Energy Storage in Earth-Abundant Dolomite Minerals
Abstract
:Featured Application
Abstract
1. Introduction
Applications of Dolomites in Concentrated Solar Power Plants (CSPs)
2. Experimental and Theoretical Methods
2.1. Materials Developed and Used
2.2. Physicochemical Characterizations of Dolomites
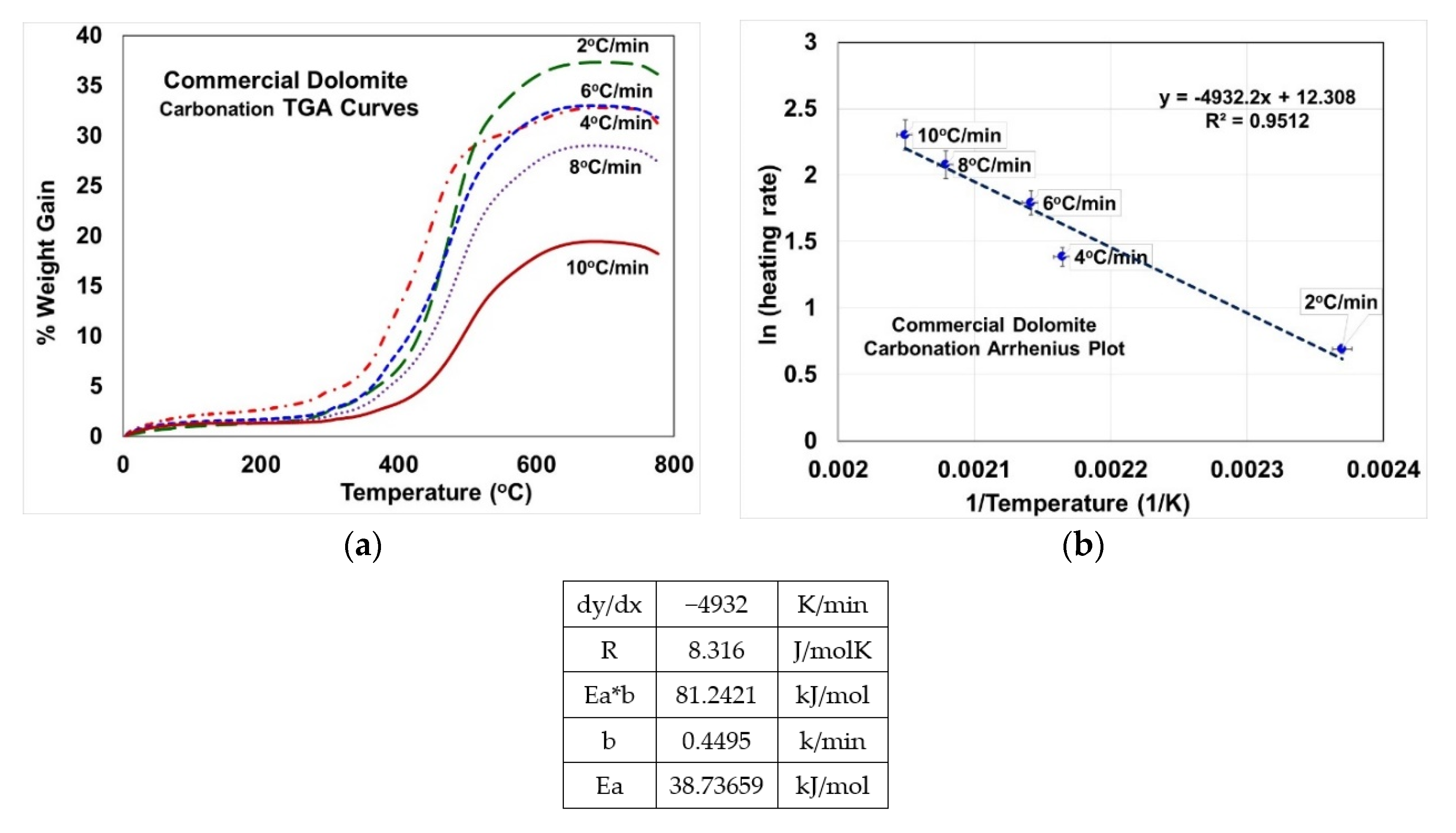
2.3. Activation Energy Calculations
3. Results and Discussion
3.1. Chemical Make-Up via X-ray Fluorescence (XRF)
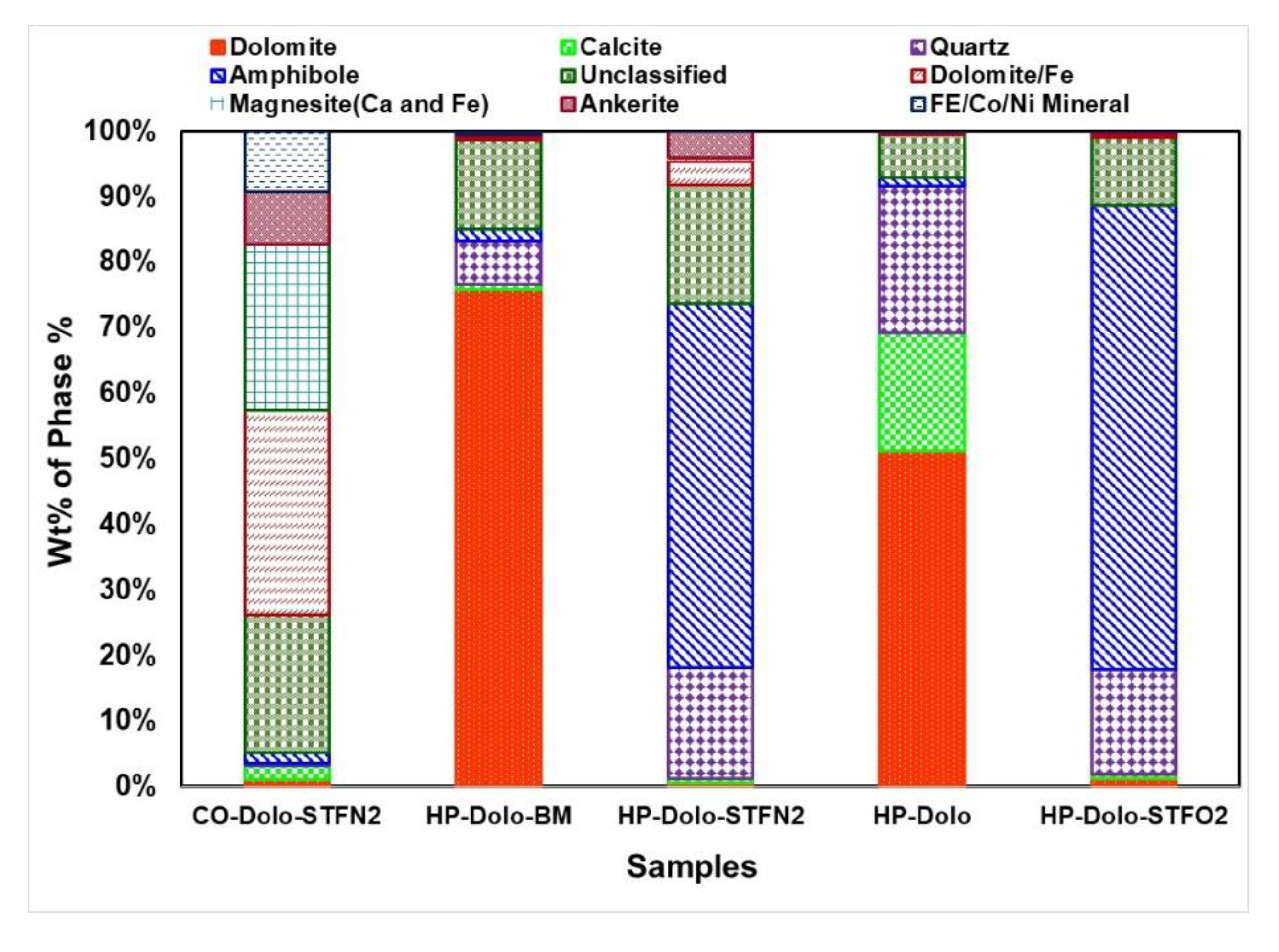
3.2. Compositional Mapping via Automated Scanning Electron Microscopy-Energy Dispersive X-ray Spectroscopy (SEM-EDX) Mineralogy
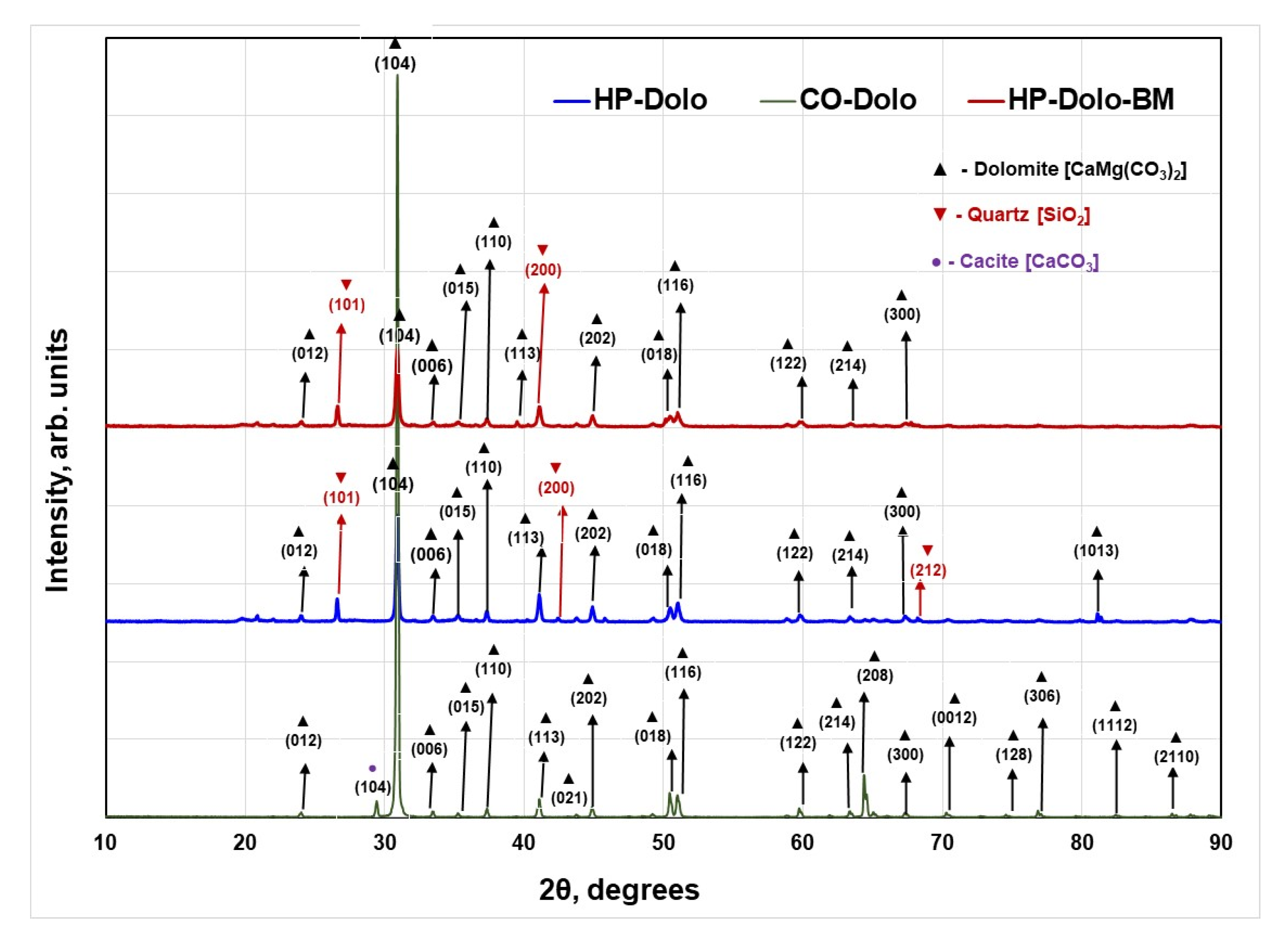
3.3. Phase Determination and Crystallite Size Analysis via X-ray Diffraction (XRD)
3.4. Chemical Environment via FTIR
3.5. Surface Area via Brunanuer-Emmett-Teller (BET) Apparatus
3.6. Activation Energy from Calcination via Thermogravimetric Analyzer (TGA)
3.7. Activation Energy from Carbonation via TGA
3.8. Calcination-Carbonation Looping Cycle via TGA
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CaMg(CO3)2 | Calcium Magnesium Carbonate |
| FIPR | Florida Industrial Phosphate Research Institute |
| TCES | Thermochemical Energy Storage |
| XRF | X-ray Fluorescence |
| XRD | X-ray Diffraction |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| Automated SEM-EDX Mineralogy | Manufacturing Company for Scanning Electron Microscopes to study Minerals |
| SEM | Scanning Electron Microscopy |
| EDX | Energy Dispersive X-ray analysis |
