1. Introduction
The current development of the global economy leads to a steady growth of energy consumption [
1], which is conditioned not only by an increase in the population, but also by the annual growth of energy consumption per capita (from 72 to 76 GJ per capita during the last 10 years). Solid fossil fuel is one of the main energy resources, accounting for more than 35% of primary energy in the structure of global energy generation [
2]. The extensive usage of coal is promoted by its rather low and stable cost, abundance of deposits, and vast stocks in many regions of the world. According to expert estimates, the global stocks of coal were over 1054 billion tons by the end of 2018 [
1]. The global consumption of coal has risen by 68% in the last 30 years [
2,
3]. This is caused not only by a greater amount of energy, generated by coal-fired thermal power plants, and energy consumption by different industries, but also by a wide use of coal as a raw material in the metallurgical, chemical, and construction industries [
4,
5,
6].
One of the negative implications of large-scale coal mining and consumption is that a large amount of coal processing waste is accumulated in the landfills of coal washing plants; it has not been reclaimed for industrial application up until recently [
7]. Such waste is referred to as a filter cake (FC). It is a slurry of fine coal dust (80–140 µm) and water (40–50%). Coal processing waste is normally stored at open-air disposal sites, thus, being exposed to strong impact of natural factors (wind, sun, and air). Storing it this way causes moisture to evaporate, while wind scatters coal dust onto the surrounding areas, polluting the land and water bodies, suitable for agricultural purposes, with acid-forming substances and heavy metals [
5]. Moreover, dry fine coal dust can ignite spontaneously [
8,
9,
10], therefore, the industrial landfills of FC are fire hazardous for coal washing plants and nearby populated areas.
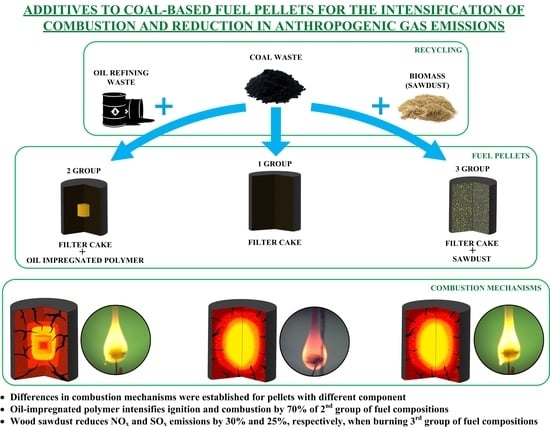
One way to diminish the impact of such waste on the environment and to satisfy the growing needs of thermal power engineering for energy resources is to involve low-grade solid fuels and coal processing waste as resources into the energy industry [
11,
12]. At the initial stage of solving this problem, it is a promising approach to develop composite compositions based on FC with the addition (if necessary) of some amount of solid or liquid components to enhance energy performance [
13,
14], and environmental [
15,
16,
17,
18], characteristics, as well as for the production of fuel briquettes and pellets for grate-firing. Using briquettes and pellets reduces the fire hazard of the fuel (as compared with coal dust). Moreover, it cuts transportation and storage costs, as well as decreases the amount of fuel falling through the grate in the boiler furnace. However, the extensive use of such solid fuel briquettes in real practice involves the problem of long and energy-consuming combustion initiation, relatively low (as compared with high-grade coals) heat of combustion, possible significant underburning, and poor physical and mechanical characteristics. However, a distinct advantage of such pellets as a fuel for local heat supply sources is their very low cost as compared with fuel oil [
19], which is a widespread energy resource of the local power industry. Even considering the ash removal costs (when using FC-based pellets as fuel), the cost of a unit of energy, produced by a local heat supply system, will be significantly lower than when using fuel oil. Moreover, oil prices and, thus, petroleum product prices are unpredictable and influenced by many factors. However, the cost of coal processing waste is made up of trans-shipment and storage expenses, while the fuel pellet production cycle (per a thermal unit) is less expensive than the fuel oil production technology [
19]. Another factor to be considered is that the FC-based pellet price is independent of the global political situation. That is why, in the medium term, this energy resource looks much more attractive than fuel oil.
