Scholar]
- Schantz, M.M.; Cleveland, D.; Heckert, N.A.; Kucklick, J.R.; Leigh, S.D.; Long, S.E.; Lynch, J.M.; Murphy, K.E.; Olfaz, R.; Pintar, A.L.; et al. Development of two fine particulate matter standard reference materials (<4 μm and <10 μm) for the determination of organic and inorganic constituents. Anal. Bioanal. Chem. 2016, 408, 4257–4266. [Google Scholar] [CrossRef]
- Aerosol Metrology for Atmospheric Science and Air Quality. Available online: https://www.euramet.org/research-innovation/search-research-projects/details/project/aerosol-metrology-for-atmospheric-science-and-air-quality/?L=0&tx_eurametctcp_project%5Baction%5D=show&tx_eurametctcp_project%5Bcontroller%5D=Project&cHash=178428d178423b178493df178420d178426f178428e178421d178427eb178490d178488a178423 (accessed on 25 July 2020).
- A Study on the Production Process of Urban Area PM2.5; National Institute of Environment Research, Incheon, Korea. 2010. Available online: http://www.prism.go.kr/homepage/entire/retrieveEntireDetail.do;jsessionid=140886EA144002BE140880A140857D140882E140891B140846A140848E140886B.node140802?cond_research_name=&cond_research_start_date=&cond_research_end_date=&research_id=1480000-201100103&pageIndex=201101697&leftMenuLevel=201100160 (accessed on 25 July 2020).
- Squizzato, S.; Masiol, M. Application of meteorology-based methods to determine local and external contributions to particulate matter pollution: A case study in Venice (Italy). Atmos. Environ. 2015, 119, 69–81. [Google Scholar] [CrossRef]
- Greenberg, R.R.; Bode, P.; De Nadai Fernandes, E.A. Neutron activation analysis: A primary method of measurement. Spectrochim. Acta B At. Spectrosc. 2011, 66, 193–241. [Google Scholar] [CrossRef]
- Cho, H.; Dasari, K.B.; Jaćimović, R.; Zeisler, R.; Sharp, N.E.; Kim, S.-H.; Sun, G.-M.; Yim, Y.-H. Application of the INAA methods for KRISS infant formula CRM analysis: Standardization of INAA at KRISS. J. Radioanal. Nucl. Chem. 2019, 322, 1537–1547. [Google Scholar] [CrossRef]
- Yu, L.L.; Browning, J.F.; Burdette, C.Q.; Caceres, G.C.; Chieh, K.D.; Davis, W.C.; Kassim, B.L.; Long, S.E.; Murphy, K.E.; Oflaz, R.; et al. Development of a kelp powder (Thallus laminariae) Standard Reference Material. Anal. Bioanal. Chem. 2018, 410, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lim, Y.; Hwang, E.; Yim, Y.-H. Development of an ID ICP-MS reference method for the determination of Cd, Hg and Pb in a cosmetic powder certified reference material. Anal. Methods 2016, 8, 796–804. [Google Scholar] [CrossRef]
- Lee, H.-S.; Kim, S.H.; Jeong, J.-S.; Lee, Y.-M.; Yim, Y.-H. Sulfur-based absolute quantification of proteins using isotope dilution inductively coupled plasma mass spectrometry. Metrologia 2015, 52, 619–627. [Google Scholar] [CrossRef]
- Kim, S.H.; Lim, Y.; Hwang, E.; Yim, Y.H. Overcoming Spectral Interferences in the Determination of Cadmium in Various Food Materials Using Isotope Dilution Inductively-coupled Plasma Mass Spectrometry. Bull. Korean Chem. Soc. 2015, 36, 936–943. [Google Scholar] [CrossRef]
- Lee, J.W.; Heo, S.W.; Kim, H.; Lim, Y.; Lee, K.-S.; Yim, Y.-H. Development of a Nutritional Supplement Certified Reference Material for Elemental Analysis. Mass Spectrom. Lett. 2018, 9, 105–109. [Google Scholar] [CrossRef]