| BET | Brunauer–Emmett–Teller Surface Area Measurement Technique |
| CSP | Concentrated Solar Power |
| CaCO3 | Calcium Carbonate or Calcite |
| CaO | Calcium Oxide |
| MgO | Magnesium Oxide |
| DAP | Diammonium Phosphate |
| MgSO4 7H2O | Magnesium Sulfate Heptahydrate |
| Al2O3 | Aluminum Oxide or Alumina |
| SiO2 | Silicon Dioxide or Silica |
| Fe2O3 | Ferrous Oxide |
| GW | Giga Watts |
| kWhth | Kilo Watt Hour Thermal |
| IPCC | Intergrovernmental Panel on Climate Chang (IPCC) |
| CCS | Carbon Capture and Storage |
| CO-Dolo | Commercial Dolomite (from Alfa Aesar) |
| HP-Dolo | Handpicked Dolomite |
| HP-Dolo-BM | Handpicked Dolomite and ball milled |
| STF | Single-zone Tube Furnace |
| CO-Dolo-STF | Commercial Dolomite Calcined in Single-zone Tube Furnace |
| HP-Dolo-STF | Handpicked Dolomite Calcined in Single-zone Tube Furnace |
| HP-Dolo-STFN2 | Handpicked Dolomite Calcined in Single-zone Tube Furnace under Nitrogen |
| HP-Dolo-STFO2 | Handpicked Dolomite Calcined in Single-zone Tube Furnace under Oxygen or air |
| KBr | Potassium Bromide |
| TGA | Thermogravimetric Analyzer |
| DSC | Differential Scanning Calorimetry |
| ASTM | American Society for Testing and Materials |
| CSM | Colorado School of Mines |
| PDXL-2 | Powder X-ray Diffraction software |
| ICSD PDF | Inorganic Crystal Structure Database Powder Diffraction File |
| FWHM | Full Width at Half Maximum |
| kJ/mol | Kilo Joule per mole |
| J/molK | Joules per mole Kelvin |
References