In the production of fuel briquettes and pellets, now there are a number of standards to comply with in order to provide regulatory values of their characteristics: ISO 17225-1:2014; DIN 51731 / DIN plus (Germany); CTI-R 04/5 (Italy); ÖNORM M7135 (Austria); SS 18 71 21 (Sweden); GOST R 57016-2016 and GOST 33103.1-2017 (Russia). The main requirements are set for the physical and mechanical characteristics (compressive strength according to GOST 21289-75, water absorption according to GOST 21290-75, stickiness, thermostability, yield of volatiles according to GOST 6382-80, mass fraction of total sulfur according to GOST 2059-75, ash content, shock resistance, lowest heat of combustion, and mechanical strength). This requires the addition of expensive binders or complicates the pellet production technology. There are research results that offer various methods of producing fuel briquettes and pellets [
20,
21]. Such studies mainly investigate their physical and mechanical characteristics, as well as analyze the thermotechnical properties of fuel compositions [
22,
23,
24]. For example, it was established that low-temperature pyrolysis affects the calorific value of pellets based on coal sludge and straw [
23], their limit of hygroscopy, emissions of gaseous combustion products, and the efficiency of boiler operation when burning the fuel in a fluidized bed at 800–900 °C. Quite often [
24,
25] the resistance of briquettes to mechanical stress is studied alongside their thermotechnical and thermophysical properties (flame temperature, calorific value, and thermal conductivity), the characteristics of solid deposits and dynamics of slagging of energy generation equipment heating surfaces. Moreover, most of the studies [
26] focus on the thermogravimetric analysis of coal pellets, containing various biomass (wood and grass waste).
However, the practical application of fuel briquettes and pellets from low-rank energy resources relies primarily on the research findings for ignition and combustion processes—ignition delay times vs. temperature in the boiler furnace, burning time, and composition of flue gases. The purpose of present work is to experimentally study the influence of a group of fuel composition additives, based on coal processing waste, on the ignition and combustion characteristics of fuel pellets, as well as anthropogenic gas emissions. Achieving this goal necessitates two types of experiments:
Combustion of single fuel pellets in a muffle furnace while recording the processes under study by a high-speed video camera and subsequent analysis of the video recordings to establish the mechanisms of physical and chemical processes, as well as temporal characteristics of ignition and combustion.
Combustion of a group of fuel pellets of identical component composition in a muffle furnace to analyze the typical anthropogenic gas emissions (CO, CO2, NOx, SOx) in flue gases.
3. Results and Discussion
Figure 3 and
Figure 4 present the video frames of ignition and combustion of a group of solid composite fuel pellets with various components and without them. It is clear (
Figure 3 and
Figure 4) that adding a single particle of porous polymer material, impregnated with different combustible liquids, or wood sawdust of different particle size to the fuel pellet composition intensifies the ignition and combustion of these compositions, as compared with pellets, produced from FC without any additional components (composition No. 1). In general, the physical and chemical processes, occurring during the heating of pellets of fuel compositions under study (
Table 1), follow almost identical patterns, since their main component is FC.
High-speed video recordings made it possible to distinguish the following main stages of the process under consideration, when single pellets are heated in motionless heated air: inert heating of the fuel; evaporation of liquid components (water or oils for compositions No. 2–No. 4); thermal decomposition of solid combustible components (FC, polymer material for compositions No. 2–No. 4, wood for compositions No. 5–No. 8); formation of gas-vapor mixture around the pellet under the conditions of diffusive-convective heat and mass transfer in the oxidizer medium; its ignition on reaching critical conditions and subsequent burnout; heating of the solid residue from the heat of the gas-phase combustion; its ignition and subsequent burnout. The gas-phase combustion develops most rapidly for FC-based fuel pellets with sawdust (
Figure 4). This result is attributed to a much higher content of volatiles in wood, as compared with FC (
Table 2). Compositions No. 5–No. 8, as compared with composition No. 1, are characterized not only by a larger (by 10–20%) area around the pellet, in which the combustion of the gas mixture occurs, but also a longer (by 20–25%) duration of this process. Additionally, the burnout times of solid components (
tb) differ by 15–20%, which is also conditioned by differences in their ultimate analyses. The content of carbon in wood is almost one third lower than in FC. Therefore, the solid residue of compositions No. 5–No. 8 burns out much faster than that of composition No. 1 (
Figure 4). Similar patterns were established when the characteristics of ignition and combustion for fuel compositions No. 2–No. 4 were compared with those for composition No. 1 (
Figure 3), even when the concentration of an additional component is only 10%. In this case, the ignition delay times and complete burnout times differ (
Figure 5) due to different combustion mechanisms. A detailed analysis of the latter will be carried out below when describing typical schemes (
Figure 6) of combustion of fuel pellets with various components.