- Avino, P.; Capannesi, G.; Rosada, A. Source identification of inorganic airborne particle fraction (PM10) at ultratrace levels by means of INAA short irradiation. Environ. Sci. Pollut. Res. 2014, 21, 4527–4538. [Google Scholar] [CrossRef]
- Müller, E.I.; Mesko, M.F.; Moraes, D.P.; Korn, M.d.G.A.; Flores, É.M.M. Chapter 4—Wet Digestion Using Microwave Heating. In Microwave-Assisted Sample Preparation for Trace Element Determination; Erico, M., Moraes, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 99–142. [Google Scholar] [CrossRef]
- Kemmouche, A.; Ali-Khodja, H.; Bencharif-Madani, F.; Mahía, P.L.; Querol, X. Comparative study of bulk and partial digestion methods for airborne PM10-bound elements in a high mineral dust urban site in Constantine, Algeria. Int. J. Environ. Anal. 2017, 97, 1132–1150. [Google Scholar] [CrossRef]
- Griepink, B.; Tölg, G. Sample digestion for the determination of elemental traces in matrices of environmental concern. Pure Appl. Chem. 1989, 61, 1139–1146. [Google Scholar] [CrossRef]
- Avinoa, P.; Capannesi, G.; Rosada, A. Heavy metal determination in atmospheric particulate matter by Instrumental Neutron Activation Analysis. Microchem. J. 2008, 88, 97–106. [Google Scholar] [CrossRef]
- Salma, I.; Zemplén-Papp, É. Instrumental neutron activation analysis for studying size-fractionated aerosols. Nucl. Instrum. Methods Phys. Res. A 1999, 435, 462–474. [Google Scholar] [CrossRef]
- Schwela, D.; Okhoya, C.N.; Markowicz, A.; Santos, F.L. Particle Air Pollution Monitoring with Nuclear Analytical Techniques: Challenges and Opportunities. Available online: http://fs.teledos.gr:2206/%3ERESEARCH%20PUBLICATIONS/NUCLEAR%20TECHNOLOGY%20-%20ENGINEERING/Elemental%20Analysis/Particle%20air%20pollution%20monitoring%20with%20nuclear%20analytical%20techniques.%20Paper.%20Schwela,%20Okhoya,%20Markowicz,%20Santos.pdf (accessed on 25 July 2020).
- Otoshi, T. Particulate Matter and Neutron Activation Analysis. In Proceedings of the 2001 Workshop on the Utilization of Research of Reactors, Beiling, China, 5–9 November 2001. [Google Scholar]
- Lestiani, D.D.; Santoso, M. Analytical Methods INAA and PIXE Applied to Characterization of Airborne Particulate Matter in Bandung, Indonesia. Atom Indones. 2011, 37, 52–56. [Google Scholar] [CrossRef]
- Dams, R.; Robbins, J.A.; Rahn, K.A.; Winchester, J.W. Nondestructive Neutron Activation Analysis of Air Pollution Particulates. Anal. Chem. 1970, 42, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Chalk, S.J. International Union of Pure and Applied Chemistry (IUPAC), Compendium of Chemical Terminology, 2nd ed. (The “Gold Book”). 2019. Available online: https://goldbook.iupac.org/terms/view/S05924 (accessed on 3 August 2020).
- De Corte, F.; Lierde, S.V. Determination and evaluation of fission k0-factors for correctionof the 235U(n,f) interference in k0-NAA. J. Radioanal. Nucl. Chem. 2001, 248, 97–101. [Google Scholar] [CrossRef]
- Kennedy, G.; Galinier, J.-L.; Zikovsky, L. Measurement of some primary nuclear interferences in neutron activation analysis with a SLOWPOKE reactor. Can. J. Chem. 1986, 64, 790. [Google Scholar] [CrossRef] [Green Version]
- De Corte, F.; Simonits, A. Vade Mecum for k0-users. DSM. Research 1994. Available online: http://www.kayzero.com/VADE%20MECUM%20FOR%20k0%20KayWin%20V3.pdf (accessed on 3 August 2020).