- Van Kauwenbergh, S.J.; Cathcart, J.B.; McClellan, G.H. Minerology and Alteration of the Phosphate Deposits of Florida; U.S. Geological Survey Bulletin: Washington, DC, USA, 1914; pp. 1–56.
- Garcia, M.; Davila, G.; Offeddu, D.; Soler, J.M.; Cama, J. Reactions during CO2 geological sequestration: Dissolution of calcite and dolomite coupled to gypsum precipitation. Rev. Soc. Esp. Mineral. 2011, 2, 93–94. [Google Scholar]
- Loomis, F.B. Field Book of Common Rocks and Minerals; e-book; G.P. Putnam’s Sons: New York, NY, USA; London, UK, 1948. [Google Scholar]
- Bucher, K.; Frey, M. Definition, Conditions and Types of Metamorphism. In Petrogenesis of Metamorphic Rocks; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- ITP Mining: Energy and Environmental Profile of the U.S. Mining Industry. December 2002. Available online: https://www.energy.gov/eere/amo/downloads/itp-mining-energy-and-environmental-profile-us-mining-industry-december-2002 (accessed on 23 September 2020).
- DAP Quality Seminar Proceedings. 1986, pp. 1–45. Available online: http://fipr.state.fl.us/wp-content/uploads/2014/12/01-000-041Final.pdf (accessed on 23 September 2020).
- Good, P.; Zanganeh, G.; Ambrosetti, G.; Barbato, M.C.; Pedretti, A.; Steinfeld, A. Towards a commercial parabolic trough CSP system using air as heat transfer fluid. Energy Procedia 2014, 49, 381–385. [Google Scholar] [CrossRef] [Green Version]
- Kakosimos, K.E.; Al-Haddad, G.; Sakellariou, K.G.; Pagkoura, C.; Konstandopoulos, A.G. Characterization of Qatar’s Carbonates for CO2 capture and thermochemical energy storage. AIP Conf. Proc. 2017, 1850, 090003. [Google Scholar]