Figure 5 presents the ignition delay times (
td) vs. burnout times of fuel pellets (
tb) when varying the heated air temperature in a wide range:
Tg = 800–1000 °C. The temperature range limit on the left (
Tg = 800 °C) corresponds to threshold (minimum) ignition conditions. At air temperatures under 800 °C, the pellets of all the eight fuel compositions (
Table 1) did not ignite. Under such conditions, moisture evaporated and solid components thermally decomposed. However, the intensity of these processes and the oxidizer temperature were not enough for the forming gas mixture to ignite. The combustible gases and vapors diffused into the environment, but their concentration around the fuel pellet was decreasing as the fuel components thermally decomposed (and the content of volatiles decreased). The ambient air temperature was not high enough for the solid residue to ignite.
The mean ignition delay times of pellets of different compositions (with various additives) decrease from 5–6 s to 2.5 s in the air temperature (
Tg) range from 800 °C to 1000 °C (
Figure 5a). Their combustion becomes more intense under such conditions. The burnout times of fuel pellet components decrease by 20–25% (
Figure 5b). It was established that the
td values for the FC-based fuel compositions under study decrease sequentially (other conditions being equal) when the following components are added to them: a polymer material particle, impregnated with oil-water emulsion (based on rapeseed oil); a polymer material particle, impregnated with rapeseed oil; wood sawdust with a particle size of 200–500 µm; wood sawdust with a particle size of 100–200 µm; wood sawdust with a particle size of 45–100 µm; wood sawdust with a particle size of less than 45 µm; a polymer material particle, impregnated with oil-water emulsion (based on turbine oil). That means that fuel pellets with the latter component (composition No. 4) are characterized by the lowest ignition delay times. The highest values of
td are typical of pellets, produced from FC without any additional components (composition No. 1).
At ambient temperatures 800–850 °C, the
td values for the compositions with various components differed by about 70% at the most (
Figure 5a). At
Tg → 1000 °C and higher, the intensity of physical and chemical processes is so high that the ignition delay times for all the fuel compositions differ by no more than 5%, thus being less than the random error of
td measurement (
Figure 5a). Under near-threshold ignition conditions, the physical and chemical processes occur less rapidly. Most of the induction period (80–90%) is made up of the thermal preparation stage, characterized largely by heat and mass transfer processes both in the condensed phase and in the gas medium. Therefore, the differences in the ignition characteristics of fuel compositions with different components are more pronounced at
Tg = 800 °C.
As it was mentioned above, the gas-phase combustion develops most rapidly for FC-based fuel pellets with additives (sawdust and porous polymer material, impregnated with combustible liquid). This result is attributed to a much higher content of volatiles (
Table 1 and
Table 2) in fuel compositions No. 2–No. 8, as compared with FC (composition No. 1) without any additives. Besides, a further analysis will show that compositions No. 2–No. 4, as compared with composition No. 1, are characterized not only by a higher content of volatiles, but also by a significant difference in the combustion mechanism. A rapid evaporation of combustible liquid leads to the emergence and formation of a developed structure of pores, whose sizes are comparable with the typical pellet size. Under such conditions, the vapors and gases with low resistance enter the oxidizing medium from the deep pellet layers through open pores. The concentration of combustible gases in the vicinity of the pellet grows rapidly, and the gas-phase ignition occurs with a short delay (as compared with fuel composition No. 1).