- Simonits, A.; De Corte, F.; Hoste, J. Single-comparator methods in reactor neutron activation analysis. J. Radioanal. Chem. 1975, 24, 31–46. [Google Scholar] [CrossRef]
- De Corte, F. The k0-Standardization Method: A Move to the Optimization of Neutron Activation Analysis. Habilitation Thesis, University of Gent, Gent, Belgium, 1987. [Google Scholar]
- Certificate of Analysis, Standard Reference Material (SRM) 1648a, Urban Particulate Matter; National Institute of Standard and Technology: Gaithersburg, MD, USA, 18 September 2015.
- Jaćimović, R.; Smodiš, B.; Bučar, T.; Stegnar, P. k0-NAA quality assessment by analysis of different certified reference materials using the KAYZERO/SOLCOI software. J. Radioanal. Nucl. Chem. 2003, 257, 659–663. [Google Scholar] [CrossRef]
- Jaćimović, R. Comparison of relative INAA and k0-INAA using soil and sediment reference materials. J. Radioanal. Nucl. Chem. 2014, 300, 663–672. [Google Scholar] [CrossRef]
- Dasari, K.B.; Cho, H.; Jaćimović, R.; Sun, G.-M.; Yim, Y.-H. Chemical Composition of Asian Dust in Daejeon, Korea, during the Spring Season. ACS Earth Space Chem. 2020, 4, 1227–1236. [Google Scholar] [CrossRef]
- Jaćimović, R.; De Corte, F.; Kennedy, G.; Vermaercke, P.; Revay, Z. The 2012 recommended k0 database. J. Radioanal. Nucl. Chem. 2014, 300, 589–592. [Google Scholar] [CrossRef]
- k0 Database 2019. 2019. Available online: http://www.kayzero.com/k0naa/k0naaorg/Nuclear_Data_SC/Nuclear_Data_SC.html (accessed on 20 August 2019).
- Simonits, A.; Östör, J.; Kálvin, S.; Fazekas, B. HyperLab: A new concept in gamma-ray spectrum analysis. J. Radioanal. Nucl. Chem. 2003, 257, 589–595. [Google Scholar] [CrossRef]
- HyperLab 2002 System, Installation and Quick Start Guide; HyperLabs Software: Budapest, Hungary, 2002.
- User’s Manual for Reactor Neutron Activation Analysis (NAA) Using the k0 Standardization Method, Version 2; Kayzero for Windows (KayWin®): Heerlen, The Netherlands, November 2005.
- Anagnostopoulos, D.F. X-ray emission spectroscopy optimization for chemical speciation in laboratory. Spectrochim. Acta B At. Spectrosc. 2018, 148, 83–91. [Google Scholar] [CrossRef]
- Sieber, J.R. Matrix-independent XRF methods for certification of standard reference materials. JCPDS Int. Cent. Diffr. Data 2002 Adv. X-Ray Anal. 2002, 45, 493–504. [Google Scholar]
- Fluxana Certified Reference Materials. Available online: https://fluxana.com/products/reference-materials/crm-catalogs (accessed on 3 August 2020).
- ISO 13528:2015. Statistical Methods for Use in Proficiency Testing by Interlaboratory Comparisons, 2nd ed.; ISO: Genève, Switzerland, 2015. [Google Scholar]
- Zeisler, R. Investigations by INAA for the development of Natural Matrix Standard Reference Materials® (SRMs) suitable for small sample analysis. J. Radioanal. Nucl. Chem. 2010, 245, 81–85. [Google Scholar] [CrossRef]
- Rousseau, R.M. Detection limit and estimate of uncertainty of analytical XRF results. Rigaku J. 2001, 18, 33–47. [Google Scholar]
- Almeida, S.M.; Ramos, C.A.; Marques, A.M.; Silva, A.V.; Freitas, M.C.; Farinha, M.M.; Reis, M.; Marques, A.P. Use of INAA and PIXE for multipollutant air quality assessment and management. J. Radioanal. Nucl. Chem. 2012, 294, 343–347. [Google Scholar] [CrossRef]