- Valverde, J.M.; Sanchez-Jimenez, P.E.; Perz-Maqueda, L.A. Ca-looping for postcombustion CO2 capture: A comparative analysis on the performances of dolomite and limestone. Appl. Energy 2015, 138, 202–215. [Google Scholar] [CrossRef] [Green Version]
- Perejon, A.; Romeo, L.M.; Lara, Y.; Lisbona, P.; Martinez, A.; Valverde, J.M. The calcium-looping technology for CO2 capture: On the important roles of energy integration and sorbent behavior. Appl. Energy 2016, 162, 787–807. [Google Scholar] [CrossRef] [Green Version]
- Benitez-Guerrero, M.; Valverde, J.M.; Sanchez-Jimenez, P.E.; Perejon, A.; Perez-Maqueda, L.A. Calcium-looping performance of mechanically modified Al2O3-CaO composites for energy storage and CO2 capture. Chem. Eng. J. 2018, 334, 2343–2355. [Google Scholar] [CrossRef]
- Perlinger, J.A.; Almendiger, J.E.; Urban, N.R.; Eisenreich, S.J. Groundwater geochemistry of aquifer thermal energy storage long-term test cycle. Water Resour. Res. 1987, 23, 2215–2226. [Google Scholar] [CrossRef]
- Rashad, M.M.; Baloumy, H.M. Chemical processing of dolomite associated with the phosphorites for production of magnesium sulfate heptahydrate. Eur. J. Miner. Process. Environ. Prot. 2005, 5, 174–183. [Google Scholar]
- Santosh, G.; Andrew, M. Demonstration of High-Temperature Calcium-Based Thermochemical Energy Storage System for Use with Concentrating Solar Power Facilities. Sun Shot, U.S. Department of Energy, CSP Program Summit 2016, 16 Slides. Available online: https://www.energy.gov/sunshot (accessed on 23 September 2020).