The experimental results enable to draw an important conclusion. Including lumber waste (wood sawdust) in the composition of FC-based pellets in the volumes not affecting the physical and mechanical characteristics of pellets leads to significant (up to 70%) reduction in the ignition delay times at relatively low (800–850 °C) temperatures. Since the emission of nitrogen oxides in combustion products of coal and coal processing waste is minimum, and this temperature range in boiler furnaces of local heat supply is the most preferred, it can be concluded that including wood sawdust in the composition of fuel pellets based on coal processing waste is highly practical.
The times of complete burnout of fuel pellets (
Figure 5b) are in good agreement with their ignition delay times. It was established that the
tb values for FC-based fuel compositions under study decrease sequentially (other conditions being equal) when the following components are added to them: wood sawdust with a particle size of 200–500, 100–200, 45–100 µm and less than 45 µm; a polymer material particle, impregnated with oil-water emulsion (based on rapeseed oil); a polymer material particle, impregnated with rapeseed oil; a polymer material particle, impregnated with oil-water emulsion (based on turbine oil). This means that fuel pellets with the latter component (composition No. 4) are characterized by the lowest burnout times of components. The highest values of
tb are typical of pellets produced from FC without any other components added to them (composition No. 1). As it will be shown below, the obtained result can be explained by the differences in the elemental composition of fuel components and in the combustion mechanisms (the combustion front of fuel pellets with pores has a rather complex structure; the developed structure of pores increases the combustion area, thus, accelerating the burnout of fuel pellet components). Solid fuel pellets with a polymer material particle, impregnated with oil-water emulsion, burn out within 62 s at relatively low ambient temperatures (
Tg = 800 °C), which is 30% lower than
tb of fuel composition No. 1 based on FC without any additional components, and at
Tg = 1000 °C, the difference in the burnout times is 45%. Thus, adding extra components (a polymer particle, impregnated with oil-water emulsion; wood sawdust) to FC intensifies both the ignition and burnout of fuel pellets.
As mentioned above, this result is explained by different combustion mechanisms of fuel compositions (
Table 1).
Figure 6 presents schemes of combustion mechanisms for three groups of FC-based fuel pellets with different components, established from analyzing the frames of high-speed video recording. The differences established in the combustion mechanisms (
Figure 6) are primarily attributed to the structure of fuel pellets. The compositions of the first and third groups (No. 1, No. 5–No. 8) have a uniform structure with a rather dense packaging of fine particles of solid components (coal dust and wood sawdust). Thus, when they are heated, the occurring processes are similar to the combustion of large (several millimeters) solid fossil fuel particles. Internal thermal stress leads to the formation of open pores (
Figure 6a,c), through which the thermal decomposition products of solid components escape from the deep layers of fuel pellets into the oxidizer medium. These pores emerge and develop primarily in the near-surface layer of the pellet, and their size is much smaller than that of the pellet itself. Notably, the shape of pellets under such conditions remains almost the same as they burn (
Figure 3a and
Figure 4). The solid residue burns out in layers. The front of combustion is formed in the near-surface layer of the pellet and moves steadily towards its deep layers.
This process is more intense for compositions No. 5–No. 8, containing wood sawdust, than it is for composition No. 1. Sawdust is actually a thermally active additive, evenly distributed throughout the pellet. The ignition temperature of wood is much lower than that of dry FC (
Table 2). Thus, in the layer-by-layer combustion of the pellet, wood particles are the first to ignite in the combustion front, and then the heat released intensifies the coal component ignition (
Figure 6c). It reduces the ignition delay of coal.