- Chutke, N.L.; Ambulkar, M.N.; Aggarwal, A.L.; Garg, A.N. Instrumental neutron activation analysis of ambient air dust particulates from metropolitan cities in India. Environ. Pollut. 1994, 85, 67–76. [Google Scholar] [CrossRef]
- Parvizimehr, A.; Baghani, A.N.; Hoseini, M.; Sorooshian, A.; Cuevas-Robles, A.; Fararouei, M.; Dehghani, M.; Delikhoon, M.; Barkhordari, A.; Shahsavan, S.; et al. On the nature of heavy metals in PM10 for an urban desert city in the Middle East: Shiraz, Iran. Microchem. J. 2020, 154, 104596. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Zhang, J.; Mao, Y.; Huang, X.; Qian, G. Evaluation for the heavy metal risk in fine particulate matter from the perspective of urban energy and industrial structure in China: A meta-analysis. J. Clean. Prod. 2020, 244, 118597. [Google Scholar] [CrossRef]
- Thurston, G.D.; Ito, K.; Lall, R. A source apportionment of U.S. fine particulate matter air pollution. Atmos. Environ. 2011, 45, 3924–3936. [Google Scholar] [CrossRef] [Green Version]
- Brahney, J.; Mahowald, N.; Ward, D.S.; Ballantyne, A.P.; Neff, J.C. Is atmospheric phosphorus pollution altering global alpine Lake stoichiometry? Glob. Biogeochem. Cycles 2015, 29, 1369–1383. [Google Scholar] [CrossRef] [Green Version]
- Mahowald, N.; Jickells, T.D.; Baker, A.R.; Artaxo, P.; Benitez-Nelson, C.R.; Bergametti, G.; Bond, T.C.; Chen, Y.; Cohen, D.D.; Herut, B.; et al. Global distribution of atmospheric phosphorus sources, concentrations and deposition rates, and anthropogenic impacts. Glob. Biogeochem. Cycles 2008, 22, GB4026. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.M.; Zhu, Q.H.; Zhang, B.; Gao, X. Concentration distribution and composition of phosphorus in street dust from small towns in Yongchuan, Chongqing. Adv. Mat. Res. 2013, 689, 556–560. [Google Scholar] [CrossRef]
- Ramírez, O.; Sánchez de la Campa, A.M.; Sánchez-Rodas, D.; de la Rosa, J.D. Hazardous trace elements in thoracic fraction of airborne particulate matter: Assessment of temporal variations, sources, and health risks in a megacity. Sci. Total Environ. 2020, 710, 136344. [Google Scholar] [CrossRef] [PubMed]
- Negral, L.; Suárez-Peña, B.; Zapico, E.; Fernández-Nava, Y.; Megido, L.; Moreno, J.; Marañón, E.; Castrillón, L. Anthropogenic and meteorological influences on PM10 metal/semi-metal concentrations: Implications for human health. Chemosphere 2020, 243, 125347. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Cuero, R.; García-Rosales, G.; Longoria-Gándara, L.C.; López-Reyes, M.C.; Ávila-Pérez, P. Application of Neutron Activation Analysis for Determination of As, Cr, Hg, and Se in Mosses in the Metropolitan Area of the Valley of Toluca, Mexico. J. Chem. 2015, 2015, 278326. [Google Scholar] [CrossRef] [Green Version]
- Air Quality Guidelines for Europe, 2nd ed.; World Health Organization: Geneva, Switzerland, 2000.
- Agents Classified by the IARC Monographs, International Agency for Research on Cancer volume 1–127. Available online: https://monographs.iarc.fr/agents-classified-by-the-iarc/ (accessed on 4 August 2020).