- International Energy Agency. IEA-ETSAP and IRENA© Technology Brief E17—January 2013; International Energy Agency: Paris, France, 2013. [Google Scholar]
- FIPR 2011–2016 Strategic Plan; Energy and Environmental Profile of the US Mining Industry. Available online: https://www.fipr.state.fl.us/wp-content/uploads/2014/09/Strategic-Plan-2011-2016-v11_final-2014-Poly-revision-final-for-Board-changes-accepted.pdf (accessed on 23 September 2020).
- Warren, J. Dolomite: Occurrence, evolution and economically important assoications. Earth Sci. Rev. 2000, 52, 1–81. [Google Scholar] [CrossRef]
- Belousouv, M.V.; Selivanov, E.N.; Gulyaeva, R.I.; Tyushnyakov, S.N.; Rakipov, D.F. Thermodynamics and kinetics of thermal dissociation of dolomite. Russ. J. Non-Ferr. Met. 2016, 57, 180–186. [Google Scholar] [CrossRef]
- Valverde, J.M.; Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A.; Quintanilla, M.A.S. Role of precalcination and regeneration conditions on postcombustion CO2 in the Ca-looping technology. Appl. Energy 2014, 125, 264–275. [Google Scholar] [CrossRef] [Green Version]
- Olszak-Humienik, M.; Jablonski, M. Thermal behavior of natural dolomite. J. Therm. Anal. Calorim. 2015, 119, 2239–2248. [Google Scholar] [CrossRef] [Green Version]
- Agrinier, P.; Deutsch, A.; Scharer, U.; Martinez, I. Fast back reactions of schock-released CO2 from carbonates: An experimental approach. Geochim. Cosmochim. Acata 2001, 65, 2615–2632. [Google Scholar] [CrossRef]
- Latchman, D. Carbon Dioxide Capture from Fossil Fuel Power Plants Using Dolomite. Master’s Thesis, University of South Florida, Tampa, FL, USA, 2010; 53p. Available online: https://scholarcommons.usf.edu/etd/1693 (accessed on 23 September 2020).
- Sayyah, M.; Lu, Y.; Masel, R.; Suslik, K.S. Mechanical Activation of CaO-Based Assorbents for CO2 capture. Chemsuschem 2013, 6, 193–198. [Google Scholar] [CrossRef]
- Song, G.; Zhu, X.; Chen, R.; Liao, Q.; Ding, Y.-D.; Chen, L. An investigation of CO2 adsorption kinetics on porous magnesium oxide. Chem. Eng. J. 2016, 283, 175–183. [Google Scholar] [CrossRef]
- De Coninck, H.C.; Loos, M.A.; Davidson, B.; Meyer, L.A. IPCC Special Report on Carbon Dioxide Capture and Storage; ECN-RX-05-209; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2005; ISBN 0-521-68551-6. [Google Scholar]
- Bayon, A.; Bader, R.; Jafarian, M.; Fedunik-Hofman, L.; Sun, Y.; Hinkley, J.; Miller, S.; Lipinski, W. Techno-economic assessment of solid-gas thermochemical energy storage systems for solar thermal power applications. Energy 2018, 149, 473–484. [Google Scholar] [CrossRef]
- Humphries, T.D.; Moller, K.T.; Rickard, W.D.A.; Veronica Sofianos, M.; Liu, S.; Buckley, C.E.; Paskevicius, M. Dolomite: A low cost thermochemical energy storage material. J. Mater. Chem. A 2019, 7, 1206. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, A.J.; Gonzalez-Aguilar, J.; Romero, M.; Coronado, J.M. Solar energy on demand: A review on high temperature heat storage systems and materials. Chem. Rev. 2019, 119, 4777–4816. [Google Scholar] [CrossRef]
- Moller, K.J.; Ibrahim, A.; Buckley, C.E.; Paskevicius, M. Inexpensive thermochemical energy storage utilizing additive enhanced limestone. J. Mater. Chem. A 2020, 8, 9646–9653. [Google Scholar] [CrossRef]