The compositions with a polymer material particle, impregnated with a combustible liquid, are characterized by a different mechanism of ignition and combustion intensification. When a fuel pellet is heated, the thermal decomposition of FC is accompanied by the endothermic evaporation of oil or water-oil emulsion. Under such conditions, the energy supplied to the pellet is spent on the phase transformation, which reduces the intensity of the process under study, on the one hand. On the other hand, the rapid evaporation of liquid in a small area, located inside the dense structure of pressed FC, results in a higher pressure of vapors in a closed space. This leads to the emergence and formation of a developed structure of pores (
Figure 6b), whose sizes are comparable with the typical pellet size. Under such conditions, the fuel pellet shape changes significantly during combustion (
Figure 3b–d). As with the other compositions, in the heating of compositions No. 2–No. 4, the vapors and gases with low resistance enter the oxidizing medium from the deep pellet layers through open pores (
Figure 6b). However, the combustion front of such pellets has a rather complex structure. The developed structure of pores increases the combustion area and, thus, accelerates the burnout of fuel pellet components. Similar processes are typical of abnormal combustion modes of composite solid fuels when fuel charges crack or detach from the engine body walls [
41].
The experimental research findings for the ignition delay times and duration of burnout of a group of fuel pellets at different heating temperatures provide a foundation for the development of an industrial technology of using such solid composite fuels to generate power by combustion, e.g., in hot-water boiler furnaces. The established temporal characteristics of ignition and combustion can be applied to calculate the thermal modes of boiler furnace operation. Moreover, it was shown that the burnout rate of fuel components may be increased or decreased by varying the component composition of fuel pellets. Thus, the energy output per a unit of time can be varied in the temperature range of 800–900 °C, typical of hot-water boiler furnaces.
In addition to the ignition and combustion characteristics, we also analyzed the concentrations of anthropogenic emissions in flue gases from fuel pellet combustion. The concentrations of carbon oxides from the combustion of compositions No. 1–No. 8 are similar (the differences do not exceed several percent): CO
2—17–18%, CO—no more than 400 ppm. These values correspond to identical characteristics of coal dust combustion in a boiler furnace [
5]. The concentrations of nitrogen and sulfur oxides from the combustion of fuel pellets with different components differ much more significantly (
Figure 7).
In
Figure 7, the NO
x and SO
x concentrations are presented per a unit of heat (emissions for identical heating value of fuel samples are compared), released from the combustion of fuel pellets of different component composition. Notably, the ratio of masses of fuel compositions from three groups (FC; FC + 10% polymer particle, impregnated with a combustible liquid; FC + 30% wood sawdust), identical in the calorific value, is 1: 0.92: 1.12. According to the ultimate analysis (
Table 2), wood contains 10 times less nitrogen and sulfur. Therefore, adding wood sawdust to FC significantly reduces the NO
x and SO
x concentrations from the combustion of these fuel compositions of identical mass. However, the heat of combustion of wood is 35% lower than that of FC (
Table 2). Therefore, the mass of the fuel composition based on FC with 30% of wood sawdust has to be 1.12 times bigger to generate the same amount of heat, as the combustion of FC does. Thus, the difference in the NO
x and SO
x concentrations for composition No. 1 and compositions No. 5–No. 8 (per a unit of heat, released from the fuel pellet combustion) is 30% and 25% (
Figure 7), respectively. Moreover, the ash content of wood is an order of magnitude lower than that of FC, so, the combustion of a larger mass of fuel pellets with it will not increase the amount of non-combustible solid residue, but will, on the contrary, somewhat decrease it.
In its turn, adding a porous polymer particle, impregnated with a combustible liquid, to the fuel pellet composition, has a positive effect on its calorific value, since the heat of combustion of oil is almost twice as high as that of FC (
Table 2 and
Table 3). Therefore, the mass of the fuel composition based on FC with 10% of porous polymer, impregnated with a combustible liquid, has to be 1.09 times smaller to generate identical amount of heat. Under such conditions, the difference in the NO
x and SO
x concentrations of the corresponding composition and FC without any components is 30% and 20% (
Figure 7), respectively. The co-firing of dry coal with used oil increases the concentrations of the oxides of nitrogen and sulfur in flue gases. This is explained by a higher combustion temperature of such fuel compositions. It was established that [
5], adding used oils to composite fuels increases the combustion temperature by 100–200 °C. For fuel compositions containing municipal solid waste, higher combustion temperature reduces the concentration of carcinogens, e.g., PCDD/Fs, despite higher NO
x and SO
x concentrations [
5].