- Ayrault, S.; Senhou, A.; Moskura, M.; Gaudry, A. Atmospheric trace element concentrations in total suspended particles near Paris, France. Atmos. Environ. 2010, 44, 3700–3707. [Google Scholar] [CrossRef]
- Eckelman, M.J.; Graedel, T.E. Silver Emissions and their Environmental Impacts: A Multilevel Assessment. Environ. Sci. Technol. 2007, 41, 6283–6289. [Google Scholar] [CrossRef]
- Sheppard, S.C.; Evenden, W.G. Critical compilation and review of plant/soil concentration ratios for uranium, thorium and lead. J. Environ. Radioact. 1988, 8, 255–285. [Google Scholar] [CrossRef]
- Nance, W.B.; Taylor, S.R. Rare earth element patterns and crustal evolution—I. Australian post-Archean sedimentary rocks. Geochim. Cosmochim. Acta 1976, 40, 1539–1551. [Google Scholar] [CrossRef]




| El. | Mass Fraction ± Expanded Uncertainty (mg/kg, k = 2) | LOD (mg/kg) | El. | Mass Fraction ± Expanded Uncertainty (mg/kg, k = 2) | LOD (mg/kg) |
|---|---|---|---|---|---|
| Ag | 0.030 | La | 0.002 | ||
| Al | 1.63 ± 0.42 | 0.09 | Mg | 1.74 ± 0.651 | 1.07 |
| As | 0.0063 | Mn | 0.0387 ± 0.0036 | 0.0045 | |
| Au | 0.00014 ± 0.00011 | 0.00005 | Mo | 0.023 | |
| Ba | 0.68 | Na | 16.4 ± 3.1 | 0.1 | |
| Br | 0.175 ± 0.024 | 0.008 | Nd | 0.04 | |
| Ca | 32.9 | Rb | 0.23 | ||
| Cd | 0.05 | Sb | 0.0025 ± 0.0004 | 0.0010 | |
| Ce | 0.03 | Sc | 0.001 | ||
| Cl | 28.2 ± 3.8 | 0.4 | Se | 0.03 | |
| Co | 0.009 | Sm | 0.00017 ± 0.00005 | 0.00015 | |
| Cr | 0.232 ± 0.050 | 0.056 | Sn | 1.4 | |
| Cs | 0.006 | Sr | 2.5 | ||
| Cu | 0.27 | Ta | 0.002 | ||
| Dy | 0.002 | Tb | 0.002 | ||
| Eu | 0.0007 | Th | 0.0041 | ||
| Fe | 3.77 ± 0.669 | 3.76 | Ti | 0.85 | |
| Ga | 0.037 | U | 0.0014 | ||
| Hf | 0.005 | V | 0.0070 | ||
| Hg | 0.017 | W | 0.013 | ||
| I | 0.028 | Yb | 0.0021 | ||
| In | 0.0005 | Zn | 0.190 ± 0.050 | 0.059 | |
| K | 3.3 | Zr | 3.4 |
| El. | NIST SRM 1648a Urban PM | KRISS Urban Dust CRM Candidate | KRISS Ash CRM Candidate | ||||
|---|---|---|---|---|---|---|---|
| k0-INAA | † NIST Values | En (k = 2) | k0-INAA | k0-INAA | |||
| Mass Fraction ± Expanded Uncertainty (mg/kg, k = 2) | Cert., Ref. or Inf. Values (mg/kg) | $ Mass Fraction ± Expanded Uncertainty (mg/kg, k = 2) | § % Dev. (KRISS/NIST) | $ Mass Fraction ± Expanded Uncertainty (mg/kg, k = 2) | § % Dev. (KRISS/NIST) | ||
| Ag | 6.94 ± 0.50 | 6.0 ± 0.3 | 1.60 | 1.27 ± 0.18 | −82% | 20.3 ± 1.5 | 192% |
| # Al | 33,528 ± 2402 | 34,300 ± 1300 | −0.28 | 49,359 ± 3847 | 47% | 10,6021 ± 7457 | 216% |
| As | 124.9 ± 8.8 | 115.5 ± 3.9 | 0.98 | 34.7 ± 2.5 | −72% | 19.4 ± 1.4 | −84% |