- Liu, M.; Steven Tay, N.H.; Bell, S.; Belusko, M.; Jacob, R.; Will, G.; Saman, W.; Bruno, F. Review on concentrating solar power plants and new developments in high temperature thermal energy storage technologies. Renew. Sustain. Energy Rev. 2016, 53, 1411–1432. [Google Scholar] [CrossRef]
- Sarrion, B.; Valverde, J.M.; Perejon, A.; Perez-Maqueda, L.; Sanchez-Jimenez, E. On the multicycle activity of natural limestone/dolomite for thermochemical energy storage of concentrated solar power. Energy Technol. 2016, 4, 1013–1019. [Google Scholar] [CrossRef]
- Reich, L.; Yue, L.; Bader, R.; Lipinski, W. Towards solar thermochemical carbon dioxide capture via calcium oxide looping: A review. Aerosol Air Qual. Res. 2014, 14, 500–514. [Google Scholar] [CrossRef]
- Valverde, J.M.; Medina, S. Limestone calcination under calcium-looping conditions CO2 capture and thermochemical energy storage in the presence of H2O: An in situ XRD analyses. Phys. Chem. Chem. Phys. 2017, 19, 7587–7596. [Google Scholar] [CrossRef]
- Medina-Carrasco, S.; Valverde, J.M. In situ XRD analysis of dolomite calcination under CO2 in a humid environment. CrystEngComm 2020. [Google Scholar] [CrossRef]
- Goldfarb, L.; Sweigart, C.M. Heat Storage System. U.S. Patent No. 4,447,347, 8 May 1984. [Google Scholar]
- Werner, S. Process of Producing Burnt Dolomite. U.S. Patent No. 2,380,480, 31 July 1945. [Google Scholar]
- Alam, T.E. Experimental Investigation of Encapsulated Phase Change Materials for Thermal Energy Storage. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, 2015; 112p. Available online: https://scholarcommons.usf.edu/etd/5632 (accessed on 23 September 2020).
- Wickramarathne, C.; Moloney, F.; Pirasaci, T.; Kamal, R.; Goswami, D.Y.; Stefanakos, E.; Dhau, J. Experimental Study on Thermal Storage Performance of Cylindrically Encapsulated PCM in a cylindrical Storage Tank with Axial Flow. In Proceedings of the ASME 2016 Power Conference Collocated with the ASME 2016 10th International Conference on Energy Sustainability and the ASME 2016 14th International Conference on Fuel Cell Science, Engineering and Technology, Charlotte, NC, USA, 26–30 June 2016; ISBN 978-0-7918-5021-3. Paper No. POWER2016-59427, V001T06A014. [Google Scholar]
- Honeyands, T.; Manuel, J.; Matthews, L.; O’Dea, D.; Pinson, D.; Leedham, J.; Zhang, G.; Li, H.; Monaghan, B.; Liu, X.; et al. Comparison of the mineralogy of iron ore sinters using of range of techniques. Minerals 2019, 9, 333. [Google Scholar] [CrossRef] [Green Version]
- ASTM E1641-16. In Standard Test Method for Decomposition Kinetics by Thermogravimetry Using the Ozawa/Flynn/Wall Method; ASTM International: West Conshohocken, PA, USA, 2016.
- Flynn, J.H.; Wall, L.A. A Quick, Direct Method for the Determination of Activation Energy from Thermogravimetric Data. Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Martin, A.L.; Inoue, H.; Yamada, Y.; Kohno, H. Sandardless XRF analysis for LOI-rich samples by scatter fundamental parameter method, Goldschmidt Conference Abstracts. Anal. Chem. 2005, p. A796. Available online: https://www.semanticscholar.org/paper/Standardless-XRF-analysis-for-LOI-rich-rock-samples-Martin-Inoue/07605bb3a20d5d76bacf6b9ff0b1669be35ec7a0#paper-header (accessed on 23 September 2020).