Apart from the effect of adding combustible components with lower content of nitrogen and sulfur or with greater calorific value to FC, the concentration of anthropogenic emissions in flue gases also depends on the chemical interaction of a large group of substances and compounds in the gas medium at relatively high temperatures [
4]. The temperature of gases in the flame can reach 1100–1400 °C [
5]. Under such conditions, the water vapors, as well as oxides of alkaline and alkaline-earth metals, [
4] can significantly reduce the anthropogenic emissions [
42]. However, a detailed mechanism of chemical reactions, explaining the content of anthropogenic emissions in flue gases from the combustion of different fuel compositions based on hydrocarbons, has not been established yet. We did not consider solving this task in our study, since it requires independent research.
The analysis of anthropogenic emissions has shown that adding some biomass (from several percent to several-dozen percent) to FC significantly reduces the concentrations of sulfur and nitrogen oxides. This reduction in anthropogenic emissions is attributed to the following groups of factors. First, replacing some part of coal with components that contain much less sulfur and nitrogen (
Table 2) reduces the NO
x and SO
x concentrations in flue gases, since solid fossil fuels are the main sources of the respective oxides [
43], forming from the oxidation of volatiles and fuel coke during combustion. The second reason is that sulfur and nitrogen oxides interact with water vapors, as well as biomass pyrolysis and gasification components. This results in the formation of salts in the ash and reduces the NO
x and SO
x concentrations in the gas medium. The redox environment, conditioned by the presence of moisture, leads to the emergence of extra H and OH radicals. They can also interact with NO
x and SO
x in line with the reactions: 2NO + 4H
2 + O
2 → N
2 + 4H
2O (at a temperature above 200 °C); SO
2 + 3H
2 → H
2S + 2H
2O (at temperatures above 1000 °C) [
42]. The metal-containing components in the ash part of the dry biomass and FC can also interact with sulfur and nitrogen oxides (CaO + SO
2 + 1/2O
2 → CaSO
4; 3SO
2 + 2Fe(OH)
3→Fe
2(SO
3)
3 + H
2O; 2Fe + 3NO → 3/2N
2 + Fe
2O
3) at temperatures above 800 °C [
44]. This results in the formation of new substances that either end up as ash residue or participate in further chemical reactions in the gas medium without forming NO
x or SO
x. The final content of NO
x in flue gases is also affected by the type of nitrogen-containing compounds in the fuel. In biomass, most of the nitrogen is in volatile components that are released and burn out at the initial stage of ignition and combustion as compounds some of which do not produce nitrogen oxides [
43]. As for coal and coal processing waste, nitrogen oxides are formed at the stage of the coke part oxidation at relatively high temperatures which are not achieved due to a decrease in temperature of fuel combustion when biomass is added [
43].
Thus, it was experimentally established that adding extra components even with relatively low concentrations (10–30%) to fuel compositions based on fine coal processing waste or dust of low-grade coal significantly enhances the combustion of fuel pellets and reduces the concentrations of anthropogenic emissions in flue gases.
The fundamental research findings provide a scientific evidence of the prospects of using multicomponent fuel pellets in real practice. They serve as a foundation for the development of an industrial technology of producing such fuel pellets, in particular for the calculation of the mass fraction of various components. The results offer the potential for the industrial recovery of abundant existing and annually produced coal processing (FC) and woodworking waste that poses a serious fire and environmental hazard if stored at open-air disposal sites. The cost of such waste-derived fuel is largely made up of transportation expenses and is much lower than that of high-quality fossil fuels. Moreover, the possibility of varying the component composition of fuel pellets by adding biomass or a highly reactive component will enable us to make more effective use of power-generating equipment. The operation of a hot-water boiler with grate firing of solid fuels can be optimized by enhancing combustion not only when employing an automatic control system, but also when changing the fuel composition to achieve the target indicators—for anthropogenic emissions (pellets with biomass) or power generation intensity (pellets with a highly reactive component).