| Au | 0.028 ± 0.006 | N/A | - | 0.279 ± 0.115 | 904% | 4.08 ± 0.32 | 14563% |
| # Ba | 680 ± 57 | N/A | - | 1361 ± 102 | 100% | 1917 ± 233 | 182% |
| Br | 520 ± 37 | 502 ± 10 | 0.47 | 57.8 ± 4.1 | −89% | 2.17 ± 0.18 | −100% |
| Ca | 56,422 ± 4074 | 58,400 ± 1900 | −0.44 | 42,076 ± 3186 | −25% | 48,097 ± 3929 | −15% |
| # Cd | 76.3 ± 5.5 | 73.7 ± 2.3 | 0.44 | 5.45 ± 1.50 | −93% | 7.22 ± 0.82 | −91% |
| Ce | 50.8 ± 3.8 | 54.6 ± 2.2 | −0.88 | 69.1 ± 5.8 | 36% | 99.1 ± 7.4 | 95% |
| # Cl | 4729 ± 334 | 4543 ± 47 | 0.55 | 12,642 ± 930 | 167% | LOD = 112 | - |
| Co | 17.3 ± 1.3 | 17.93 ± 0.68 | −0.42 | 19.1 ± 1.4 | 10% | 16.8 ± 1.2 | −3.0% |
| Cr | 397 ± 29 | 402 ± 13 | −0.16 | 219 ± 18 | −45% | 238 ± 17 | −40% |
| Cs | 3.38 ± 0.24 | 3.4 ± 0.2 | −0.06 | 4.67 ± 0.33 | 38% | 5.30 ± 0.38 | 57% |
| # Cu | 682 ± 90 | 610 ± 70 | 0.63 | 854 ± 100 | 25% | 2054 ± 252 | 201% |
| # Dy | 3.37 ± 0.30 | N/A | - | 3.40 ± 0.42 | 0.9% | 3.95 ± 0.48 | 17% |
| Eu | 1.52 ± 0.11 | N/A | - | 1.15 ± 0.09 | −24% | 1.32 ± 0.11 | −13% |
| Fe | 38,162 ± 2767 | 39,200 ± 2100 | −0.30 | 39,139 ± 2997 | 2.6% | 39,446 ± 2838 | 3.4% |
| # Ga | 17.5 ± 1.3 | N/A | - | LOD = 18.1 | - | 24.6 ± 2.5 | 41% |
| Hf | 4.15 ± 0.30 | 5.2 | - | 7.40 ± 0.53 | 78% | 4.74 ± 0.35 | 14% |
| Hg | 1.49 ± 0.14 | 1.323 ± 0.064 | 1.07 | 0.447 ± 0.074 | −70% | LOD = 0.5 | - |
| # I | 20.5 ± 3.5 | N/A | - | 27.2 ± 5.2 | 33% | LOD = 18 | - |
| # In | 0.986 ± 0.077 | N/A | - | 1.08 ± 0.08 | 10% | 0.547 ± 0.091 | −45% |
| K | 9986 ± 763 | 10,560 ± 490 | −0.63 | 16,739 ± 1486 | 68% | 29630 ± 2470 | 197% |
| La | 35.3 ± 2.6 | 39 ± 3 | −0.94 | 36.4 ± 2.9 | 3.1% | 55.3 ± 4.0 | 56% |
| # Mg | 7992 ± 708 | 8130 ± 120 | −0.19 | 10,331 ± 733 | 29% | 18,834 ± 1916 | 136% |
| # Mn | 790 ± 56 | 790 ± 44 | −0.01 | 682 ± 52 | −14% | 1170 ± 82 | 48% |
| Mo | 18.4 ± 1.3 | N/A | - | 25.9 ± 2.0 | 41% | 26.0 ± 1.9 | 41% |
| Na | 4295 ± 308 | 4240 ± 60 | 0.17 | 14,302 ± 1033 | 233% | 8499 ± 601 | 98% |
| Nd | 27.2 ± 2.2 | N/A | - | 27.6 ± 2.3 | 1.4% | 35.2 ± 3.4 | 29% |
| Rb | 48.1 ± 3.5 | 51.0 ± 1.5 | −0.76 | 95.3 ± 6.9 | 98% | 114 ± 8 | 137% |
| Sb | 44.6 ± 3.2 | 45.4 ± 1.4 | −0.23 | 73.3 ± 5.2 | 64% | 9.78 ± 0.71 | −78% |
| Sc | 6.54 ± 0.47 | 6 to 120 | - | 7.70 ± 0.59 | 18% | 8.12 ± 0.58 | 24% |
| Se | 26.1 ± 1.9 | 28.4 ± 1.1 | −1.06 | 6.01 ± 0.49 | −77% | 2.13 ± 0.15 | −92% |
| Sm | 4.32 ± 0.31 | 4.3 ± 0.3 | 0.04 | 4.75 ± 0.34 | 10% | 6.80 ± 0.55 | 58% |
| # Sn | 151 ± 13 | N/A | - | 138 ± 17 | −8.4% | 146 ± 13 | −2.9% |