- King, B.-S.; Vivit, D. Loss on-ignition correction in the XRF analysis of silicate rocks. X-Ray Spectrom. 1988, 17, 4. [Google Scholar] [CrossRef]
- Warne, S.S.J.; Morgan, D.J.; Milodowski, A.E. Thermal analysis studies of the dolomite, ferroan dolomite, ankerite series. Part 1. Iron content recognition and determination by variable atmosphere DTA. Thermochim. Acta 1981, 51, 105–111. [Google Scholar] [CrossRef]
- Miser, D.E.; Swinnea, J.S.; Steinfink, H. TEM observations and X-ray crystal-structure refinement of a twinned dolomite with a modulated microstructure. Am. Mineral. 1987, 72, 188–193. [Google Scholar]
- Sakher, E.; Loudjani, N.; Bechiheub, M.; Bououdina, M. Influence of milling time on structural and microstructural parameters of Ni50Ti50 prepared by mechanical alloying using Rietveld analysis. J. Nanomater. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Database of ATR-FT-IR Spectra of Various Materials, Dolomite in Mid-IR Region, Institute of Chemistry, University Tartu, Estonia. Available online: https://lisa.chem.ut.ee/IR_spectra/paint/fillers/dolomite/ (accessed on 23 September 2020).
- Ji, J.; Ge, Y.; Balsam, W.; Damuth, J.E.; Chen, J. Rapid identification of dolomite using a Fourier Transform Infrared Spectrophotometer (FTR): A fast method for identifying Heinrich events in IODP site U1308. Mar. Geol. 2009, 258, 60–68. [Google Scholar] [CrossRef]
- Shokri, B.; Firouzjah, M.A.; Hosseini, S.I. FTIR Analysis of Silicon Dioxide Thin Film Deposited by Metal Organic-Based PECVD. Available online: https://www.ispc-conference.org/ispcproc/papers/791.pdf (accessed on 23 September 2020).
- Chan, R.K.; Murthi, K.S.; Harrison, D. Thermogravimetric analysis of Ontario limestones and dolomites. II Reactivity of sulfur dioxide with calcined samples. Can. J. Chem. 1970, 48, 2979. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Ma, K.; Abdala, A.A.; Lu, L.J.; Tanakov, I.; Biswal, S.L.; Hirasaki, G.J. Adsorption of a switchable cationic surfactant on natural carbonate minerals. In Proceedings of the Society of Petroleum Engineers—19th SPE Improved Oil Recovery Symposium, IOR 2014, Tulsa, OK, USA, 12–16 April 2014; Society of Petroleum Engineers (SPE): Richardson, TX, USA, 2014; Volume 1, pp. 163–178. [Google Scholar]





| Elements or Compounds | CO-Dolo wt% | CO-Dolo-STF wt% | HP-Dolo wt% | HP-Dolo-STF wt% | Remarks |
|---|---|---|---|---|---|
| CaO | 67.90 | 34.26 | 36.60 | 32.00 | ~50% reduction in CaO content in HP-Dolo. After carbonation in STF, the CaO content increased in the range from 3.5 to 17 wt% |
| MgO | 19.60 | 9.80 | 19.20 | 10.90 | More or less equal amount of MgO in both samples. After carbonation in STF, the MgO content is reduced in the range from 1.7 to 8.3 wt% |
| SiO2 | 1.64 | 0.40 | 32.00 | 24.10 | HP-Dolo contains large amount of silica (sand type). After carbonation in STF, the SiO2 content reduced up to 8 wt%. |
| P2O5 | 0.52 | 0.60 | 1.61 | 0.83 | P2O5 in trace amounts in all samples |
| Fe2O3 | 9.94 | 9.33 | 2.53 | 3.88 | Fe2O3 is greater in CO-Dolo due to purification procedures. |
| Al2O3 | - | - | 6.66 | 5.07 | Alumina Al2O3 is found in Earth-mined phosphates, HP-Dolo. |
| CaO + MgO combined | 87.50 | 44.06 | 55.8 | 42.90 | Added row 1 and row 2 values. ~30% mass reduction in HP-Dolo due to unreactive SiO2. After carbonation in STF, this reactive mixture of CaO+MgO increased by 3–8 wt%. |