| Sr | 226 ± 18 | 215 ± 17 | 0.43 | LOD = 94.2 | - | 416 ± 31 | 84% |
| Ta | 6.47 ± 0.49 | N/A | - | 1.01 ± 0.08 | −84% | 1.22 ± 0.10 | −81% |
| Tb | 0.518 ± 0.037 | N/A | - | 0.837 ± 0.060 | 62% | 0.723 ± 0.052 | 40% |
| Th | 7.19 ± 0.51 | 7 to 107 | - | 12.7 ± 0.9 | 77% | 15.6 ± 1.3 | 116% |
| # Ti | 3855 ± 315 | 4021 ± 86 | −0.51 | 3815 ± 335 | −1.0% | 4712 ± 352 | 22% |
| U | 5.36 ± 0.39 | N/A | - | 2.96 ± 0.29 | −45% | 94.6 ± 6.9 | 1665% |
| # V | 131 ± 9 | 127 ± 11 | 0.29 | 64.9 ± 5.0 | −50% | 74.1 ± 5.1 | −44% |
| # W | 5.55 ± 0.54 | 4.6 ± 0.3 | 1.54 | 28.4 ± 2.2 | 412% | 14.5 ± 1.1 | 160% |
| Yb | 1.71 ± 0.13 | N/A | - | 1.85 ± 0.14 | 7.8% | 1.70 ± 0.12 | −0.9% |
| Zn | 4623 ± 334 | 4800 ± 270 | −0.41 | 21,126 ± 1578 | 357% | 2852 ± 207 | −38% |
| Zr | 155 ± 16 | N/A | - | 314 ± 29 | 102% | 226 ± 20 | 46% |
| E1. | NIST SRM 1648a Urban PM | KRISS Urban Dust CRM Candidate | KRISS Ash CRM Candidate | ||
|---|---|---|---|---|---|
| WD-XRF | NIST Certified Values | WD-XRF | WD-XRF | ID-ICP/MS | |
| Mass Fraction ± Expanded Uncertainty (mg/kg, k = 2) | |||||
| P | 8410 ± 588 | N/A | 1100 ± 177 | 70,907 ± 3686 | N/A |
| Si | 124,500 ± 6400 | † 128,000 ± 4000 | 138,000 ± 7070 | 182,360 ± 9243 | N/A |
| Ni | 90 ± 15 | 81.1 ± 6.8 | 90 ± 16 | 110.46 ± 29 | 95.2 ± 6.7 |
| Pb | N/A | 6550 ± 330 | <150 | 164.9 ± 27 | 145.2 ± 2.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.; Dasari, K.B.; Lim, M.C.; Sun, G.M.; Jaćimović, R.; Yim, Y.-H. Application of k0-INAA Method in Preliminary Characterization of KRISS Urban Airborne Particulate Matter Certified Reference Material. Appl. Sci. 2020, 10, 6649. https://doi.org/10.3390/app10196649
Cho H, Dasari KB, Lim MC, Sun GM, Jaćimović R, Yim Y-H. Application of k0-INAA Method in Preliminary Characterization of KRISS Urban Airborne Particulate Matter Certified Reference Material. Applied Sciences. 2020; 10(19):6649. https://doi.org/10.3390/app10196649
Chicago/Turabian StyleCho, Hana, Kishore B. Dasari, Myung Chul Lim, Gwang Min Sun, Radojko Jaćimović, and Yong-Hyeon Yim. 2020. "Application of k0-INAA Method in Preliminary Characterization of KRISS Urban Airborne Particulate Matter Certified Reference Material" Applied Sciences 10, no. 19: 6649. https://doi.org/10.3390/app10196649
APA StyleCho, H., Dasari, K. B., Lim, M. C., Sun, G. M., Jaćimović, R., & Yim, Y.-H. (2020). Application of k0-INAA Method in Preliminary Characterization of KRISS Urban Airborne Particulate Matter Certified Reference Material. Applied Sciences, 10(19), 6649. https://doi.org/10.3390/app10196649