| Loss on Ignition (LOI) | 0.00 | 45.00 | 0.00 | 22.00 | Loss on Ignition is due to the CO2 release during calcination. |
| Sample Category Number(s) | Sample(s) Name | Dolomite Pure or Dolomite (with Fe) Concentration (as Reference Phase) [wt% of Phase%] | Other Major/Minor Phases [wt% of Phase%] |
|---|---|---|---|
| 1 | Commercial + Hand Crushed STF (N2 ambient) [CO-Dolo-STFN2] | Dolomite (Pure): 0.91 Dolomite (with Fe): 29.38 | Magnesite (with Ca and Fe): 23.70 Magnesite (low Ca and Fe) 2.28 Ankerite (pure): 7.56 Cr-Ni Ankerite: 3.19 Calcite: 2.09 Fe-Co-Ni Mineral: 8.69 Quartz: 0.36 Amphibole: 1.46 Unclassified: 19.74 |
| 2. | Hand Picked + Hand Crushed [HP-Dolo] | Dolomite (Pure): 49.60 Dolomite (with Fe): 0.08 | Calcite: 17.46 Quartz: 21.67 Amphibole: 1.26 Unclassified: 6.36 |
| 3 | Hand Picked + Ball Milled (Acetone) [HP-Dolo-BM] | Dolomite (Pure): 74.90 Dolomite (with Fe): 0.53 | Calcite: 0.82 Quartz: 6.42 Amphibole: 1.85 Unclassified: 13.37 |
| 4 | Hand Picked + Crushed STF (N2 ambient) [HP-Dolo-STFN2] | Dolomite (Pure): 0.50% Dolomite (with Fe): 3.51 | Ankerite: 3.71 Quartz: 15.98 Anorthite: 4.42 Amphibole: 51.93 Unclassified: 16.92 |
| 5 | Hand Picked + Hand Crushed STF (Air ambient) [HP-Dolo-STFO2] | Dolomite (Pure): 1.15 Dolomite (with Fe): 0.31 | Ankerite: 0.48 Quartz: 14.26 Anorthite: 8.01 Amphibole: 63.47 Unclassified: 9.33 |
| Co-Dolo | HP-Dolo | HPO-Dolo-BM | Dolomite | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Average Crystallite Size = 552.5 nm | Average Crystallite Size = 448.3 nm | Average Crystallite Size = 354.0 nm | ICSD—202162 | ||||||
| 2θ | d-value | FWMH | 2θ | d-value | FWHM | 2θ | d-value | FWHM | (hkl) |
| 30.909 | 2.89075 | 0.1344 | 30.9 | 2.89125 | 0.188 | 30.906 | 2.89099 | 0.2253 | 104 |
| 41.078 | 2.19557 | 0.1932 | 41.076 | 2.19565 | 0.1856 | 41.072 | 2.19588 | 0.2378 | 113 |
| 44.841 | 2.01966 | 0.1696 | 44.878 | 2.01805 | 0.1829 | 44.868 | 2.01849 | 0.2226 | 202 |
| 50.430 | 1.80814 | 0.1463 | 50.450 | 1.80746 | 0.2309 | 50.458 | 1.80721 | 0.2937 | 018 |
| 50.982 | 1.78985 | 0.1809 | 51.008 | 1.7890 | 0.2195 | 51.005 | 1.78911 | 0.3062 | 116 |
| Lattice Parameters (Calculated for hkl = (300) and (006)) a = b = 4.8163(2) Å c = 16.0748(7) Å Unit Cell Volume = 322.93 (Å)3 | Lattice Parameters (Calculated for hkl = (300) and (006)) a = b = 4.8146(5) Å c = 16.0504 Å Unit Cell Volume = 322.22 (Å)3 | Lattice Parameters (Calculated for hkl = (300) and (006)) a = b = 4.8187(8) Å c = 16.0513 Å Unit Cell Volume = 322.78 (Å)3 | a = b = 4.811 Å c = 16.0462 Å Volume = 321.659 (Å)3 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinivasan, S.; Dodson, D.; Charles, M.B.J.; Wallen, S.L.; Albarelli, G.; Kaushik, A.; Hickman, N.; Chaudhary, G.R.; Stefanakos, E.; Dhau, J. Energy Storage in Earth-Abundant Dolomite Minerals. Appl. Sci. 2020, 10, 6679. https://doi.org/10.3390/app10196679
Srinivasan S, Dodson D, Charles MBJ, Wallen SL, Albarelli G, Kaushik A, Hickman N, Chaudhary GR, Stefanakos E, Dhau J. Energy Storage in Earth-Abundant Dolomite Minerals. Applied Sciences. 2020; 10(19):6679. https://doi.org/10.3390/app10196679
Chicago/Turabian StyleSrinivasan, Sesha, Dominic Dodson, Mc Ben Joe Charles, Scott L. Wallen, Gary Albarelli, Ajeet Kaushik, Nicoleta Hickman, Ganga Ram Chaudhary, Elias Stefanakos, and Jaspreet Dhau. 2020. "Energy Storage in Earth-Abundant Dolomite Minerals" Applied Sciences 10, no. 19: 6679. https://doi.org/10.3390/app10196679






